Abstract
Bisphenol A (BPA) is an endocrine-disrupting compound, and the binding mechanism of BPA with carrier proteins has drawn widespread attention. Halogen substitutions can significantly impact the properties of BPA, resulting in various effects for human health. Here, we selected tetrabromobisphenol A (TBBPA) and tetrachlorobisphenol A (TCBPA) to investigate the interaction between different halogen-substituted BPAs and human serum albumin (HSA). TBBPA/TCBPA spontaneously occupied site I and formed stable binary complexes with HSA. Compared to TCBPA, TBBPA has higher binding affinity to HSA. The effect of different halogen substituents on the negatively charged surface area of BPA was an important reason for the higher binding affinity of TBBPA to HSA compared to TCBPA. Hydrogen bonds and van der Waals forces were crucial in the TCBPA–HSA complex, while the main driving factor for the formation of the TBBPA–HSA complex was hydrophobic interactions. Moreover, the presence of TBBPA/TCBPA changed the secondary structure of HSA. Amino acid residues such as Lys199, Lys195, Phe211, Arg218, His242, Leu481, and Trp214 were found to play crucial roles in the binding process between BPA compounds and HSA. Furthermore, the presence of halogen substituents facilitated the binding of BPA compounds with HSA.
1. Introduction
Bisphenol compounds (BPs) are a class of basic chemicals used in the production of polycarbonates and epoxy resins. Among them, BPA is the most used in industry, including in epoxy resins, polycarbonate plastics, dental products, medical devices, electronics, and more [1]. Meanwhile, halogenated derivatives of BPA, such as TBBPA and TCBPA were commonly used as flame retardants [2]. As the commercial applications of BPs have expanded, researchers have increasingly focused on the potential toxicity of BPs, including endocrine-disrupting activity, genetic toxicity, and acute toxicity [3]. Studies have indicated that long-term exposure to BPs may lead to various diseases [4]. Furthermore, BPs can accumulate in the human body through various routes, including skin contact, dietary exposure, and inhalation of dust [5]. Once in the bloodstream, BPs bind to HSA and are transported to tissues and organs through the circulatory system [6]. Hence, the adverse effects of BPs on the body are closely linked to their affinity for binding with HSA. While the impact of BPs on the endocrine system has been extensively studied [7], many mechanisms of BPs, particularly their binding mechanisms with HSA, remain unclear.
With between 55% and 60% of all the proteins in plasma being HSA, it is the most prevalent protein in the plasma of the circulatory system. HSA plays multiple physiological roles, including transportation, pH regulation, and maintenance of blood osmotic pressure [8]. Crucially, HSA is important for the distribution of small molecules [9,10], and it serves as a storage and transport system for both exogenous and endogenous small molecules in the body, including fatty acids, hormones, drugs, amino acids, and proteins [11,12]. HSA contains three similar domains (I, II, and III), each of which are further divided into two subdomains (IA, IB, IIA, IIB, IIIA, and IIIB) [13,14]. Overall, there are seven binding sites in HSA, and the two important binding sites are Sudlow’s sites, namely site I and site II, located in the IIA and IIIA subdomains, respectively [15,16]. Even though these domains have similarities in their amino acid sequence and structure, each domain exhibits different ligand binding affinities and functions. For example, Sudlow’s I preferentially binds with bulky heterocyclic anions such as warfarin, whereas Sudlow’s II has a preferential binding affinity for aromatic carboxylates such as ibuprofen [17]. As the primary reservoir for small molecules in the blood, HSA can bind with ligands in a dissociated state, forming ligand–HSA complexes. The affinity between HSA and ligands is usually expressed by the binding constant (Ka), which represents an important factor influencing the metabolism, distribution, and free concentration of ligands [18], and it significantly affects the half-life of ligands in the human body. A higher Ka value indicates lower tissue absorption of the ligand and a longer elimination half-life in the body [19]. Moreover, the binding of ligands to HSA can change the conformation of HSA, leading to the disruption of its biological function [20,21]. Additionally, toxic small molecules can also be transported, and they are released to target organs with the help of HSA [8,22]. Therefore, the Ka between ligands and HSA can serve as a useful parameter for predicting the potential toxicity of compounds and evaluating their toxicity.
To date, there has been limited research on the interaction between BPA and its analogues with serum albumin molecules. Xie et al. found that hydrophobic interactions were present in the BPA–HSA interaction [23]. Through hydrophobic interactions, BPA could accumulate in the aromatic groups of BSA and adjacent DNA base pairs [24]. Furthermore, Mathew et al. reported the interaction between BPS/BPA with serum albumin and analyzed whether BPS could be a potential replacement for BPA [25]. These studies not only provide new insights into the non-covalent interactions between BPA-like compounds with biomacromolecules but also help elucidate the toxic mechanisms of these harmful chemicals.
However, the mechanism of interaction between halogen-substituted BPA compounds and HSA has not been thoroughly investigated. Therefore, we selected different halogen-substituted BPAs (TBBPA and TCBPA) as representative compounds to thoroughly explore and analyze the binding characteristics of TBBPA/TCBPA with HSA using various spectroscopic techniques and computer simulations. Furthermore, the obtained data combined with theoretical calculation were used to investigate the specific impact of various halogen substitutions on the interaction between HSA and BPA. The results of this research will provide valuable data support for better understanding the distribution, transport, and potential threats to human health posed by different BPA compounds in the human body.
2. Results and Discussion
2.1. Formation of Complexes
UV-visible absorption spectroscopy is widely used to determine the formation of a ligand–receptor complex [26,27]. The formation of a complex often leads to significant changes in the absorption intensity or maximum absorption peak position of the receptor’s UV-visible absorption spectrum [11]. As shown in Figure 1, HSA exhibits two characteristic absorption peaks at 215 nm and 280 nm, respectively. The peak at 215 nm originates from the n → π* transition of the C=O bond in the peptide backbone of HSA, while another absorption peak corresponds to the information of aromatic amino acid residues in the HSA structure [28].
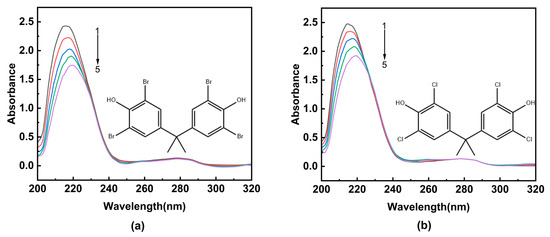
Figure 1.
The UV-visible absorption spectra of HSA in the presence of ligands. (a) TBBPA; (b) TCBPA. [HSA] was 4.0 × 10−6 M, and [TBBPA] and [TCBPA] were varied as indicated: [TBBPA] = 0, 1.0, 2.0, 3.0, 4.0 × 10−5 M from curves 1 → 5; [TCBPA] = 0, 1.0, 2.0, 3.0, 4.0 × 10−6 M from curves 1 → 5.
