Elevated Levels of Circulating lncRNAs LIPCAR and MALAT1 Predict an Unfavorable Outcome in Acute Coronary Syndrome Patients
Abstract
1. Introduction
2. Results
2.1. Plasma Biochemical Parameters of SA, UA, and STEMI Patients
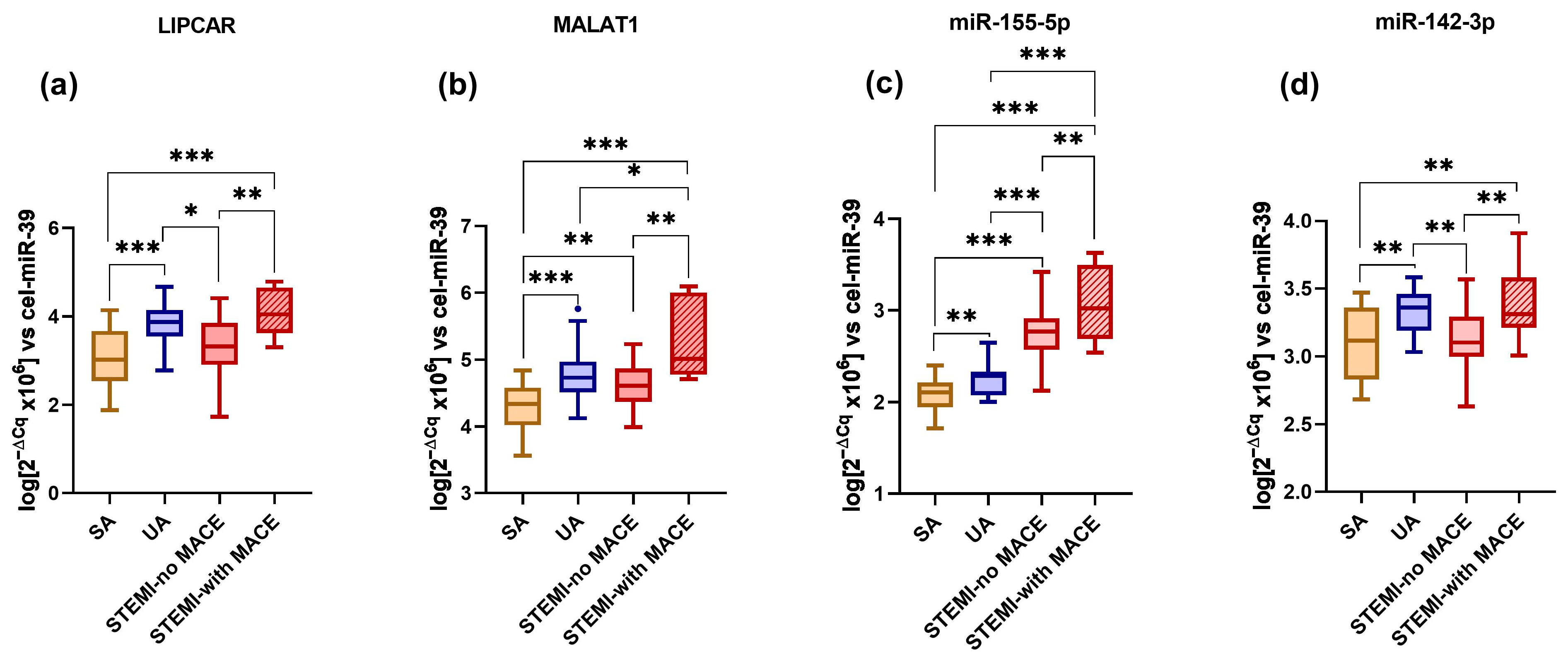
2.2. Distribution of ncRNAs in the Plasma of SA, UA, and STEMI Patients
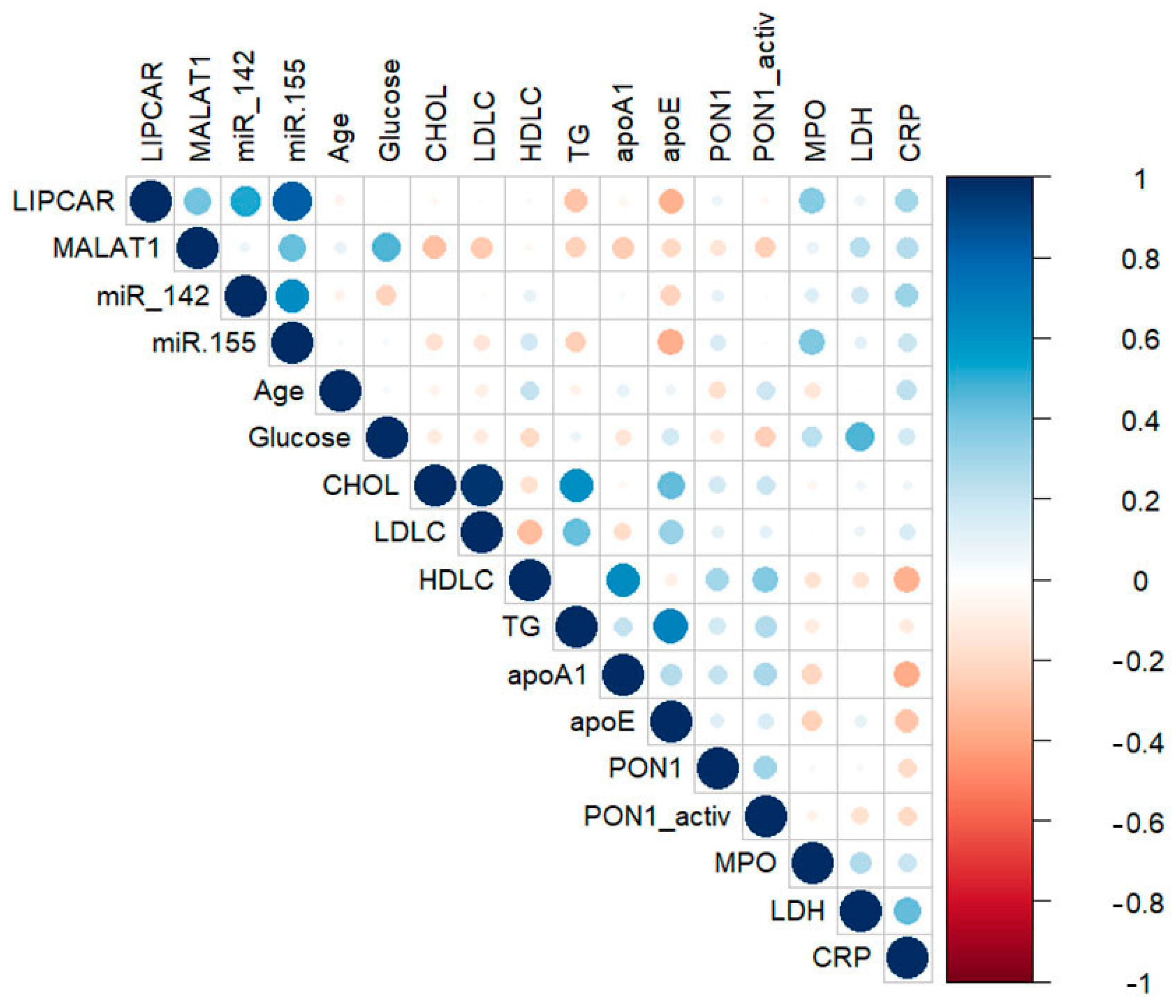
2.3. Correlations between lncRNAs, miRNAs, and Main Biochemical Parameters in Plasma of CAD Patients
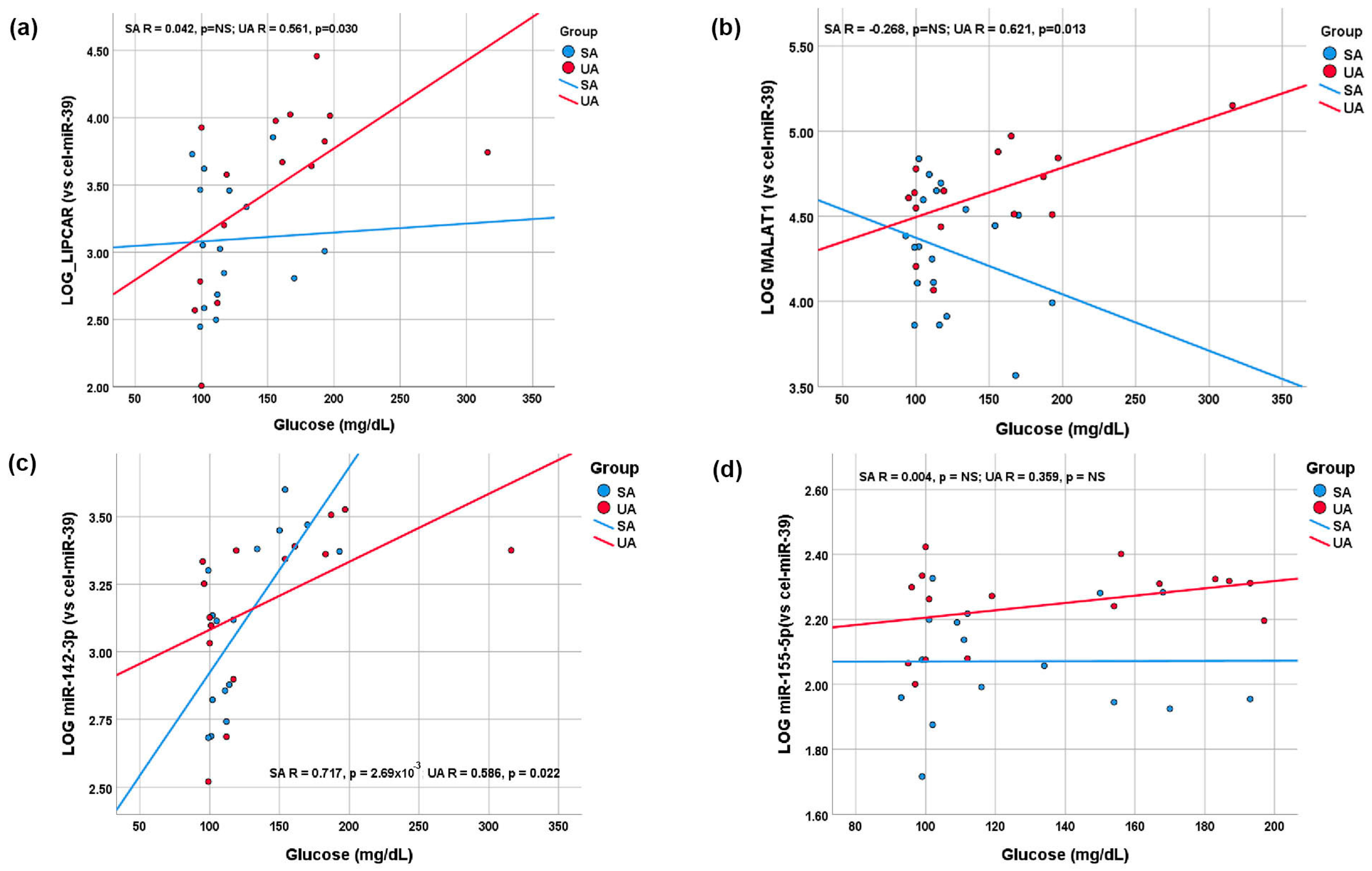
2.4. Correlations between lncRNAs, miRNAs, and Main Parameters in Plasma of STEMI Patients
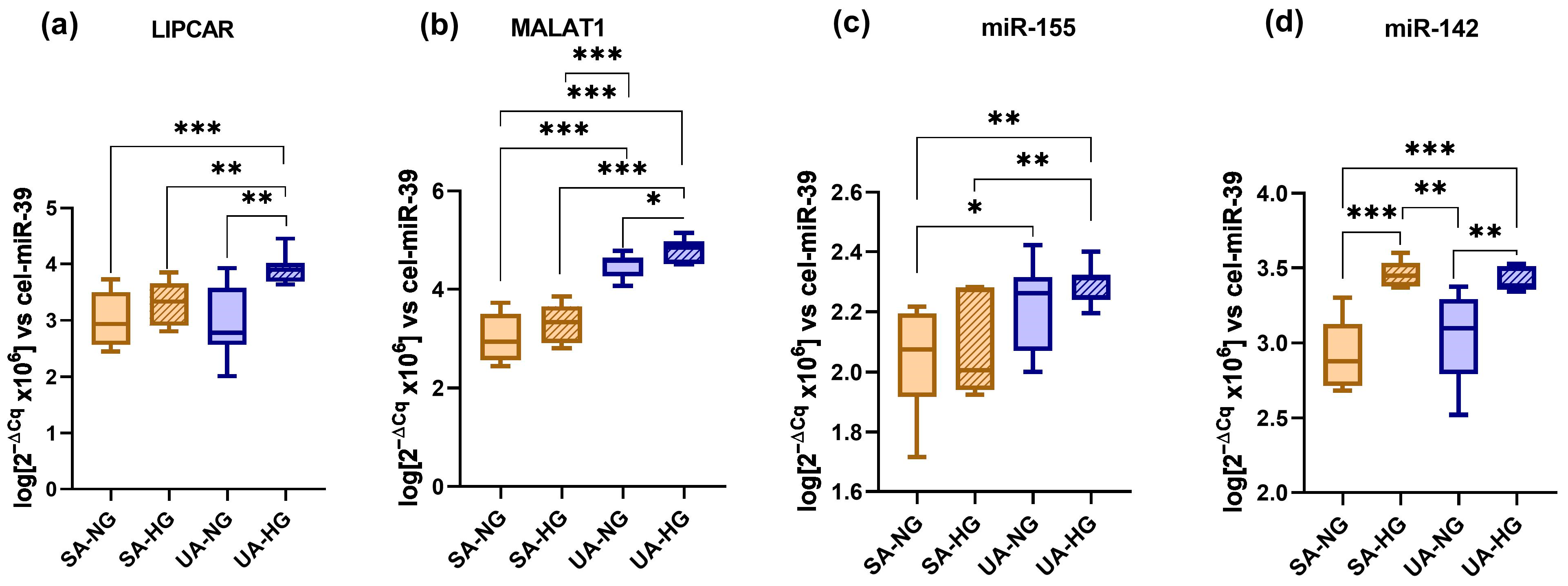
2.5. Plasma lncRNA and miRNA Levels as Predictors for Vulnerable CAD Patients: The Impact of Hyperglycemia
2.6. Plasma lncRNA and miRNA Levels as Predictors for MACE Occurrence in STEMI Patients
3. Discussion
4. Materials and Methods
4.1. Study Design: SA, UA, and STEMI Patients
4.2. Determination of Plasma Parameters of SA, UA, and STEMI Patients
4.3. Analysis of ncRNAs in the Plasma of SA, UA, and STEMI Patients
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Carnuta, M.G.; Stancu, C.S.; Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; et al. Dysfunctional high-density lipoproteins have distinct composition, diminished anti-inflammatory potential and discriminate acute coronary syndrome from stable coronary artery disease patients. Sci. Rep. 2017, 7, 7295. [Google Scholar] [CrossRef]
- Huarte, M.; Rinn, J.L. Large non-coding RNAs: Missing links in cancer? Hum. Mol. Genet. 2010, 19, R152–R161. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Beutner, F.; Scholz, M.; Gielen, S.; Gabel, G.; Bergert, H.; Schuler, G.; Thiery, J.; Teupser, D. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 620–627. [Google Scholar] [CrossRef]
