Molecular Mechanisms of Migraine: Nitric Oxide Synthase and Neuropeptides
Abstract
1. Introduction
2. Basic Pathophysiology
3. Migraine Triggering Models
4. Functional Neuroimaging
5. Migraine Pathophysiology
6. Nitric Oxide (NO)
6.1. Preclinical Evidence for NO Mechanisms in Migraine
6.2. Human Evidence for NO Mechanisms in Migraine
6.3. Therapeutic Scope
7. Calcitonin Gene-Related Peptide (CGRP)
7.1. Preclinical Evidence for CGRP in Migraine
7.2. Clinical Evidence for CGRP in Migraine
7.3. Therapeutic Scope
8. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP)
8.1. Preclinical Evidence for PACAP in Migraine
8.2. Clinical Evidence for PACAP in Migraine
8.3. Therapeutic Scope
9. Vasoactive Intestinal Peptide (VIP)
9.1. Preclinical Evidence for the Role of VIP in Migraine
9.2. Clinical Evidence for the Role of VIP in Migraine
9.3. Therapeutic Scope
10. Neuropeptide Y (NPY)
10.1. Preclinical Evidence for the Role of NPY in Migraine
10.2. Clinical Evidence for the Role of NPY in Migraine
10.3. Therapeutic Scope
11. Conclusions
12. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karsan, N.; Goadsby, P.J. Biological insights from the premonitory symptoms of migraine. Nat. Rev. Neurol. 2018, 14, 699–710. [Google Scholar] [CrossRef]
- Viana, M.; Sances, G.; Linde, M.; Ghiotto, N.; Guaschino, E.; Allena, M.; Terrazzino, S.; Nappi, G.; Goadsby, P.J.; Tassorelli, C. Clinical features of migraine aura: Results from a prospective diary-aided study. Cephalalgia 2017, 37, 979–989. [Google Scholar] [CrossRef]
- Polderman, T.J.; Benyamin, B.; de Leeuw, C.A.; Sullivan, P.F.; van Bochoven, A.; Visscher, P.M.; Posthuma, D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 2015, 47, 702–709. [Google Scholar] [CrossRef]
- Gormley, P.; Anttila, V.; Winsvold, B.S.; Palta, P.; Esko, T.; Pers, T.H.; Farh, K.H.; Cuenca-Leon, E.; Muona, M.; Furlotte, N.A.; et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 2016, 48, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Anttila, V.; Stefansson, H.; Kallela, M.; Todt, U.; Terwindt, G.M.; Calafato, M.S.; Nyholt, D.R.; Dimas, A.S.; Freilinger, T.; Müller-Myhsok, B.; et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat. Genet. 2010, 42, 869–873. [Google Scholar] [CrossRef]
- Chasman, D.I.; Schürks, M.; Anttila, V.; de Vries, B.; Schminke, U.; Launer, L.J.; Terwindt, G.M.; van den Maagdenberg, A.M.; Fendrich, K.; Völzke, H.; et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 2011, 43, 695–698. [Google Scholar] [CrossRef]
- Choquet, H.; Yin, J.; Jacobson, A.S.; Horton, B.H.; Hoffmann, T.J.; Jorgenson, E.; Avins, A.L.; Pressman, A.R. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Commun. Biol. 2021, 4, 864. [Google Scholar] [CrossRef]
- Hautakangas, H.; Winsvold, B.S.; Ruotsalainen, S.E.; Bjornsdottir, G.; Harder, A.V.E.; Kogelman, L.J.A.; Thomas, L.F.; Noordam, R.; Benner, C.; Gormley, P.; et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 2022, 54, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Anttila, V.; Winsvold, B.S.; Gormley, P.; Kurth, T.; Bettella, F.; McMahon, G.; Kallela, M.; Malik, R.; de Vries, B.; Terwindt, G.; et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat. Genet. 2013, 45, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.B.; Rasmussen, B.K.; Fenger, K.; Olesen, J. Migraine without aura and migraine with aura are distinct clinical entities: A study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia 1996, 16, 239–245. [Google Scholar] [CrossRef]
- Grangeon, L.; Lange, K.S.; Waliszewska-Prosół, M.; Onan, D.; Marschollek, K.; Wiels, W.; Mikulenka, P.; Farham, F.; Gollion, C.; Ducros, A. Genetics of migraine: Where are we now? J. Headache Pain 2023, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.; Dodick, D.W. Migraine. Nat. Rev. Dis. Primers 2022, 8, 2. [Google Scholar] [CrossRef]
- Karsan, N.; Bose, R.P.; O’Daly, O.; Zelaya, F.; Goadsby, P.J. Regional cerebral perfusion during the premonitory phase of triggered migraine: A double-blind randomized placebo-controlled functional imaging study using pseudo-continuous arterial spin labeling. Headache 2023, 63, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Viana, M.; Linde, M.; Sances, G.; Ghiotto, N.; Guaschino, E.; Allena, M.; Terrazzino, S.; Nappi, G.; Goadsby, P.J.; Tassorelli, C. Migraine aura symptoms: Duration, succession and temporal relationship to headache. Cephalalgia 2016, 36, 413–421. [Google Scholar] [CrossRef]
- Viana, M.; Sances, G.; Linde, M.; Nappi, G.; Khaliq, F.; Goadsby, P.J.; Tassorelli, C. Prolonged migraine aura: New insights from a prospective diary-aided study. J. Headache Pain 2018, 19, 77. [Google Scholar] [CrossRef]
- Schankin, C.J.; Viana, M.; Goadsby, P.J. Persistent and Repetitive Visual Disturbances in Migraine: A Review. Headache 2017, 57, 1–16. [Google Scholar] [CrossRef]
- Rasmussen, B.K.; Olesen, J. Migraine with aura and migraine without aura: An epidemiological study. Cephalalgia 1992, 12, 221–228. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; á Dunga, B.O.; Olesen, J. Human models of migraine—Short-term pain for long-term gain. Nat. Rev. Neurol. 2017, 13, 713–724. [Google Scholar] [CrossRef]