Upon increasing the concentration of TBBPA/TCBPA, a notable decrease in the intensity of the characteristic absorption peak at 215 nm of HSA is observed, accompanied by a redshift. This observation suggests that TBBPA/TCBPA can bind to HSA, and this bind process change is conformational in the peptide chain of HSA [29,30]. Furthermore, a slight decrease is observed in absorbance at 280 nm, indicating the exposure of aromatic amino acids to a more polar environment [31]. Although the shape of the maximum peak at 280 nm remains largely unchanged, these findings revealed HSA can interact with TBBPA/TCBPA, leading to the formation of TBBPA/TCBPA–HSA complexes.
2.2. Interaction Study
Fluorescence spectroscopy is a valuable tool for exploring the binding characteristics between a ligand and receptor [32]. The primary source of HSA’s intrinsic fluorescence is aromatic amino acids, including tryptophan (Trp) residues, phenylalanine (Phe), and tyrosine (Tyr) [33,34]. Among them, Phe is often disregarded due to its poor quantum yield, and therefore, the fluorescence intensity mostly reflects the contributions of Trp and Tyr residues [30]. These residues are located in the binding cavity of HSA, and their fluorescence intensities are sensitive to the presence of ligands. Consequently, fluorescence spectroscopy is well suited for investigating the interactions between ligands and HSA.
Figure 2 depicts the effect of TBBPA/TCBPA on the fluorescence spectra of HSA at 298 K. The maximum emission wavelength of HSA is observed at 342 nm, and its fluorescence intensity systematically decreases as the concentrations of TBBPA/TCBPA increase, accompanied by a slight blue shift in the maximum wavelength. These observations indicate the presence of interactions between TBBPA/TCBPA and HSA, and the microenvironment surrounding Tyr and Trp residues were altered by ligands [11,28]. These results confirm the formation of complexes between TBBPA/TCBPA and HSA, which is in line with the findings from the UV-visible absorption spectroscopy experiments. Moreover, it is noteworthy that TBBPA exhibits a stronger fluorescence-quenching ability compared to TCBPA, indicating a higher binding affinity between TBBPA and HSA.
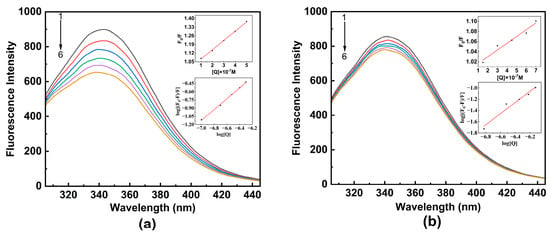
Figure 2.
The fluorescence spectra of HSA with ligands at 298 K. (a) TBBPA; (b) TCBPA. The insets of the figure show the Stern–Volmer and double logarithmic equation curves for TBBPA–HSA and TCBPA–HSA. [HSA] = 1.0 × 10−6 M; [TBBPA] = 0, 1.0, 2.0, 3.0, 4.0, 5.0 × 10−6 M; [TCBPA] = 0, 1.5, 3.0, 4.5, 6.0, 7.5 × 10−6 M.
Fluorescence quenching may occur by dynamic quenching, static quenching, or mixed quenching [35]. To investigate the detailed quenching mechanism between HSA and TBBPA/TCBPA, Equations (1) and (2) [32] can be utilized. If the value of Kq is greater than 2.0 × 1010 M−1·s−1 (the maximum scattering collision quenching rate constant for dynamic quenching), it indicates static quenching [28]. Conversely, if the value is below this threshold, it indicates dynamic quenching. It should be noted that the values of Kq for TBBPA/TCBPA with HSA are significantly larger than 2.0 × 1010 M−1·s−1, revealing that the fluorescence quenching between TBBPA/TCBPA and HSA corresponds to the static quenching mechanism (Table 1).

Table 1.
Stern–Volmer quenching parameters.
In addition to Kq, the trend of Ksv with changes in temperature is another important parameter for distinguishing the source of fluorescence quenching [36]. Table 1 presents the Ksv values for TBBPA/TCBPA–HSA under different temperatures. In the TBBPA group, the Ksv values decline with increasing temperature, providing further evidence for the static quenching mechanism. Conversely, the Ksv value for TCBPA–HSA increases with temperature, indicating the occurrence of dynamic quenching in the binding process between TCBPA and HSA [30]. In summary, TBBPA–HSA exhibits a static quenching mechanism, while TCBPA–HSA exhibits a mixed quenching mechanism.
Through thermodynamic experiments, it is possible to comprehend the interaction between TBBPA/TCBPA and HSA better. Ka and n were calculated using Equation (3) and are presented in Table 2. At any temperature, the value of n tends to be close to 1, showing a stable binary complex was formed between TBBPA/TCBPA and HSA. In vivo, binding constants for drug–protein interactions typically fall within the range of 104~106 M−1 [20]. Binding constants in the range of 104 M−1 and 105~106 M−1 generally signify moderate and strong binding strengths, respectively, between the ligand and HSA [37]. The Ka values for TBBPA–HSA and TCBPA–HSA were 6.245 × 105 and 2.040 × 105 M−1 at 298 K, respectively, indicating the strong binding affinity in both cases. Notably, the Ka value of TBBPA–HSA is three times higher than that of TCBPA–HSA, suggesting a higher binding affinity of TBBPA to HSA.

Table 2.
Thermodynamic parameters of present work.
In general, ligands primarily exist in either the bound state, where they are mainly bound to plasma proteins, or the free state in the bloodstream [38]. The bound ligand serves as a reservoir or storage, while the free ligand exerts its toxicological and/or pharmacological effects [39]. Hence, the Ka of ligand–HSA was considered to be a useful parameter to access the toxicity of similar compounds [35,37]. For example, the toxicity of aromatic organophosphate flame retardants was inversely proportional to their binding affinity with HSA [35]. The Ka values of TBBPA–HSA are significantly higher than those of TCBPA at different temperatures, indicating a greater stability of TBBPA–HSA compared to TCBPA–HSA. Consequently, the proportion of free TBBPA in the bloodstream is lower than that of TCBPA under the same intake conditions. This suggests that TCBPA may have a higher toxicity in the body compared to TBBPA [40,41]. Moreover, stronger binding results in a longer half-life and slower metabolism in the body, potentially enhancing toxic side effects [42]. Therefore, TBBPA is more likely to accumulate in the plasma with a longer elimination half-life compared to TCBPA. Overall, the long-term effects of TBBPA on HSA should be taken into consideration. These findings emphasize the importance of not overlooking the impact of TBBPA/TCBPA on human health.