- Cakmak, H.A.; Demir, M. MicroRNA and Cardiovascular Diseases. Balkan Med. J. 2020, 37, 60–71. [Google Scholar] [CrossRef]
- Islas, J.F.; Moreno-Cuevas, J.E. A MicroRNA Perspective on Cardiovascular Development and Diseases: An Update. Int. J. Mol. Sci. 2018, 19, 2075. [Google Scholar] [CrossRef]
- Colpaert, R.M.W.; Calore, M. MicroRNAs in Cardiac Diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef]
- Bhat, S.A.; Ahmad, S.M.; Mumtaz, P.T.; Malik, A.A.; Dar, M.A.; Urwat, U.; Shah, R.A.; Ganai, N.A. Long non-coding RNAs: Mechanism of action and functional utility. Noncoding RNA Res. 2016, 1, 43–50. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1471. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 2016, 1402, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, P.; Lipovich, L.; Grander, D.; Morris, K.V. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim. Biophys. Acta 2014, 1840, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd. LIPCAR: A mitochondrial lnc in the noncoding RNA chain? Circ. Res. 2014, 114, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Y.; Wang, M.; Wang, L.; Zhang, W.; Ge, Z.R. Circulating LIPCAR is a potential biomarker of heart failure in patients post-acute myocardial infarction. Exp. Biol. Med. 2021, 246, 2589–2594. [Google Scholar] [CrossRef]