- Iversen, H.K.; Olesen, J.; Tfelt-Hansen, P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain 1989, 38, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. The trigeminovascular system and migraine: Studies characterising cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993, 33, 48–56. [Google Scholar] [CrossRef]
- Lassen, L.H.; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP may play a causative role in migraine. Cephalalgia 2002, 22, 54–61. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Steinberg, A.; Frederiksen, S.D.; Blixt, F.W.; Warfvinge, K.; Edvinsson, L. Expression of messenger molecules and receptors in rat and human sphenopalatine ganglion indicating therapeutic targets. J. Headache Pain 2016, 17, 78. [Google Scholar] [CrossRef]
- Akerman, S.; Goadsby, P.J. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci. Transl. Med. 2015, 7, 308ra157. [Google Scholar] [CrossRef]
- Tuka, B.; Helyes, Z.; Markovics, A.; Bagoly, T.; Szolcsanyi, J.; Szabo, N.; Toth, E.; Kincses, Z.T.; Vecsei, L.; Tajti, J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013, 33, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Zagami, A.S.; Edvinsson, L.; Goadsby, P.J. Pituitary adenylate cyclase activating polypeptide and migraine. Ann. Clin. Transl. Neurol. 2014, 1, 1036–1040. [Google Scholar] [CrossRef]
- Schytz, H.W.; Birk, S.; Wienecke, T.; Kruuse, C.; Olesen, J.; Ashina, M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain J. Neurol. 2009, 132, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Miller, S.; Martins-Oliveira, M.; Akerman, S.; Supronsinchai, W.; Sun, H.; Shi, L.; Wang, J.; Zhu, D.; Lehto, S.; et al. PAC1 receptor blockade reduces central nociceptive activity: New approach for primary headache? Pain 2020, 161, 1670–1681. [Google Scholar] [CrossRef]
- Lundbeck. Lundbeck Announces Positive Phase II Proof of Concept Results with Lu AG09222 in Migraine Prevention. Available online: https://news.cision.com/h--lundbeck-a-s/r/lundbeck-announces-positive-phase-ii-proof-of-concept-results-with-lu-ag09222-in-migraine-prevention,c3754245 (accessed on 10 June 2023).
- Rahmann, A.; Wienecke, T.; Hansen, J.M.; Fahrenkrug, J.; Olesen, J.; Ashina, M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia 2008, 28, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Al-Karagholi, M.A.; De Icco, R.; Coskun, H.; Elbahi, F.A.; Lopez-Lopez, C.; Snellman, J.; Hannibal, J.; Amin, F.M.; Ashina, M. Effect of Vasoactive Intestinal Polypeptide on Development of Migraine Headaches: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2118543. [Google Scholar] [CrossRef]
- Amin, F.M.; Hougaard, A.; Schytz, H.W.; Asghar, M.S.; Lundholm, E.; Parvaiz, A.I.; de Koning, P.J.; Andersen, M.R.; Larsson, H.B.; Fahrenkrug, J.; et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain J. Neurol. 2014, 137, 779–794. [Google Scholar] [CrossRef]
- Asghar, M.S. Effects of glyceryl trinitrate and calcitonin gene-related peptide on BOLD signal and arterial diameter: Methodological studies by fMRI and MRA. Dan. Med. J. 2012, 59, B4489. [Google Scholar] [CrossRef]
- Amin, F.M.; Asghar, M.S.; Hougaard, A.; Hansen, A.E.; Larsen, V.A.; de Koning, P.J.; Larsson, H.B.; Olesen, J.; Ashina, M. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: A cross-sectional study. Lancet Neurol. 2013, 12, 454–461. [Google Scholar] [CrossRef]
- Christiansen, I.; Thomsen, L.L.; Daugaard, D.; Ulrich, V.; Olesen, J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia 1999, 19, 660–667, discussion 626. [Google Scholar] [CrossRef]
- Hansen, J.M.; Hauge, A.W.; Olesen, J.; Ashina, M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010, 30, 1179–1186. [Google Scholar] [CrossRef]
- Hansen, J.M.; Thomsen, L.L.; Olesen, J.; Ashina, M. Familial hemiplegic migraine type 1 shows no hypersensitivity to nitric oxide. Cephalalgia 2008, 28, 496–505. [Google Scholar] [CrossRef]
- Hansen, J.M.; Thomsen, L.L.; Olesen, J.; Ashina, M. Calcitonin gene-related peptide does not cause the familial hemiplegic migraine phenotype. Neurology 2008, 71, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Thomsen, L.L.; Marconi, R.; Casari, G.; Olesen, J.; Ashina, M. Familial hemiplegic migraine type 2 does not share hypersensitivity to nitric oxide with common types of migraine. Cephalalgia 2008, 28, 367–375. [Google Scholar] [CrossRef]
- Hansen, J.M.; Thomsen, L.L.; Olesen, J.; Ashina, M. Calcitonin gene-related peptide does not cause migraine attacks in patients with familial hemiplegic migraine. Headache 2011, 51, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, H.; Al-Karagholi, M.A.; Walker, C.S.; Arngrim, N.; Rees, T.; Petersen, J.; Siow, A.; Mørch-Rasmussen, M.; Tan, S.; O’Carroll, S.J.; et al. Amylin Analog Pramlintide Induces Migraine-like Attacks in Patients. Ann. Neurol. 2021, 89, 1157–1171. [Google Scholar] [CrossRef]
- Ghanizada, H.; Al-Karagholi, M.A.; Arngrim, N.; Mørch-Rasmussen, M.; Walker, C.S.; Hay, D.L.; Ashina, M. Effect of Adrenomedullin on Migraine-Like Attacks in Patients with Migraine: A Randomized Crossover Study. Neurology 2021, 96, e2488–e2499. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.; Ghanizada, H.; Nielsen, C.A.W.; Hougaard, A.; Ashina, M. Opening of ATP sensitive potassium channels causes migraine attacks with aura. Brain J. Neurol. 2021, 144, 2322–2332. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.; Hansen, J.M.; Guo, S.; Olesen, J.; Ashina, M. Opening of ATP-sensitive potassium channels causes migraine attacks: A new target for the treatment of migraine. Brain J. Neurol. 2019, 142, 2644–2654. [Google Scholar] [CrossRef]