To obtain more understanding of the binding forces between TBBPA/TCBPA and HSA, the ΔG, ΔS, and ΔH between HSA and TBBPA/TCBPA were calculated using Equation (4). A negative value of ΔG indicates the spontaneous formation of TBBPA/TCBPA-HSA complexes (Table 2) [43]. The values of ΔH and ΔS provide information about the binding forces between macromolecules and ligands [44,45]. For TBBPA–HSA, the ΔH is 19.19 kJ·moL−1, and the ΔS is 175.4 J·moL−1·K−1, indicating hydrophobic interactions being the main driving force [46]. Conversely, for TCBPA–HSA, the ΔH is −55.35 kJ·moL−1, and the ΔS is −84.86 J·moL−1·K−1. Therefore, hydrogen bonds and van der Waals forces play significant roles in the formation of TCBPA–HSA complexes [18,47].
To further analyze the differences in the binding ability between TBBPA and TCBPA with HSA, we employed Multiwfn 3.8 software [48] to calculate and analyze the molecular surface electrostatic potential of these halogen-substituted BPAs. Table 3 and Figure 3 display the calculated results. The different halogen substitutions on BPA structure can impact the distribution and the size of positive and negative surface areas of the electron cloud. Figure 3 illustrates that the negative surface area of TBBPA (157.29 Å2) is larger than that of TCBPA (143.55 Å2). These differences arising from varying halogen substitutions lead to significant alterations in the molecular surface electrostatic potential, indicating that a more stable complex between HSA and TBBPA would be formed if the latter had a higher negative surface area [49]. This finding aligns with the observed increase in Ka values.

Table 3.
Detailed data of surface charge for TBBPA and TCBPA.
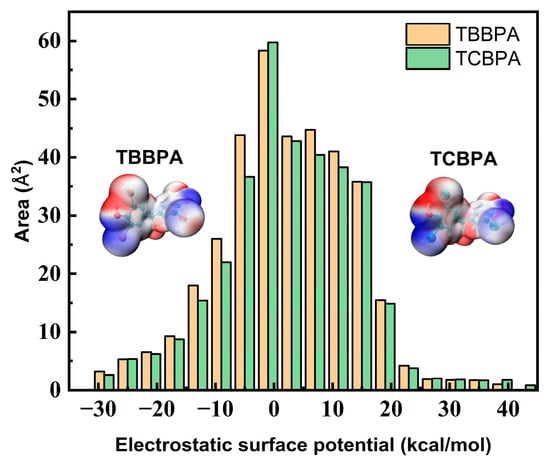
Figure 3.
Surface charge and distribution of TBBPA and TCBPA.
2.3. Binding Site Analysis
HSA contains two major binding sites for ligand [50]. Among them, site I encompasses Trp and Tyr residues, while site II only contains Tyr residue. Synchronous fluorescence spectroscopy is a highly sensitive and selective technique that provides relevant insights into the interaction of ligands with aromatic amino acids. In the experiment, the monochromators were set for synchronous scans at fixed wavelength differences (∆λ) between the excitation wavelengths (λex) and the measured emission wavelengths (λem). Synchronous fluorescence spectra of Tyr and Trp residues were obtained, with Δλ being 15 and 60 nm, respectively (Δλ = λem − λex). Hence, the effects of TBBPA/TCBPA on Trp and Tyr residues were explored by synchronous fluorescence spectroscopy. Upon the addition of TBBPA/TCBPA, a significant decline in the fluorescence intensity of Trp is observed, while the fluorescence intensity of Tyr experiences a slight decrease (Figure 4). These phenomena indicate that the binding site of HSA with TBBPA/TCBPA is close to Trp [51], and the microenvironment surrounding Trp is influenced by the presence of TBBPA/TCBPA. In summary, TBBPA/TCBPA enters site I and binds to HSA.
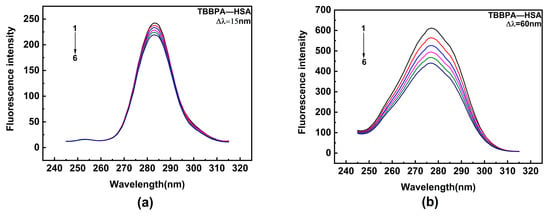
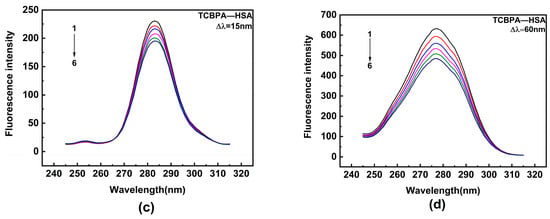
Figure 4.
Synchronous fluorescence spectra. (a,b) TBBPA–HSA; (c,d) TCBPA-HSA. Here, [HSA] = 1.0 × 10−6 M; [TCBPA] = 0, 1.5, 3.0, 4.5, 6.0, 7.5 × 10−6 M; [TBBPA] = 0, 1.0, 2.0, 3.0, 4.0, 5.0 × 10−6 M.
HSA possesses distinct hydrophobic cavities for ligand binding, and through site-specific competition experiments, we can identify the specific binding sites of ligands on HSA [10,52]. In this work, three representative site-specific competition probes were chosen to determine the specific binding sites of TBBPA/TCBPA with HSA [37]. Table 4 presents the results, showing that the presence or absence of ibuprofen or digoxin has little impact on the Ka values of TBBPA/TCBPA with HSA (p > 0.05, n = 3). However, the Ka values of TBBPA/TCBPA with HSA noticeably decrease upon the addition of phenylbutazone (p < 0.01, n = 3). This suggests competition between phenylbutazone and TBBPA/TCBPA for binding sites. Hence, it can be said that the primary binding site of TBBPA/TCBPA with HSA is site I [53], which aligns with the findings from synchronous fluorescence spectroscopy.

Table 4.
The Ka values of TBBPA/TCBPA–HSA with competing probes.
2.4. Conformational Changes Study
To investigate the conformational changes induced by TBBPA/TCBPA in HSA, both qualitative and quantitative analyses were conducted using 3D fluorescence spectra and CD spectra.