- Hu, N.; Zeng, X.; Tang, F.; Xiong, S. Exosomal long non-coding RNA LIPCAR derived from oxLDL-treated THP-1 cells regulates the proliferation of human umbilical vein endothelial cells and human vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2021, 575, 65–72. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Chen, H.; Wei, X.; Xu, X. Expression of Long Noncoding RNA LIPCAR Promotes Cell Proliferation, Cell Migration, and Change in Phenotype of Vascular Smooth Muscle Cells. Med. Sci. Monit. 2019, 25, 7645–7651. [Google Scholar] [CrossRef]
- Zhang, X.; Hamblin, M.H.; Yin, K.J. The long noncoding RNA Malat1: Its physiological and pathophysiological functions. RNA Biol. 2017, 14, 1705–1714. [Google Scholar] [CrossRef]
- Yan, Y.; Song, D.; Song, X.; Song, C. The role of lncRNA MALAT1 in cardiovascular disease. IUBMB Life 2020, 72, 334–342. [Google Scholar] [CrossRef]
- Huangfu, N.; Xu, Z.; Zheng, W.; Wang, Y.; Cheng, J.; Chen, X. LncRNA MALAT1 regulates oxLDL-induced CD36 expression via activating beta-catenin. Biochem. Biophys. Res. Commun. 2018, 495, 2111–2117. [Google Scholar] [CrossRef]
- Huang, C.; Han, J.; Wu, Y.; Li, S.; Wang, Q.; Lin, W.; Zhu, J. Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization. Mol. Med. Rep. 2018, 18, 509–515. [Google Scholar] [CrossRef]
- Zheng, B.; Yin, W.N.; Suzuki, T.; Zhang, X.H.; Zhang, Y.; Song, L.L.; Jin, L.S.; Zhan, H.; Zhang, H.; Li, J.S.; et al. Exosome-Mediated miR-155 Transfer from Smooth Muscle Cells to Endothelial Cells Induces Endothelial Injury and Promotes Atherosclerosis. Mol. Ther. 2017, 25, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Barbalata, T.; Scarlatescu, A.I.; Sanda, G.M.; Toma, L.; Stancu, C.S.; Dorobantu, M.; Micheu, M.M.; Sima, A.V.; Niculescu, L.S. Mitochondrial DNA Together with miR-142-3p in Plasma Can Predict Unfavorable Outcomes in Patients after Acute Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 9947. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, L.; Zhu, X.; Wang, Y.; Li, Q.; Ma, J.; Li, H. MALAT1 overexpression attenuates AS by inhibiting ox-LDL-stimulated dendritic cell maturation via miR-155-5p/NFIA axis. Cell Cycle 2020, 19, 2472–2485. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Xiang, L.; Chen, Z.; Liu, X.; Ou, H.; Zhou, J.; Yang, D. MALAT1 functions as a competing endogenous RNA to regulate SMAD5 expression by acting as a sponge for miR-142-3p in hepatocellular carcinoma. Cell Biosci. 2019, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, W.; Long, Q.Q.; Zhang, J.; Li, Y.F.; Liu, D.C.; Yan, J.J.; Yang, Z.J.; Wang, L.S. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci. Rep. 2017, 7, 7491. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo-Calvo, D.; Kenneweg, F.; Bang, C.; Toro, R.; van der Meer, R.W.; Rijzewijk, L.J.; Smit, J.W.; Lamb, H.J.; Llorente-Cortes, V.; Thum, T. Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci. Rep. 2016, 6, 37354. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.F.; Yang, X.C.; Xu, L.; Li, W.M.; Xia, K.; Zhang, D.P.; Wu, R.N.; Gan, T. Circulating Long Noncoding RNA LIPCAR Acts as a Novel Biomarker in Patients with ST-Segment Elevation Myocardial Infarction. Med. Sci. Monit. 2018, 24, 5064–5070. [Google Scholar] [CrossRef]
- Li, R.; Jin, J.; Liu, E.; Zhang, J. A novel circulating biomarker lnc-MALAT1 for acute myocardial infarction: Its relationship with disease risk, features, cytokines, and major adverse cardiovascular events. J. Clin. Lab. Anal. 2022, 36, e24771. [Google Scholar] [CrossRef]