- Weiller, C.; May, A.; Limmroth, V.; Juptner, M.; Kaube, H.; Schayck, R.V.; Coenen, H.H.; Diener, H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995, 1, 658–660. [Google Scholar] [CrossRef]
- Bahra, A.; Matharu, M.S.; Buchel, C.; Frackowiak, R.S.; Goadsby, P.J. Brainstem activation specific to migraine headache. Lancet 2001, 357, 1016–1017. [Google Scholar] [CrossRef] [PubMed]
- Denuelle, M.; Fabre, N.; Payoux, P.; Chollet, F.; Geraud, G. Hypothalamic activation in spontaneous migraine attacks. Headache 2007, 47, 1418–1426. [Google Scholar] [CrossRef]
- Afridi, S.K.; Matharu, M.S.; Lee, L.; Kaube, H.; Friston, K.J.; Frackowiak, R.S.; Goadsby, P.J. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain J. Neurol. 2005, 128, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Moragues, N.; Ciofi, P.; Lafon, P.; Odessa, M.F.; Tramu, G.; Garret, M. cDNA cloning and expression of a gamma-aminobutyric acid A receptor epsilon-subunit in rat brain. Eur. J. Neurosci. 2000, 12, 4318–4330. [Google Scholar] [PubMed]
- Bonnert, T.P.; McKernan, R.M.; Farrar, S.; le Bourdellès, B.; Heavens, R.P.; Smith, D.W.; Hewson, L.; Rigby, M.R.; Sirinathsinghji, D.J.; Brown, N.; et al. theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc. Natl. Acad. Sci. USA 1999, 96, 9891–9896. [Google Scholar] [CrossRef]
- Ray, B.S.; Wolff, H.G. Experimental studies on headache. Pain sensitive structures of the head and their significance in headache. Arch. Surg. 1940, 51, 813–856. [Google Scholar] [CrossRef]
- Uddman, R.; Goadsby, P.J.; Jansen, I.; Edvinsson, L. PACAP, a VIP-like peptide: Immunohistochemical localization and effect upon cat pial arteries and cerebral blood flow. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1993, 13, 291–297. [Google Scholar] [CrossRef]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: Trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef]
- Edvinsson, L.; Brodin, E.; Jansen, I.; Uddman, R. Neurokinin A in cerebral vessels: Characterization, localization and effects in vitro. Regul. Pept. 1988, 20, 181–197. [Google Scholar] [CrossRef]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurol. 2011, 12, 570–584. [Google Scholar] [CrossRef]
- Hoffmann, J.; Baca, S.M.; Akerman, S. Neurovascular mechanisms of migraine and cluster headache. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 573–594. [Google Scholar] [CrossRef]
- Maniyar, F.H.; Sprenger, T.; Monteith, T.; Schankin, C.; Goadsby, P.J. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain J. Neurol. 2014, 137, 232–241. [Google Scholar] [CrossRef]
- Karsan, N.; Bose, P.R.; O’Daly, O.; Zelaya, F.O.; Goadsby, P.J. Alterations in Functional Connectivity During Different Phases of the Triggered Migraine Attack. Headache 2020, 60, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.H.; Menz, M.M.; Haaker, J.; May, A. The migraineur’s brain networks: Continuous resting state fMRI over 30 days. Cephalalgia 2020, 40, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.H.; May, A. The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain J. Neurol. 2016, 139, 1987–1993. [Google Scholar] [CrossRef]
- Giffin, N.J.; Ruggiero, L.; Lipton, R.B.; Silberstein, S.D.; Tvedskov, J.F.; Olesen, J.; Altman, J.; Goadsby, P.J.; Macrae, A. Premonitory symptoms in migraine: An electronic diary study. Neurology 2003, 60, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Tassorelli, C.; Greco, R.; Morocutti, A.; Costa, A.; Nappi, G. Nitric oxide-induced neuronal activation in the central nervous system as an animal model of migraine: Mechanisms and mediators. Funct. Neurol. 2001, 16, 69–76. [Google Scholar]
- Tassorelli, C.; Joseph, S.A. Systemic nitroglycerin induces fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995, 682, 167–181. [Google Scholar] [CrossRef]
- Akerman, S.; Karsan, N.; Bose, P.; Hoffmann, J.R.; Holland, P.R.; Romero-Reyes, M.; Goadsby, P.J. Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity. Brain J. Neurol. 2019, 142, 103–119. [Google Scholar] [CrossRef]
- Zhang, X.; Kainz, V.; Zhao, J.; Strassman, A.M.; Levy, D. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Ann. Neurol. 2013, 73, 741–750. [Google Scholar] [CrossRef]
- Tassorelli, C.; Costa, A.; Blandini, F.; Joseph, S.A.; Nappi, G. Effect of nitric oxide donors on the central nervous system--nitroglycerin studies in the rat. Funct. Neurol. 2000, 15 (Suppl. S3), 19–27. [Google Scholar]
- Lambert, G.A.; Donaldson, C.; Boers, P.M.; Zagami, A.S. Activation of trigeminovascular neurons by glyceryl trinitrate. Brain Res. 2000, 887, 203–210. [Google Scholar] [CrossRef]
- Pardutz, A.; Krizbai, I.; Multon, S.; Vecsei, L.; Schoenen, J. Systemic nitroglycerin increases nNOS levels in rat trigeminal nucleus caudalis. Neuroreport 2000, 11, 3071–3075. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Romero-Reyes, M.; Karsan, N.; Bose, P.; Hoffmann, J.R.; Holland, P.R.; Goadsby, P.J. Therapeutic targeting of nitroglycerin-mediated trigeminovascular neuronal hypersensitivity predicts clinical outcomes of migraine abortives. Pain 2021, 162, 1567–1577. [Google Scholar] [CrossRef]