Three-dimensional fluorescence spectra allow for qualitative assessment of protein conformational changes [12,31]. Two characteristic peaks of HSA can be observed in 3D spectra (Figure 5). Peak 1 represents the properties of Trp and Tyr residues [54], and peak 2 reflects the fluorescence spectrum associated with the protein–peptide chain structure, which is closely related to the secondary structure of protein [55]. Table 5 presented the complete data of the 3D fluorescence spectra. In the TBBPA and TCBPA groups, the intensity of peak 1 significantly decreases, and the presence of ligands induced blue shift. This indicates the insertion of TBBPA/TCBPA into the binding sites of HSA, leading to changes in the microenvironment surrounding Trp and Tyr residues [56]. Moreover, the Stokes shift of peak 2 changes by 6 nm and 1 nm, respectively, while the intensity of peak 2 decreases to 575.5 and 692.6. These observations suggest that TBBPA/TCBPA induces changes in the secondary structure of HSA, and TBBPA exerts a stronger impact on HSA conformation compared to TCBPA.
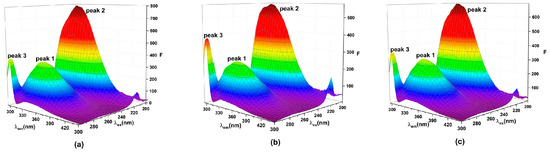
Figure 5.
Three-dimensional fluorescence spectra. (a) HSA; (b) TBBPA-HSA; (c) TCBPA-HSA. [HSA] = [TBBPA] = [TCBPA] = 1.0 × 10−6 M.

Table 5.
Relevant data from the three-dimensional fluorescence spectra.
CD spectroscopy can quantitatively analyze the specific changes of protein secondary structure, providing more particular information regarding the conformational changes of HSA upon interaction with TBBPA/TCBPA [10]. HSA displays two negative peaks at 208 nm (π → π*) and 220 nm (n → π*), representing the characteristic α-helical structure of HSA (Figure 6) [57]. The CD peak intensity at 220 nm of HSA undergoes significant changes in the presence of TBBPA/TCBPA (Figure 6), indicating alterations in the spatial conformation of HSA induced by TBBPA/TCBPA and modifications to HSA’s secondary structure [58]. Moreover, the effect of TBBPA on the CD spectrum of HSA is more pronounced compared to TCBPA, revealing a greater impact of TBBPA on HSA conformation.
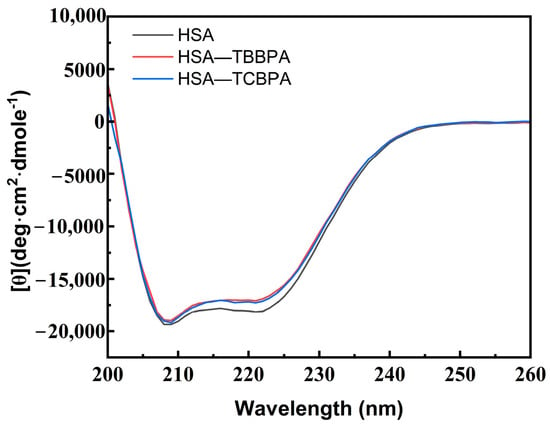
Figure 6.
CD spectra of HSA with and without TBBPA/TCBPA. [HSA] = [TBBPA] = [TCBPA] = 1.0 × 10−6 M.
Subsequently, the data were analyzed using the CONTINLL program [59]. The findings show that the α-helix content in free HSA is 51.4%, the β-turn content is 14.1%, the β-sheet content is 7.7%, and the random coil content is 26.9% (Table 6). Upon the addition of ligands, a similar change was observed in different groups. Specifically, the α-helix content slightly decreased from 51.4% to 48.2% and 50.4% upon interaction with TBBPA and TCBPA, respectively. These quantitative data demonstrate that the presence of TBBPA/TCBPA can cause changes in HSA conformation, with TBBPA exhibiting a more significant effect on HSA conformation compared to TCBPA. Furthermore, changes in protein structure may impact its physiological function [60]. Therefore, the conformational alterations in HSA caused by TBBPA/TCBPA may disrupt the bioactivity of HSA in the human body.

Table 6.
Relevant data of CD spectra.
2.5. Molecular Docking
Molecular docking was employed in this study to explore the binding modes of HSA with TBBPA/TCBPA, providing additional information at the molecular level [32,61]. TBBPA/TCBPA insert into the IIA subdomain of HSA (site I), which was confirmed by site competition experiments. As a result, the docking center was set at site I in this work. The conformation of the TBBPA/TCBPA–HSA complex was simulated by the AutoDock Vina program [62]. These data support the conclusion that TBBPA/TCBPA can spontaneously enter site I of HSA. The docking results revealed that the binding energies for TBBPA–HSA and TCBPA–HSA were −7.5 kcal/mol and −7.4 kcal/mol, respectively. This qualitatively indicates that the affinity of TBBPA for HSA is higher than that of TCBPA, which is consistent with the previous findings from the solution experiments.
Figure 7 illustrates the optimal docking models of HSA–TBBPA and HSA–TCBPA, where TBBPA/TCBPA is surrounded by numerous residues upon entering the HSA cavity. The π electrons on the phenyl rings of TBBPA and TCBPA are involved in pi–pi interactions with Trp214. This is probably what causes the fluorescence-quenching mechanism of TBBPA/TCBPA in HSA (Trp214 being a major contributor to HSA fluorescence intensity [63]). It is worth noting that TBBPA/TCBPA interacts with residues Lys199, Lys195, Ser202, Phe211, Arg218, Arg222, His242, Leu481, and Trp214. These results suggest that these amino acid residues may play a crucial role in the binding of BPA-like compounds to HSA. Moreover, the halogen substituents on TBBPA and TCBPA engage in pi–alkyl interactions with certain amino acid residues of HSA, indicating the presence of halogen substituents favors the formation of stable complexes between BPA-like compounds and proteins. According to the literature, the Ka value for BPA–HSA is at the level of 103 [23], while the Ka values obtained in this experiment are at the level of 105. Therefore, these data partially support the aforementioned conclusions.
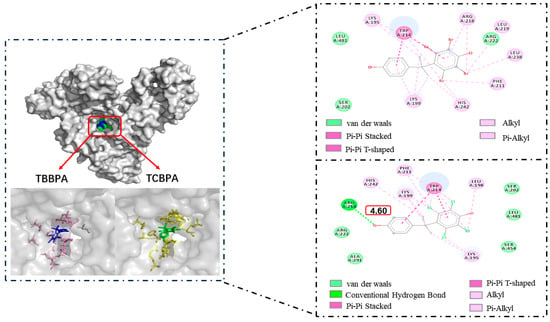
Figure 7.
Molecular docking of TBBPA/TCBPA–HSA.