- Vausort, M.; Wagner, D.R.; Devaux, Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ. Res. 2014, 115, 668–677. [Google Scholar] [CrossRef]
- Lv, F.; Liu, L.; Feng, Q.; Yang, X. Long non-coding RNA MALAT1 and its target microRNA-125b associate with disease risk, severity, and major adverse cardiovascular event of coronary heart disease. J. Clin. Lab. Anal. 2021, 35, e23593. [Google Scholar] [CrossRef]
- Kong, X.; Wang, J.; Cao, Y.; Zhang, H.; Lu, X.; Wang, Y.; Bo, C.; Wang, T.; Li, S.; Tian, K.; et al. The long noncoding RNA MALAT-1 functions as a competing endogenous RNA to regulate MSL2 expression by sponging miR-338-3p in myasthenia gravis. J. Cell. Biochem. 2019, 120, 5542–5550. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Salmena, L.; Zhang, J.; Carver, B.; Haveman, W.J.; Pandolfi, P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010, 465, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, G.; Chen, Z. MALAT1 promoted cell proliferation and migration via MALAT1/miR-155/MEF2A pathway in hypoxia of cardiac stem cells. J. Cell. Biochem. 2019, 120, 6384–6394. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Chen, L.D.; Huang, J.C.; Zeng, A.M.; Huang, J.F.; Lin, Q.C. Long non-coding RNA MALAT1 affects intermittent hypoxia-induced endothelial injury by regulating miR-142-3p/HMGB1. Sleep Breath. 2022, 26, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, L.S.; Simionescu, N.; Sanda, G.M.; Carnuta, M.G.; Stancu, C.S.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; Simionescu, M.; et al. MiR-486 and miR-92a Identified in Circulating HDL Discriminate between Stable and Vulnerable Coronary Artery Disease Patients. PLoS ONE 2015, 10, e0140958. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Barbalata, T.; Moraru, O.E.; Stancu, C.S.; Devaux, Y.; Simionescu, M.; Sima, A.V.; Niculescu, L.S. Increased miR-142 Levels in Plasma and Atherosclerotic Plaques from Peripheral Artery Disease Patients with Post-Surgery Cardiovascular Events. Int. J. Mol. Sci. 2020, 21, 9600. [Google Scholar] [CrossRef]



| Parameters | SA (n = 23) | UA (n = 21) | STEMI | |
|---|---|---|---|---|
| no MACE (n = 38) | with MACE (n = 12) | |||
| Total cholesterol (mg/dL) | 168 ± 7.52 | 204 ± 11.17 * | 181 ± 7.85 | 182 ± 11.38 |
| HDL-C (mg/dL) | 43 ± 1.74 | 45.11 ± 3.25 | 25.83 ± 1.09 ***,### | 24.62 ± 1.39 ***,### |
| LDL-C (mg/dL) | 95 ± 6.72 | 114 ± 8.13 | 127 ± 7.65 * | 127 ± 10.81 * |
| Triglycerides (mg/dL) | 166 ± 15.23 | 164 ± 17.73 | 140 ± 6.98 | 155 ± 17.13 |
| ApoA-I (mg/dL) | 151 ± 9.11 | 146 ± 8.10 | 98 ± 5.99 ***,### | 92 ± 9.17 ***,### |
| ApoE (mg/dL) | 2.16 ± 0.15 | 2.89 ± 0.28 * | 2.32 ± 0.15 # | 2.31± 0.21 |
| Glucose (mg/dL) | 122 ± 6.23 | 135 ± 8.49 | 100 ± 3.05 **,### | 122 ± 7.67 $$ |
| PON1 protein (μg/mL) | 4.40 ± 0.39 | 3.65 ± 0.33 | 2.76 ± 0.12 ***,## | 2.58 ± 0.20 **,# |
| PON1 activity (U/L) | 446 ± 61.61 | 364 ± 61.47 | 380 ± 44.92 | 290 ± 68.53 |
| MPO protein (μg/mL) | 36.7 ± 2.84 | 42.7 ± 4.22 | 56.5 ± 6.16 * | 67.1 ± 12.52 **,# |
| CRP (μg/mL) | 17.0 ± 2.69 | 35.5 ± 6.99 * | 22.6 ± 2.29 # | 36.6 ± 4.40 is **,$$ |
| LDH (U/L) | 1547 ± 94.16 | 1990 ± 213.6 | 4454 ± 259.08 ***,### | 6589 ± 700.15 is ***,###,$$ |
| Area Under the ROC Curve—UA vs. SA Analysis | ||||||
|---|---|---|---|---|---|---|
| Test Result Variable(s) | Glycemia | Area | Std. Error a | p-Value b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | |||||