- Karsan, N.; Goadsby, P.J. New Oral Drugs for Migraine. CNS Drugs 2022, 36, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, K.L.; Bulmer, D.C.; Goadsby, P.J. Fos expression in the trigeminocervical complex of the cat after stimulation of the superior sagittal sinus is reduced by L-NAME. Neurosci. Lett. 1999, 266, 173–176. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Abbasnejad, M.; Raoof, M.; Esmaeili-Mahani, S.; Kooshki, R. The involvement of orexin 1 and cannabinoid 1 receptors within the ventrolateral periaqueductal gray matter in the modulation of migraine-induced anxiety and social behavior deficits of rats. Peptides 2021, 146, 170651. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Smith, M.L.; McGuire, B.; Tarash, I.; Evans, C.J.; Charles, A. Characterization of a novel model of chronic migraine. Pain 2014, 155, 269–274. [Google Scholar] [CrossRef]
- Koulchitsky, S.; Fischer, M.J.; De Col, R.; Schlechtweg, P.M.; Messlinger, K. Biphasic response to nitric oxide of spinal trigeminal neurons with meningeal input in rat--possible implications for the pathophysiology of headaches. J. Neurophysiol. 2004, 92, 1320–1328. [Google Scholar] [CrossRef]
- Fischer, M.J.; Koulchitsky, S.; Messlinger, K. The nonpeptide calcitonin gene-related peptide receptor antagonist BIBN4096BS lowers the activity of neurons with meningeal input in the rat spinal trigeminal nucleus. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5877–5883. [Google Scholar] [CrossRef]
- Karsan, N.; Bose, P.R.; Thompson, C.; Newman, J.; Goadsby, P.J. Headache and non-headache symptoms provoked by nitroglycerin in migraineurs: A human pharmacological triggering study. Cephalalgia 2020, 40, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef] [PubMed]
- Chizh, B.; Palmer, J.; Lai, R.; Guillard, F.; Bullman, J.; Baines, A.; Napolitano, A.; Appleby, J. A randomised, two-period cross-over study to investigate the efficacy of the Trpv1 antagonist SB-705498 in acute migraine. Eur. J. Pain 2009, 13, S202a-S202. [Google Scholar] [CrossRef]
- Messlinger, K.; Lennerz, J.K.; Eberhardt, M.; Fischer, M.J. CGRP and NO in the trigeminal system: Mechanisms and role in headache generation. Headache 2012, 52, 1411–1427. [Google Scholar] [CrossRef]
- Akerman, S.; Williamson, D.J.; Kaube, H.; Goadsby, P.J. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002, 137, 62–68. [Google Scholar] [CrossRef]
- Fletcher, A.; McLoone, P.; Bulpitt, C. Quality of life on angina therapy: A randomised controlled trial of transdermal glyceryl trinitrate against placebo. Lancet 1988, 2, 4–8. [Google Scholar] [CrossRef]
- Sicuteri, F.; Del Bene, E.; Poggioni, M.; Bonazzi, A. Unmasking latent dysnociception in healthy subjects. Headache 1987, 27, 180–185. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Lippton, H.; Edwards, J.C.; Baricos, W.H.; Hyman, A.L.; Kadowitz, P.J.; Gruetter, C.A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: Evidence for the involvement of S-nitrosothiols as active intermediates. J. Pharmacol. Exp. Ther. 1981, 218, 739–749. [Google Scholar] [PubMed]
- Thomsen, L.L.; Iversen, H.K.; Brinck, T.A.; Olesen, J. Arterial supersensitivity to nitric oxide (nitroglycerin) in migraine sufferers. Cephalalgia 1993, 13, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.L.; Kruuse, C.; Iversen, H.K.; Olesen, J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur. J. Neurol. 1994, 1, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Tassorelli, C.; Joseph, S.A.; Buzzi, M.G.; Nappi, G. The effects on the central nervous system of nitroglycerin—Putative mechanisms and mediators. Prog. Neurobiol. 1999, 57, 607–624. [Google Scholar] [CrossRef]
- Schoonman, G.G.; van der Grond, J.; Kortmann, C.; van der Geest, R.J.; Terwindt, G.M.; Ferrari, M.D. Migraine headache is not associated with cerebral or meningeal vasodilatation—A 3T magnetic resonance angiography study. Brain J. Neurol. 2008, 131, 2192–2200. [Google Scholar] [CrossRef]
- Iversen, H.K.; Olesen, J. Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs. Cephalalgia 1996, 16, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, K.L.; Kaube, H.; Goadsby, P.J. Sumatriptan can inhibit trigeminal afferents by an exclusively neural mechanism. Brain J. Neurol. 1996, 119 Pt 5, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Lassen, L.H.; Ashina, M.; Christiansen, I.; Ulrich, V.; Grover, R.; Donaldson, J.; Olesen, J. Nitric oxide synthase inhibition: A new principle in the treatment of migraine attacks. Cephalalgia 1998, 18, 27–32. [Google Scholar] [CrossRef]
- Palmer, J.E.; Guillard, F.L.; Laurijssens, B.E.; Wentz, A.L.; Dixon, R.M. A randomised, single-blind, placebo-controlled, adaptive clinical trial of GW274150, a selective iNOS inhibitor, in the treatment of acute migraine. Cephalalgia 2009, 29, 124. [Google Scholar]