3. Materials and Methods
3.1. Instruments and Software
Steady-state fluorescence measurements (with a single-cell Peltier accessory temperature control system) were conducted by using a Cary Eclipse fluorescence spectrophotometer (Agilent Technologies, Palo Alto, CA, USA). pH measurements were performed using an EL20 precision pH meter (Mettler Toledo Inc., Schwerzenbach, Switzerland). UV-visible absorption data detection was performed using a GENESYS 50 UV-Visible spectrophotometer (Thermo Scientific, Carlsbad, CA, USA). Protein secondary structure analysis was carried out by using a J-1700 circular dichroism spectrometer (JASCO, Ishikawa-machi, Tokyo, Japan). AutoDock Vina 1.1.2 and Discovery Studio Client software 2018 were utilized for building the ligand (TBBPA/TCBPA)–receptor (HSA) models and analyzing their interactions [62]. The Multiwfn software 3.8 was employed to calculate the surface charge distribution of TBBPA and TCBPA [48].
3.2. Experimental Chemicals
TBBPA, TCBPA, and site-specific competitors (ibuprofen, digitonin, and phenylbutazone) were supplied by Shanghai Macklin Biochemical Co., Ltd., Shanghai, China. TBBPA and TCBPA were dissolved in absolute methanol to prepare stock solutions (1.0 × 10−2 M) and stored at −20 °C before use. HSA and Tris were obtained from Sigma-Aldrich, St. Louis, MI, USA and Shanghai Yuan Ye Biological Technology Co., Ltd., Shanghai, China, respectively. The stock solutions were diluted to appropriate concentrations according to experimental requirements. A Tris-HCl buffer solution containing 0.9% NaCl (pH 7.4) was used to imitate the physiological environment in the human body. All reagents used in this work were of analytical grade or higher.
3.3. Experimental Methods
3.3.1. UV Absorption Experiment
The concentration of HSA was set at 4.0 × 10−6 M, while the concentration of the ligands (TBBPA/TCBPA) was varied as follows: [TBBPA] = 0, 1.0, 2.0, 3.0, 4.0 × 10−5 M; [TCBPA] = 0, 1.0, 2.0, 3.0, 4.0 × 10−6 M. UV absorption spectra of HSA were recorded in the range of 200–320 nm. The corresponding concentration solutions of the ligands were used as the blank solution, which were used to eliminate the effect of TBBPA/TCBPA on the UV-vis absorption spectra.
3.3.2. Fluorescence–Thermodynamic Experiment
Prior to taking steady-state fluorescence measurements, the HSA and ligand mixture solution was given 2 min to equilibrate. At different temperatures (298, 303, and 310 K), fluorescence emission spectra of HSA were recorded in the range of 305–445 nm while varying the concentration of TBBPA/TCBPA. The concentration of HSA was fixed at 1.0 × 10−6 M. For TBBPA, it was sequentially added to achieve a concentration range from 0 to 5.0 × 10−7 M, with each change being 1.0 × 10−7 M. For TCBPA, it was sequentially added to achieve a concentration range from 0 to 7.5 × 10−7 M, with each change being 1.5 × 10−7 M.
To account for the ligand concentration in the study system, which was much lower than the 10−5 M level, the inner-filter effect of the ligand was neglected. The Stern–Volmer equation and double logarithmic equation (Equations (1)–(3)) were used to analyze the data of fluorescence spectra. Thermodynamic parameters were obtained using Equation (4) to determine the driving forces in the binding process.
where F0 and F are the fluorescence intensities of the study system with and without ligand, respectively; [Q] is the concentration of the ligand; Ksv and Kq are the Stern–Volmer quenching constant and the quenching rate constant of the biomolecule (M−1 s−1), respectively; τ0 (6.30 ns) is the fluorescence lifetime value of free HSA [64]; Ka and n are the binding constant and the number of binding sites of the ligand–receptor, respectively; ΔG, ΔH, and ΔS are the changes in Gibbs free energy, enthalpy, and entropy during the ligand–receptor binding process, respectively. In addition, R and T are the gas constant at room temperature and the temperature in Kelvin, respectively.
3.3.3. Synchronous Fluorescence Experiment
In this study, the wavelength intervals (Δλ = λem − λex) were fixed at 15 to gather fluorescence characteristic information from the Tyr residues, whereas Δλ was fixed at 60 to gather fluorescence characteristic information from the Trp residues [65]. The data were recorded in the wavelength range of 245–315 nm.
3.3.4. Site-Specific Competitive Experiment
The site-specific competitive experiment was conducted following the methodology described in the literature [37], using digitonin, ibuprofen, and phenylbutazone as site-specific probes. First, a 1:1 complex of the site-specific probe with HSA was prepared in the buffer solution. Then, TBBPA/TCBPA was added, and the influence of the site-specific probes on the binding ability of TBBPA/TCBPA with HSA was explored by measuring the changes in fluorescence intensity. This experiment was conducted under the same test conditions as the thermodynamic experiment, except at room temperature.
3.3.5. Three-Dimensional (3D) Fluorescence Experiment
The concentration of the HSA solution was fixed at 1.0 × 10−6 M. The effect of TBBPA/TCBPA on the 3D fluorescence spectra of HSA was measured when the concentrations of ligands were 1.0 × 10−6 M. The instrumental parameters were then set as follows: the step sizes were 1 nm; the excitation and emission slit widths were set to 10 nm; excitation wavelength range and emission wavelength range were 200–300 nm and 295–445 nm, respectively.
3.3.6. Circular Dichroism (CD) Spectroscopy Experiment
CD spectra of HSA with and without TBBPA/TCBPA were measured in the range of 200–260 nm, and the CONTINLL program [59] was utilized to calculate the secondary structure content of HSA. The concentration of HSA and the ligands was set as 1.0 × 10−6 M. The instrumental parameters were set as follows: path length of 1 mm and scanning speed of 100 nm min−1.
3.3.7. Molecular Docking Calculation
The 1H9Z crystal structure in the RCSB Protein Data Bank (http://www.rcsb.org/pdb accessed on 15 May 2023) was used as the three-dimensional structure of HSA. In this structure, the protonation states of residues were at pH 7.0 [66]. All water molecules and ligands in the 1H9Z crystal structure were removed, and Kollman charges and polar hydrogens were added to residues by using MGLTools-1.5.6 software. The structures of TBBPA and TCBPA were built using Chem Office and optimized under MMFF94 force field conditions, and then converted into pdbqt format. The optimal configurations of the TBBPA/TCBPA–HSA complexes were constructed using AutoDock Vina 1.1.2 according to the official tutorial (https://vina.scripps.edu/tutorial/ accessed on 15 May 2023). Finally, the results were showed in the form of pictures with the help of PyMOL-2.5 and Discovery Studio Visualizer 2018 Client. Additionally, the ligand conformations constructed and optimized using Chem Office were used to calculate the surface charge and distribution of the ligands using Multiwfn software 3.8 [48].