| LIPCAR | Normoglycemic group | 0.486 | 0.152 | 0.925 | 0.188 | 0.783 |
| Hyperglycemic group | 0.900 | 0.101 | 7.79 × 10−5 | 0.702 | 1.098 | |
| MALAT1 | Normoglycemic group | 0.587 | 0.129 | 0.502 | 0.334 | 0.839 |
| Hyperglycemic group | 0.952 | 0.058 | 6.66 × 10−15 | 0.839 | 1.066 | |
| miR-142-3p | Normoglycemic group | 0.622 | 0.137 | 0.372 | 0.354 | 0.891 |
| Hyperglycemic group | 0.367 | 0.176 | 0.448 | 0.022 | 0.711 | |
| miR-155-5p | Normoglycemic group | 0.717 | 0.121 | 0.074 | 0.479 | 0.954 |
| Hyperglycemic group | 0.905 | 0.086 | 2.32 × 10−6 | 0.737 | 1.073 | |
| Independent-Group Area Difference Under the ROC Curve | ||||||
|---|---|---|---|---|---|---|
| Test Result Variable(s) | Asymptotic | AUC Difference | Std. Error Difference b | Asymptotic 95% Confidence Interval | ||
| z | p-Value a | Lower Bound | Upper Bound | |||
| LIPCAR | −2.272 | 0.023 | −0.414 | 0.182 | −0.772 | −0.057 |
| MALAT1 | −2.588 | 0.010 | −0.366 | 0.141 | −0.643 | −0.089 |
| miR-142-3p | 1.147 | 0.251 | 0.256 | 0.223 | −0.181 | 0.692 |
| miR-155-5p | −1.268 | 0.205 | −0.188 | 0.148 | −0.479 | 0.103 |
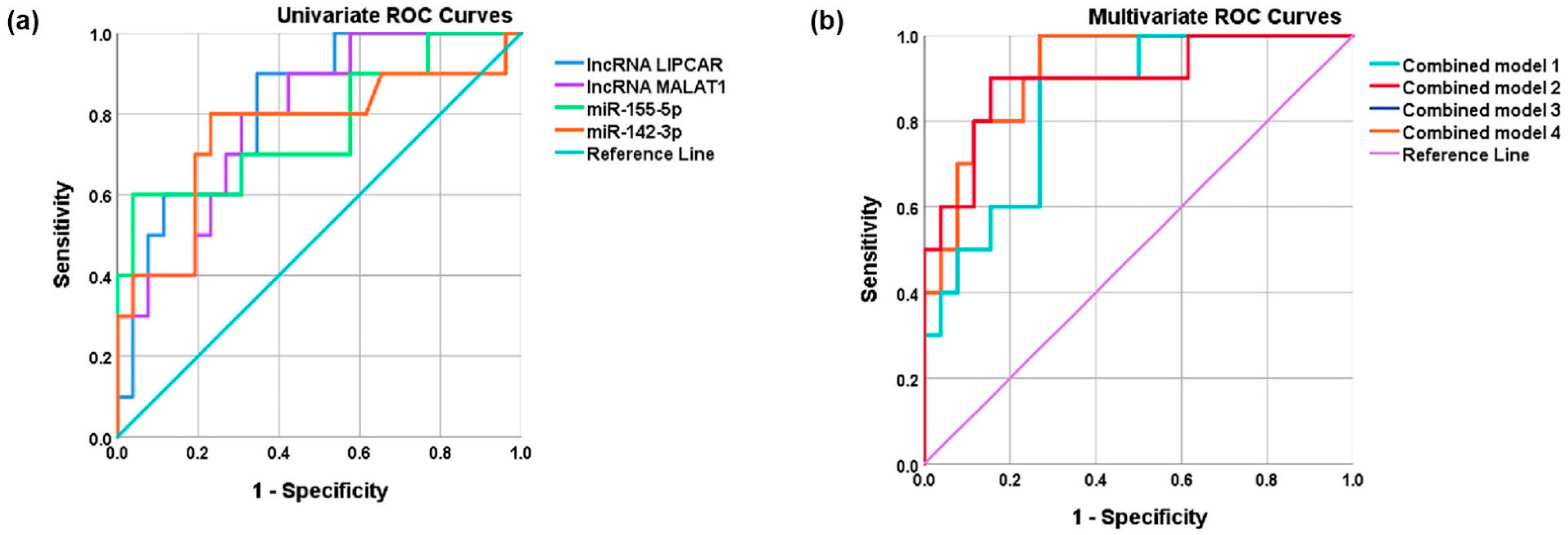
| Area Under the Curve | |||||
|---|---|---|---|---|---|
| Test Result Variable(s) * | Area | Standard Error a | p-Value b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||||
| Univariate models | |||||
| LIPCAR | 0.815 | 0.074 | 0.004 | 0.670 | 0.961 |
| MALAT1 | 0.792 | 0.078 | 0.007 | 0.640 | 0.945 |
| miR-155-5p | 0.769 | 0.098 | 0.013 | 0.577 | 0.962 |
| miR-142-3p | 0.756 | 0.103 | 0.019 | 0.554 | 0.958 |
| Multivariate models | |||||