- Høivik, H.O.; Laurijssens, B.E.; Harnisch, L.O.; Twomey, C.K.; Dixon, R.M.; Kirkham, A.J.; Williams, P.M.; Wentz, A.L.; Lunnon, M.W. Lack of efficacy of the selective iNOS inhibitor GW274150 in prophylaxis of migraine headache. Cephalalgia 2010, 30, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, A.; Hauge, A.W.; Guo, S.; Tfelt-Hansen, P. The nitric oxide synthase inhibitor and serotonin-receptor agonist NXN-188 during the aura phase of migraine with aura: A randomized, double-blind, placebo-controlled cross-over study. Scand. J. Pain 2013, 4, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Alberti, A.; Codini, M.; Floridi, A.; Gallai, V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 2000, 20, 907–918. [Google Scholar] [CrossRef]
- Juhasz, G.; Zsombok, T.; Modos, E.A.; Olajos, S.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Vitrai, J.; Bagdy, G. NO-induced migraine attack: Strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain 2003, 106, 461–470. [Google Scholar] [CrossRef]
- Juhasz, G.; Zsombok, T.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Bagdy, G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 2005, 25, 179–183. [Google Scholar] [CrossRef]
- Afridi, S.K.; Kaube, H.; Goadsby, P.J. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain 2004, 110, 675–680. [Google Scholar] [CrossRef]
- Abrams, J. Pharmacology of nitroglycerin and long-acting nitrates. Am. J. Cardiol. 1985, 56, 12a–18a. [Google Scholar] [CrossRef]
- Bhatt, D.K.; Gupta, S.; Jansen-Olesen, I.; Andrews, J.S.; Olesen, J. NXN-188, a selective nNOS inhibitor and a 5-HT1B/1D receptor agonist, inhibits CGRP release in preclinical migraine models. Cephalalgia 2013, 33, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Medve, R.A.; Andrews, J.S. Effects of fixed dose combination of nNOS inhibition and 5HT agonism on progression of migraine with and without aura. Cephalalgia 2009, 29, 126. [Google Scholar]
- Reuter, U.; Bolay, H.; Jansen-Olesen, I.; Chiarugi, A.; Sanchez del Rio, M.; Letourneau, R.; Theoharides, T.C.; Waeber, C.; Moskowitz, M.A. Delayed inflammation in rat meninges: Implications for migraine pathophysiology. Brain J. Neurol. 2001, 124, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Zagami, A.S.; Goadsby, P.J.; Edvinsson, L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 1990, 16, 69–75. [Google Scholar] [CrossRef]
- Lennerz, J.K.; Ruhle, V.; Ceppa, E.P.; Neuhuber, W.L.; Bunnett, N.W.; Grady, E.F.; Messlinger, K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J. Comp. Neurol. 2008, 507, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Gaspar, R.C.; Roberts, R.; Chen, T.B.; Zeng, Z.; Villarreal, S.; Edvinsson, L.; Salvatore, C.A. Localization of CGRP receptor components and receptor binding sites in rhesus monkey brainstem: A detailed study using in situ hybridization, immunofluorescence, and autoradiography. J. Comp. Neurol. 2016, 524, 90–118. [Google Scholar] [CrossRef]
- Eftekhari, S.; Edvinsson, L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci. 2011, 12, 112. [Google Scholar] [CrossRef]
- Eftekhari, S.; Salvatore, C.A.; Calamari, A.; Kane, S.A.; Tajti, J.; Edvinsson, L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010, 169, 683–696. [Google Scholar] [CrossRef]
- Csati, A.; Tajti, J.; Tuka, B.; Edvinsson, L.; Warfvinge, K. Calcitonin gene-related peptide and its receptor components in the human sphenopalatine ganglion—Interaction with the sensory system. Brain Res. 2012, 1435, 29–39. [Google Scholar] [CrossRef]
- Csati, A.; Tajti, J.; Kuris, A.; Tuka, B.; Edvinsson, L.; Warfvinge, K. Distribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience 2012, 202, 158–168. [Google Scholar] [CrossRef]
- Eftekhari, S.; Salvatore, C.A.; Johansson, S.; Chen, T.B.; Zeng, Z.; Edvinsson, L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015, 1600, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Kanje, M.; Longmore, J.; Tajti, J.; Uddman, R.; Edvinsson, L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: Co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001, 909, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Thalakoti, S.; Patil, V.V.; Damodaram, S.; Vause, C.V.; Langford, L.E.; Freeman, S.E.; Durham, P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 2007, 47, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, J.C.A.; Warfvinge, K.; Krause, D.N.; Blixt, F.W.; Sheykhzade, M.; Edvinsson, L.; Haanes, K.A. C-fibers may modulate adjacent Aδ-fibers through axon-axon CGRP signaling at nodes of Ranvier in the trigeminal system. J. Headache Pain 2019, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.L.; Russo, A.F. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 3423–3429. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Storer, R.J.; Akerman, S.; Goadsby, P.J. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br. J. Pharmacol. 2004, 142, 1171–1181. [Google Scholar] [CrossRef]
- Summ, O.; Charbit, A.R.; Andreou, A.P.; Goadsby, P.J. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain J. Neurol. 2010, 133, 2540–2548. [Google Scholar] [CrossRef]
- Pozo-Rosich, P.; Storer, R.J.; Charbit, A.R.; Goadsby, P.J. Periaqueductal gray calcitonin gene-related peptide modulates trigeminovascular neurons. Cephalalgia 2015, 35, 1298–1307. [Google Scholar] [CrossRef]