4. Conclusions
This study performed a combination of multispectral, theoretical calculation, and molecular docking to generate credible and comprehensive experimental data. The investigation focused on the interaction mechanisms between HSA and TBBPA/TCBPA, as well as the impact of different halogen substitutions on their binding process.
The results reveal that TBBPA/TCBPA can effectively insert into site I of HSA, forming stable binary complexes. Hydrophobic interactions primarily drive the formation of the TBBPA–HSA complex, whereas the TCBPA–HSA complex is derived by hydrogen bonding and van der Waals forces. The binding constant of TBBPA–HSA exceeds that of TCBPA–HSA by threefold. The presence of TBBPA/TCBPA induces conformational changes and alters the microenvironment of HSA, with TBBPA exhibiting a stronger influence on the secondary structure compared to TCBPA. These structural modifications in HSA imply potential interference with its biological activity, thus highlighting the associated health hazards for humans.
Furthermore, the inclusion of halogen substituents facilitates the stability of the BPA-like compounds–HSA complex. The differential impact of various halogen substitutions on the negative electrostatic surface area of BPA emerges as a significant factor relative to the higher binding affinity of TBBPA with HSA when compared to TCBPA. Notably, the stronger binding between TBBPA and HSA results in an extended half-life and slower metabolism within the body, warranting additional attention towards the long-lasting effects of TBBPA on humans. Thus, this study enhances our understanding of the binding mechanisms between halogenated BPA compounds and HSA, providing valuable theoretical support for managing human health risks.
Author Contributions
Conceptualization, H.Z. and B.C.; methodology, H.Z.; software, R.C.; validation, H.Z., R.C. and C.C.; formal analysis, H.Z.; investigation, H.Z.; resources, B.C.; data curation, R.C.; writing—original draft preparation, H.Z., R.C. and C.C.; writing—review and editing, H.Z., L.G., P.D. and L.D.; visualization, L.G., P.D. and L.D.; supervision, B.C.; project administration, B.C.; funding acquisition, B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (22064012, 31700403 and 21907048) and Natural Science Foundation of Jiangxi Province (20202BAB203009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ikhlas, S.; Usman, A.; Ahmad, M. Comparative study of the interactions between bisphenol-A and its endocrine disrupting analogues with bovine serum albumin using multi-spectroscopic and molecular docking studies. J. Biomol. Struct. Dyn. 2019, 37, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.-B.; et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 2015, 83, 183–191. [Google Scholar]
- Lo, H.S.; Po, B.H.K.; Li, L.; Wong, A.Y.M.; Kong, R.Y.C.; Li, L.; Tse, W.K.F.; Wong, C.K.C.; Cheung, S.G.; Lai, K.P. Bisphenol A and its analogues in sedimentary microplastics of Hong Kong. Mar. Pollut. Bull. 2021, 164, 112090. [Google Scholar] [PubMed]
- Hu, Q.; Guan, X.-Q.; Song, L.-L.; Wang, H.-N.; Xiong, Y.; Liu, J.-L.; Yin, H.; Cao, Y.-F.; Hou, J.; Yang, L.; et al. Inhibition of pancreatic lipase by environmental xenoestrogens. Ecotox. Environ. Saf. 2020, 192, 110305. [Google Scholar] [CrossRef]
- Liao, S.; Wu, X.; Xie, Z. Determination of some estrogens by flow injection analysis with acidic potassium permanganate–formaldehyde chemiluminescence detection. Anal. Chim. Acta 2005, 537, 189–195. [Google Scholar] [CrossRef]
- Das, P.; Chaudhari, S.K.; Das, A.; Kundu, S.; Saha, C. Interaction of flavonols with human serum albumin: A biophysical study showing structure–activity relationship and enhancement when coated on silver nanoparticles. J. Biomol. Struct. Dyn. 2019, 37, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Fénichel, P.; Chevalier, N. Is Testicular Germ Cell Cancer Estrogen Dependent? The Role of Endocrine Disrupting Chemicals. Endocrinology 2019, 160, 2981–2989. [Google Scholar]
- Rabbani, G.; Ahn, S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019, 123, 979–990. [Google Scholar]
- Murugan Sreedevi, S.; Vinod, S.M.; Krishnan, A.; Perumal, T.; Ali Alasmary, F.; Salem Alsaiari, N.; Govindasamy, M.; Rajendran, K. Molecular Docking approach on the effect of Site- Selective and Site-Specific Drugs on the Molecular Interactions of Human Serum Albumin (HSA) -Acridinedione dye complex. Arab. J. Chem. 2023, 16, 104701. [Google Scholar] [CrossRef]
- Perera, N.L.D.; Miksovska, J.; O’Shea, K.E. Elucidation of specific binding sites and extraction of toxic Gen X from HSA employing cyclodextrin. J. Hazard. Mater. 2022, 425, 127765. [Google Scholar]
- Kooravand, M.; Asadpour, S.; Haddadi, H.; Farhadian, S. An insight into the interaction between malachite green oxalate with human serum albumin: Molecular dynamic simulation and spectroscopic approaches. J. Hazard. Mater. 2021, 407, 124878. [Google Scholar] [PubMed]
- Zargar, S.; Wani, T.A. Exploring the binding mechanism and adverse toxic effects of persistent organic pollutant (dicofol) to human serum albumin: A biophysical, biochemical and computational approach. Chem.-Biol. Interact. 2021, 350, 109707. [Google Scholar]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975, 11, 824. [Google Scholar] [PubMed]
- Sudlow, G.D.; Birkett, D.J.; Wade, D.N. Further characterization of specific drug binding sites on HSA. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar] [PubMed]
- Liu, C.; Yang, W.; Gao, Q.; Du, J.; Luo, H.; Liu, Y.; Yang, C. Differential recognition and quantification of HSA and BSA based on two red-NIR fluorescent probes. J. Lumin. 2018, 197, 193–199. [Google Scholar]
- Kragh-Hansen, U.; Chuang, V.T.G.; Otagiri, M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol. Pharm. Bull. 2002, 25, 695. [Google Scholar]