| Multivariate model 1 (LIPCAR and MALAT1) | 0.842 | 0.068 | 0.002 | 0.710 | 0.975 |
| Multivariate model 2 (LIPCAR, MALAT1, and miR-155-5p) | 0.896 | 0.064 | 2.75 × 10−4 | 0.771 | 1.000 |
| Multivariate model 3 LIPCAR, MALAT1, and miR-142-3p) | 0.919 | 0.045 | 1.18 × 10−4 | 0.832 | 1.000 |
| Multivariate model 4 (lncRNAs LIPCAR and MALAT1, plus miR-142-3p and miR-155-5p) | 0.919 | 0.045 | 1.18 × 10−4 | 0.832 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalata, T.; Niculescu, L.S.; Stancu, C.S.; Pinet, F.; Sima, A.V. Elevated Levels of Circulating lncRNAs LIPCAR and MALAT1 Predict an Unfavorable Outcome in Acute Coronary Syndrome Patients. Int. J. Mol. Sci. 2023, 24, 12076. https://doi.org/10.3390/ijms241512076
Barbalata T, Niculescu LS, Stancu CS, Pinet F, Sima AV. Elevated Levels of Circulating lncRNAs LIPCAR and MALAT1 Predict an Unfavorable Outcome in Acute Coronary Syndrome Patients. International Journal of Molecular Sciences. 2023; 24(15):12076. https://doi.org/10.3390/ijms241512076
Chicago/Turabian StyleBarbalata, Teodora, Loredan S. Niculescu, Camelia S. Stancu, Florence Pinet, and Anca V. Sima. 2023. "Elevated Levels of Circulating lncRNAs LIPCAR and MALAT1 Predict an Unfavorable Outcome in Acute Coronary Syndrome Patients" International Journal of Molecular Sciences 24, no. 15: 12076. https://doi.org/10.3390/ijms241512076
APA StyleBarbalata, T., Niculescu, L. S., Stancu, C. S., Pinet, F., & Sima, A. V. (2023). Elevated Levels of Circulating lncRNAs LIPCAR and MALAT1 Predict an Unfavorable Outcome in Acute Coronary Syndrome Patients. International Journal of Molecular Sciences, 24(15), 12076. https://doi.org/10.3390/ijms241512076