- Recober, A.; Kuburas, A.; Zhang, Z.; Wemmie, J.A.; Anderson, M.G.; Russo, A.F. Role of calcitonin gene-related peptide in light-aversive behavior: Implications for migraine. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F.; Kuburas, A.; Kaiser, E.A.; Raddant, A.C.; Recober, A. A Potential Preclinical Migraine Model: CGRP-Sensitized Mice. Mol. Cell. Pharmacol. 2009, 1, 264–270. [Google Scholar] [PubMed]
- Recober, A.; Kaiser, E.A.; Kuburas, A.; Russo, A.F. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology 2010, 58, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Kainz, V.; Jakubowski, M.; Gooley, J.J.; Saper, C.B.; Digre, K.; Burstein, R. A neural mechanism for exacerbation of headache by light. Nat. Neurosci. 2010, 13, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Winborn, C.S.; Marquez de Prado, B.; Russo, A.F. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2693–2703. [Google Scholar] [CrossRef]
- Roon, K.I.; Olesen, J.; Diener, H.C.; Ellis, P.; Hettiarachchi, J.; Poole, P.H.; Christianssen, I.; Kleinermans, D.; Kok, J.G.; Ferrari, M.D. No acute antimigraine efficacy of CP-122,288, a highly potent inhibitor of neurogenic inflammation: Results of two randomized, double-blind, placebo-controlled clinical trials. Ann. Neurol. 2000, 47, 238–241. [Google Scholar] [CrossRef]
- May, A.; Goadsby, P.J. Substance P receptor antagonists in the therapy of migraine. Expert. Opin. Investig. Drugs 2001, 10, 673–678. [Google Scholar] [CrossRef]
- Russo, A.F. Calcitonin gene-related peptide (CGRP): A new target for migraine. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 533–552. [Google Scholar] [CrossRef]
- Ho, T.W.; Edvinsson, L.; Goadsby, P.J. CGRP and its receptors provide new insights into migraine pathophysiology. Nat. Rev. Neurol. 2010, 6, 573–582. [Google Scholar] [CrossRef]
- Tfelt-Hansen, P.; Le, H. Calcitonin gene-related peptide in blood: Is it increased in the external jugular vein during migraine and cluster headache? A review. J. Headache Pain 2009, 10, 137–143. [Google Scholar] [CrossRef]
- Ashina, M.; Bendtsen, L.; Jensen, R.; Schifter, S.; Olesen, J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain 2000, 86, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Cernuda-Morollon, E.; Larrosa, D.; Ramon, C.; Vega, J.; Martinez-Camblor, P.; Pascual, J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013, 81, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.A.; Lassen, L.H.; Birk, S.; Lesko, L.; Olesen, J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin. Pharmacol. Ther. 2005, 77, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.E.; Younis, S.; Deen, M.; Khan, S.; Ghanizada, H.; Ashina, M. Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. J. Headache Pain 2018, 19, 105. [Google Scholar] [CrossRef]
- Guo, S.; Vollesen, A.L.; Olesen, J.; Ashina, M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 2016, 157, 2773–2781. [Google Scholar] [CrossRef]
- Asghar, M.S.; Hansen, A.E.; Amin, F.M.; van der Geest, R.J.; Koning, P.; Larsson, H.B.; Olesen, J.; Ashina, M. Evidence for a vascular factor in migraine. Ann. Neurol. 2011, 69, 635–645. [Google Scholar] [CrossRef]
- Trasforini, G.; Margutti, A.; Portaluppi, F.; Menegatti, M.; Ambrosio, M.R.; Bagni, B.; Pansini, R.; Degli Uberti, E.C. Circadian profile of plasma calcitonin gene-related peptide in healthy man. J. Clin. Endocrinol. Metab. 1991, 73, 945–951. [Google Scholar] [CrossRef]
- Portaluppi, F.; Trasforini, G.; Margutti, A.; Vergnani, L.; Ambrosio, M.R.; Rossi, R.; Bagni, B.; Pansini, R.; degli Uberti, E.C. Circadian rhythm of calcitonin gene-related peptide in uncomplicated essential hypertension. J. Hypertens. 1992, 10, 1227–1234. [Google Scholar] [CrossRef]
- Fox, A.W.; Davis, R.L. Migraine chronobiology. Headache 1998, 38, 436–441. [Google Scholar] [CrossRef]
- Ho, T.W.; Ferrari, M.D.; Dodick, D.W.; Galet, V.; Kost, J.; Fan, X.; Leibensperger, H.; Froman, S.; Assaid, C.; Lines, C.; et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: A randomised, placebo-controlled, parallel-treatment trial. Lancet 2008, 372, 2115–2123. [Google Scholar] [CrossRef]
- Ho, T.W.; Connor, K.M.; Zhang, Y.; Pearlman, E.; Koppenhaver, J.; Fan, X.; Lines, C.; Edvinsson, L.; Goadsby, P.J.; Michelson, D. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 2014, 83, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Aurora, S.K.; Loeys, T.; Ashina, M.; Jones, C.; Giezek, H.; Massaad, R.; Williams-Diaz, A.; Lines, C.; Ho, T.W. Long-term tolerability of telcagepant for acute treatment of migraine in a randomized trial. Headache 2011, 51, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Shapiro, R.E.; Diener, H.C.; Lucas, S.; Kost, J.; Fan, X.; Fei, K.; Assaid, C.; Lines, C.; Ho, T.W. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 2009, 73, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.J.; Aurora, S.K.; Dodick, D.W.; Goadsby, P.J.; Ge, Y.J.; Bachman, R.; Taraborelli, D.; Fan, X.; Assaid, C.; Lines, C.; et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 2011, 31, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Diener, H.C.; Husstedt, I.W.; Goadsby, P.J.; Hall, D.; Meier, U.; Pollentier, S.; Lesko, L.M. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004, 350, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Croop, R.; Lipton, R.B.; Kudrow, D.; Stock, D.A.; Kamen, L.; Conway, C.M.; Stock, E.G.; Coric, V.; Goadsby, P.J. Oral rimegepant for preventive treatment of migraine: A phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 51–60. [Google Scholar] [CrossRef]