- Sand, K.M.K.; Bern, M.; Nilsen, J.; Noordzij, H.T.; Sandlie, I.; Andersen, J.T. Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics. Front. Immunol. 2015, 5, 682. [Google Scholar] [PubMed]
- Zhu, C.; Liu, F.; Wei, Y.; Zhang, F.; Pan, T.; Ye, Y.; Shen, Y. Evaluating the potential risk by probing the site-selective binding of rutin-Pr(III) complex to human serum albumin. Food Chem. Toxicol. 2021, 148, 111927. [Google Scholar] [PubMed]
- Zhou, Z.; Hu, X.; Hong, X.; Zheng, J.; Liu, X.; Gong, D.; Zhang, G. Interaction characterization of 5−hydroxymethyl−2−furaldehyde with human serum albumin: Binding characteristics, conformational change and mechanism. J. Mol. Liq. 2020, 297, 111835. [Google Scholar] [CrossRef]
- Wang, B.; Qin, Q.; Chang, M.; Li, S.; Shi, X.; Xu, G. Molecular interaction study of flavonoids with human serum albumin using native mass spectrometry and molecular modeling. Anal. Bioanal. Chem. 2018, 410, 827–837. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.; Liu, R.; Sun, Y.; Li, X.; Fan, J. The binding affinity of phthalate plasticizers-protein revealed by spectroscopic techniques and molecular modeling. Food Chem. Toxicol. 2014, 71, 244–253. [Google Scholar]
- Abdelhameed, A.S.; Bakheit, A.H.; AlRabiah, H.K.; Hassan, E.S.G.; Almutairi, F.M. Molecular interactions of AL3818 (anlotinib) to human serum albumin as revealed by spectroscopic and molecular docking studies. J. Mol. Liq. 2019, 273, 259–265. [Google Scholar]
- Xie, X.; Wang, X.; Xu, X.; Sun, H.; Chen, X. Investigation of the interaction between endocrine disruptor bisphenol A and human serum albumin. Chemosphere 2010, 80, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Zhang, X.; Fei, X.-C.; Wang, S.-L.; Gao, H.-W. Binding of bisphenol A and acrylamide to BSA and DNA: Insights into the comparative interactions of harmful chemicals with functional biomacromolecules. J. Hazard. Mater. 2010, 182, 877–885. [Google Scholar]
- Mathew, M.; Sreedhanya, S.; Manoj, P.; Aravindakumar, C.T.; Aravind, U.K. Exploring the Interaction of Bisphenol-S with Serum Albumins: A Better or Worse Alternative for Bisphenol A? J. Phys. Chem. B 2014, 118, 3832–3843. [Google Scholar] [PubMed]
- Raghu, M.S.; Kumar, K.Y.; Veena, K.; Kumar, C.B.P.; Almalki, A.S.; Mani, G.; Alasmary, F.A.; Prashanth, M.K. Synthesis, characterization, antimicrobial and interaction studies of pteridines with human serum albumin: A combined multi-spectroscopic and computational study. J. Mol. Struct. 2022, 1250, 131857. [Google Scholar]
- Hashemi-Shahraki, F.; Shareghi, B.; Farhadian, S. The interaction of Naphthol Yellow S (NYS) with pepsin: Insights from spectroscopic to molecular dynamics studies. Int. J. Biol. Macromol. 2020, 165, 1842–1851. [Google Scholar] [PubMed]
- Gu, J.; Huang, X.; Liu, H.; Dong, D.; Sun, X. A mutispectroscopic study on the structure–affinity relationship of the interactions of bisphenol analogues with bovine serum albumin. Chemosphere 2022, 291, 132769. [Google Scholar]
- Tang, L.; Zuo, H.; Shu, L. Comparison of the interaction between three anthocyanins and human serum albumins by spectroscopy. J. Lumin. 2014, 153, 54–63. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Dehghan, G.; Shaghaghi, M.; Panahi, Y.; Jouyban, A.; Yekta, R. Multispectral and molecular docking studies on the interaction of human serum albumin with iohexol. J. Mol. Liq. 2017, 248, 459–467. [Google Scholar]
- Ren, G.; Sun, H.; Guo, J.; Fan, J.; Li, G.; Xu, S. Molecular mechanism of the interaction between resveratrol and trypsin via spectroscopy and molecular docking. Food Funct. 2019, 10, 3291–3302. [Google Scholar] [PubMed]
- Tan, C.; Zhang, T.; Wang, G.; Zhang, L.; Liu, C.; Li, W.; Li, J.; Lu, R. Study of the interaction mechanism between human serum albumin and Ti3C2Tx with different degrees of oxidation by multi-spectroscopic method and molecular docking. J. Hazard. Mater. Adv. 2023, 9, 100236. [Google Scholar]
- Qadeer, A.; Rabbani, G.; Zaidi, N.; Ahmad, E.; Khan, J.M.; Khan, R.H. 1-Anilino-8-Naphthalene Sulfonate (ANS) Is Not a Desirable Probe for Determining the Molten Globule State of Chymopapain. PLoS ONE 2012, 7, e50633. [Google Scholar]
- Ahmad, P.; Islam, B.U.; Allarakha, S.; Rabbani, G.; Dixit, K.; Moinuddin; Khan, R.H.; Siddiqui, S.A.; Ali, A. Preferential recognition of peroxynitrite-modified human serum albumin by circulating autoantibodies in cancer. Int. J. Biol. Macromol. 2015, 72, 875–882. [Google Scholar] [CrossRef]
- Ma, X.; Kuang, L.; Wang, X.; Zhang, Z.; Chen, C.; Ding, P.; Chi, B.; Xu, J.; Tuo, X. Investigation on the interaction of aromatic organophosphate flame retardants with human serum albumin via computer simulations, multispectroscopic techniques and cytotoxicity assay. Int. J. Biol. Macromol. 2023, 247, 125741. [Google Scholar]
- Ungor, D.; Bélteki, R.; Horváth, K.; Dömötör, O.; Csapó, E. Fluorescence Quenching of Tyrosine-Ag Nanoclusters by Metal Ions: Analytical and Physicochemical Assessment. Int. J. Mol. Sci. 2022, 23, 9775. [Google Scholar]
- Lv, X.; Jiang, Z.; Zeng, G.; Zhao, S.; Li, N.; Chen, F.; Huang, X.; Yao, J.; Tuo, X. Comprehensive insights into the interactions of dicyclohexyl phthalate and its metabolite to human serum albumin. Food Chem. Toxicol. 2021, 155, 112407. [Google Scholar] [PubMed]
- Siddiqui, S.; Ameen, F.; ur Rehman, S.; Sarwar, T.; Tabish, M. Studying the interaction of drug/ligand with serum albumin. J. Mol. Liq. 2021, 336, 116200. [Google Scholar]
- Abdelaziz, M.A.; Shaldam, M.; El-Domany, R.A.; Belal, F. Multi-Spectroscopic, thermodynamic and molecular dynamic simulation studies for investigation of interaction of dapagliflozin with bovine serum albumin. Spectrochim. Acta A 2022, 264, 120298. [Google Scholar]
- Liao, T.; Zhang, Y.; Huang, X.; Jiang, Z.; Tuo, X. Multi-spectroscopic and molecular docking studies of human serum albumin interactions with sulfametoxydiazine and sulfamonomethoxine. Spectrochim. Acta A 2021, 246, 119000. [Google Scholar]
- Port, M.; Idée, J.-M.; Medina, C.; Robic, C.; Sabatou, M.; Corot, C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: A critical review. BioMetals 2008, 21, 469–490. [Google Scholar]
- Cao, H.; Liu, X.; Ulrih, N.P.; Sengupta, P.K.; Xiao, J. Plasma protein binding of dietary polyphenols to human serum albumin: A high performance affinity chromatography approach. Food Chem. 2019, 270, 257–263. [Google Scholar] [CrossRef]
- Mondal, M.; Krishna, R.; Sakthivel, N. Molecular interaction between human serum albumin (HSA) and phloroglucinol derivative that shows selective anti-proliferative potential. J. Lumin. 2017, 192, 990–998. [Google Scholar] [CrossRef]
- Maurya, N.; ud din Parray, M.; Maurya, J.K.; Kumar, A.; Patel, R. Interaction of promethazine and adiphenine to human hemoglobin: A comparative spectroscopic and computational analysis. Spectrochim. Acta A 2018, 199, 32–42. [Google Scholar]
- Khatun, S.; Riyazuddeen; Kumar, A.; Subbarao, N. Thermodynamics, molecular modelling and denaturation studies on exploring the binding mechanism of tetramethylpyrazine with human serum albumin. JChTh 2020, 140, 105915. [Google Scholar]
- Zhang, G.; Zhao, N.; Hu, X.; Tian, J. Interaction of alpinetin with bovine serum albumin: Probing of the mechanism and binding site by spectroscopic methods. Spectrochim. Acta A 2010, 76, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Sardar, P.S.; Roy, P.; Dasgupta, S.; Bose, A. Investigation on the interaction of Rutin with serum albumins: Insights from spectroscopic and molecular docking techniques. J. Photochem. Photobiol. B Biol. 2018, 183, 101–110. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar]
- Ma, J.; Yang, B.; Hu, X.; Gao, Y.; Qin, C. The binding mechanism of benzophenone-type UV filters and human serum albumin: The role of site, number, and type of functional group substitutions. Environ. Pollut. 2023, 324, 121342. [Google Scholar] [PubMed]
- Majid, N.; Siddiqi, M.K.; Alam, A.; Malik, S.; Ali, W.; Khan, R.H. Cholic acid inhibits amyloid fibrillation: Interplay of protonation and deprotonation. Int. J. Biol. Macromol. 2022, 221, 900–912. [Google Scholar]
- Feng, J.; Zhao, X.; Yan, Y.; Chen, H.; Liu, J.; Li, X.; Na, R.; Li, Q.X. Interactions between stipuol enantiomers and human serum albumin. Food Chem. 2022, 385, 132686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, N.; Zhang, Y.; Pan, J.; Gong, D. Binding mechanism of 4−octylphenol with human serum albumin: Spectroscopic investigations, molecular docking and dynamics simulation. Spectrochim. Acta A 2021, 255, 119662. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A.; Alsaif, N.A.; Khayyat, A.I.A. A Comprehensive Investigation of Interactions between Antipsychotic Drug Quetiapine and Human Serum Albumin Using Multi-Spectroscopic, Biochemical, and Molecular Modeling Approaches. Molecules 2022, 27, 2589. [Google Scholar] [CrossRef]
- Maurya, N.; Maurya, J.K.; Singh, U.K.; Dohare, R.; Zafaryab, M.; Moshahid Alam Rizvi, M.; Kumari, M.; Patel, R. In Vitro Cytotoxicity and Interaction of Noscapine with Human Serum Albumin: Effect on Structure and Esterase Activity of HSA. Mol. Pharm. 2019, 16, 952–966. [Google Scholar] [CrossRef]
- Gan, R.; Zhao, L.; Sun, Q.; Tang, P.; Zhang, S.; Yang, H.; He, J.; Li, H. Binding behavior of trelagliptin and human serum albumin: Molecular docking, dynamical simulation, and multi-spectroscopy. Spectrochim. Acta A 2018, 202, 187–195. [Google Scholar] [CrossRef]
- Xiang, H.; Sun, Q.; Wang, W.; Li, S.; Xiang, X.; Li, Z.; Liao, X.; Li, H. Study of conformational and functional changes caused by binding of environmental pollutant tonalide to human serum albumin. Chemosphere 2021, 270, 129431. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Razlansari, M. Exploring the binding mechanisms of inorganic magnetic nanocarrier containing L-Dopa with HSA protein utilizing multi spectroscopic techniques. J.Biomol. Struct. Dyn. 2021, 39, 7160–7167. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hao, H.; Li, Q.; Liu, C.; Lei, J.; Yu, F.; Chen, K.; Liu, Y.; Huang, T. The interaction mechanism between fludarabine and human serum albumin researched by comprehensive spectroscopic methods and molecular docking technique. Spectrochim. Acta A 2020, 233, 118170. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X.; Jiang, Z.; Tuo, X. Investigation of the binding properties between levamlodipine and HSA based on MCR-ALS and computer modeling. Spectrochim. Acta A 2021, 245, 118929. [Google Scholar] [CrossRef]
- Alsaif, N.A.; Wani, T.A.; Bakheit, A.H.; Zargar, S. Multi-spectroscopic investigation, molecular docking and molecular dynamic simulation of competitive interactions between flavonoids (quercetin and rutin) and sorafenib for binding to human serum albumin. Int. J. Biol. Macromol. 2020, 165, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J.Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, X.; Yi, X.; Zhong, S. Mechanistic and conformational studies on the interaction of anesthetic sevoflurane with human serum albumin by multispectroscopic methods. J. Mol. Liq. 2017, 241, 577–583. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, H.; Xu, L.; Cai, R.; Chen, C.; Chi, B.; Tuo, X. An insight into the interaction between Indisulam and human serum albumin: Spectroscopic method, computer simulation and in vitro cytotoxicity assay. Bioorg. Chem. 2022, 127, 106017. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Rehman, M.T.; Rabbani, G.; Khan, P.; AlAjmi, M.F.; Hassan, M.I.; Muteeb, G.; Kim, J. Insight of the Interaction between 2,4-thiazolidinedione and Human Serum Albumin: A Spectroscopic, Thermodynamic and Molecular Docking Study. Int. J. Mol. Sci. 2019, 20, 2727. [Google Scholar] [CrossRef]
- Petitpas, I.; Bhattacharya, A.A.; Twine, S.; East, M.; Curry, S. Crystal structure analysis of warfarin binding to human serum albumin: Anatomy of drug site I. J. Biol. Chem. 2001, 276, 22804–22809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).