- Hutchinson, S.; Schim, J.; Lipton, R.; Croop, R.; Jensen, C.; Thiry, A.; Stock, E.; Conway, C.; Lovegren, M.; Coric, V.; et al. Oral rimegepant 75 mg is safe and well tolerated in adults with migraine and cardiovascular risk factors: Results of a multicenter, long-term, open-label safety study. In Proceedings of the American Academy of Neurology 2021 Virtual Annual Meeting, Virtual, 17–21 April 2021. [Google Scholar]
- Croop, R.; Goadsby, P.J.; Stock, D.A.; Conway, C.M.; Forshaw, M.; Stock, E.G.; Coric, V.; Lipton, R.B. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: A randomised, phase 3, double-blind, placebo-controlled trial. Lancet 2019, 394, 737–745. [Google Scholar] [CrossRef]
- Lipton, R.B.; Coric, V.; Stock, E.G.; Stock, D.A.; Morris, B.A.; McCormack, T.J. Rimegepant 75 mg, an oral calcitonin gene-related peptide antagonist, for the acute treatment of migraine: Two phase 3, double-blind, randomized, placebo-controlled trials. Cephalalgia 2018, 38, 18–19. [Google Scholar]
- Hutchinson, S.; Silberstein, S.D.; Blumenfeld, A.M.; Lipton, R.B.; Lu, K.; Yu, S.Y.; Severt, L. Safety and efficacy of ubrogepant in participants with major cardiovascular risk factors in two single-attack phase 3 randomized trials: ACHIEVE I and II. Cephalalgia 2021, 41, 979–990. [Google Scholar] [CrossRef]
- Hutchinson, S.; Dodick, D.W.; Treppendahl, C.; Bennett, N.L.; Yu, S.Y.; Guo, H.; Trugman, J.M. Ubrogepant for the Acute Treatment of Migraine: Pooled Efficacy, Safety, and Tolerability from the ACHIEVE I and ACHIEVE II Phase 3 Randomized Trials. Neurol. Ther. 2021, 10, 235–249. [Google Scholar] [CrossRef]
- Ailani, J.; Lipton, R.B.; Hutchinson, S.; Knievel, K.; Lu, K.; Butler, M.; Yu, S.Y.; Finnegan, M.; Severt, L.; Trugman, J.M. Long-Term Safety Evaluation of Ubrogepant for the Acute Treatment of Migraine: Phase 3, Randomized, 52-Week Extension Trial. Headache 2020, 60, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Lipton, R.B.; Ailani, J.; Halker Singh, R.B.; Shewale, A.R.; Zhao, S.; Trugman, J.M.; Yu, S.Y.; Viswanathan, H.N. Ubrogepant, an Acute Treatment for Migraine, Improved Patient-Reported Functional Disability and Satisfaction in 2 Single-Attack Phase 3 Randomized Trials, ACHIEVE I and II. Headache 2020, 60, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Dodick, D.W.; Ailani, J.; Lu, K.; Finnegan, M.; Szegedi, A.; Trugman, J.M. Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine: The ACHIEVE II Randomized Clinical Trial. Jama 2019, 322, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Lipton, R.B.; Ailani, J.; Lu, K.; Finnegan, M.; Trugman, J.M.; Szegedi, A. Ubrogepant for the Treatment of Migraine. N. Engl. J. Med. 2019, 381, 2230–2241. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Lipton, R.B.; Ailani, J.; Silberstein, S.D.; Tassorelli, C.; Guo, H.; Lu, K.; Dabruzzo, B.; Miceli, R.; Severt, L.; et al. Time course of efficacy of atogepant for the preventive treatment of migraine: Results from the randomized, double-blind ADVANCE trial. Cephalalgia 2022, 42, 3–11. [Google Scholar] [CrossRef]
- Boinpally, R.; Jakate, A.; Butler, M.; Periclou, A. Atogepant and sumatriptan: No clinically relevant drug-drug interactions in a randomized, open-label, crossover trial. Pain Manag. 2022, 12, 499–508. [Google Scholar] [CrossRef]
- Pozo-Rosich, P.; Ailani, J.; Ashina, M.; Goadsby, P.J.; Lipton, R.B.; Reuter, U. Atogepant for the preventive treatment of chronic migraine: Results from the PROGRESS phase 3 trial. Neurology 2023, 100 (17 Supplement 2), 3980. [Google Scholar]
- Goadsby, P.J.; Dodick, D.W.; Ailani, J.; Trugman, J.M.; Finnegan, M.; Lu, K.; Szegedi, A. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: A double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020, 19, 727–737. [Google Scholar] [CrossRef]
- Croop, R.; Madonia, J.; Stock, D.A.; Thiry, A.; Forshaw, M.; Murphy, A.; Coric, V.; Lipton, R.B. Zavegepant nasal spray for the acute treatment of migraine: A Phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial. Headache 2022, 62, 1153–1163. [Google Scholar] [CrossRef]
- Lipton, R.B.; Croop, R.; Stock, D.A.; Madonia, J.; Forshaw, M.; Lovegren, M.; Mosher, L.; Coric, V.; Goadsby, P.J. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: A phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023, 22, 209–217. [Google Scholar] [CrossRef]
- Woodhead, J.L.; Siler, S.Q.; Howell, B.A.; Watkins, P.B.; Conway, C. Comparing the Liver Safety Profiles of Four Next-Generation CGRP Receptor Antagonists to the Hepatotoxic CGRP Inhibitor Telcagepant Using Quantitative Systems Toxicology Modeling. Toxicol. Sci. 2022, 188, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Blumenfeld, A.; Jensen, C.M.; Croop, R.; Thiry, A.; L’Italien, G.; Morris, B.A.; Coric, V.; Goadsby, P.J. Efficacy of rimegepant for the acute treatment of migraine based on triptan treatment experience: Pooled results from three phase 3 randomized clinical trials. Cephalalgia 2023, 43, 3331024221141686. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.M.; Goadsby, P.J.; Dodick, D.W.; Hutchinson, S.; Liu, C.; Finnegan, M.; Trugman, J.M.; Szegedi, A. Efficacy of ubrogepant based on prior exposure and response to triptans: A post hoc analysis. Headache 2021, 61, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.; Goadsby, P.J.; Schwedt, T.; Lipton, R.; Liu, C.; Lu, K.; Yu, S.Y.; Severt, L.; Finnegan, M.; Trugman, J.M. Ubrogepant for the acute treatment of migraine when administered during the prodrome of migraine when administered during the prodrome (premonitory phase): Results from a phase 3, randomized, double-blind, placebo-controlled, crossover study. Headache 2023, 63 (S1), 177. [Google Scholar]
- Goadsby, P.J.; Ailani, J.; Dodick, D.; Starling, A.; Liu, C.; Yu, S.Y.; Brand-Schieber, E.; Finnegan, M.; Trugman, J.M. Efficacy of Ubrogepant for the Treatment of Migraine Symptoms During the Prodrome (Premonitory Phase): Results From the PRODROME Trial. Headache 2023, 63 (S1), 137. [Google Scholar]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef]
- Sun, H.; Dodick, D.W.; Silberstein, S.; Goadsby, P.J.; Reuter, U.; Ashina, M.; Saper, J.; Cady, R.; Chon, Y.; Dietrich, J.; et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. Neurol. 2016, 15, 382–390. [Google Scholar] [CrossRef]
- Bigal, M.E.; Edvinsson, L.; Rapoport, A.M.; Lipton, R.B.; Spierings, E.L.; Diener, H.C.; Burstein, R.; Loupe, P.S.; Ma, Y.; Yang, R.; et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015, 14, 1091–1100. [Google Scholar] [CrossRef]
- Bigal, M.E.; Walter, S.; Bronson, M.; Alibhoy, A.; Escandon, R. Cardiovascular and hemodynamic parameters in women following prolonged CGRP inhibition using LBR-101, a monoclonal antibody against CGRP. Cephalalgia 2014, 34, 968–976. [Google Scholar] [CrossRef]
- Dodick, D.W.; Goadsby, P.J.; Silberstein, S.D.; Lipton, R.B.; Olesen, J.; Ashina, M.; Wilks, K.; Kudrow, D.; Kroll, R.; Kohrman, B.; et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014, 13, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Skljarevski, V.; Oakes, T.M.; Zhang, Q.; Ferguson, M.B.; Martinez, J.; Camporeale, A.; Johnson, K.W.; Shan, Q.; Carter, J.; Schacht, A.; et al. Effect of Different Doses of Galcanezumab vs Placebo for Episodic Migraine Prevention: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 187–193. [Google Scholar] [CrossRef]
- Oakes, T.M.M.; Skljarevski, V.; Zhang, Q.; Kielbasa, W.; Hodsdon, M.E.; Detke, H.C.; Camporeale, A.; Saper, J.R. Safety of galcanezumab in patients with episodic migraine: A randomized placebo-controlled dose-ranging Phase 2b study. Cephalalgia 2018, 38, 1015–1025. [Google Scholar] [CrossRef]
- Stauffer, V.L.; Dodick, D.W.; Zhang, Q.; Carter, J.N.; Ailani, J.; Conley, R.R. Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1080–1088. [Google Scholar] [CrossRef]
- Skljarevski, V.; Matharu, M.; Millen, B.A.; Ossipov, M.H.; Kim, B.K.; Yang, J.Y. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 2018, 38, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology 2018, 91, e2211–e2221. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132. [Google Scholar] [CrossRef]
- Ashina, S.; Melo-Carrillo, A.; Toluwanimi, A.; Bolo, N.; Szabo, E.; Borsook, D.; Burstein, R. Galcanezumab effects on incidence of headache after occurrence of triggers, premonitory symptoms, and aura in responders, non-responders, super-responders, and super non-responders. J. Headache Pain 2023, 24, 26. [Google Scholar] [CrossRef]
- Edvinsson, L. CGRP blockers in migraine therapy: Where do they act? Br. J. Pharmacol. 2008, 155, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.M.; Hougaard, A.; Cramer, S.P.; Christensen, C.E.; Wolfram, F.; Larsson, H.B.W.; Ashina, M. Intact blood-brain barrier during spontaneous attacks of migraine without aura: A 3T DCE-MRI study. Eur. J. Neurol. 2017, 24, 1116–1124. [Google Scholar] [CrossRef]
- Hougaard, A.; Amin, F.M.; Christensen, C.E.; Younis, S.; Wolfram, F.; Cramer, S.P.; Larsson, H.B.W.; Ashina, M. Increased brainstem perfusion, but no blood-brain barrier disruption, during attacks of migraine with aura. Brain J. Neurol. 2017, 140, 1633–1642. [Google Scholar] [CrossRef]
- Schankin, C.J.; Maniyar, F.H.; Seo, Y.; Kori, S.; Eller, M.; Chou, D.E.; Blecha, J.; Murphy, S.T.; Hawkins, R.A.; Sprenger, T.; et al. Ictal lack of binding to brain parenchyma suggests integrity of the blood-brain barrier for 11C-dihydroergotamine during glyceryl trinitrate-induced migraine. Brain J. Neurol. 2016, 139, 1994–2001. [Google Scholar] [CrossRef]
- Christensen, S.L.; Ernstsen, C.; Olesen, J.; Kristensen, D.M. No central action of CGRP antagonising drugs in the GTN mouse model of migraine. Cephalalgia 2020, 40, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Ziegeler, C.; Mehnert, J.; Asmussen, K.; May, A. Central effects of erenumab in migraine patients: An event-related functional imaging study. Neurology 2020, 95, e2794–e2802. [Google Scholar] [CrossRef] [PubMed]
- VanderPluym, J.; Dodick, D.W.; Lipton, R.B.; Ma, Y.; Loupe, P.S.; Bigal, M.E. Fremanezumab for preventive treatment of migraine: Functional status on headache-free days. Neurology 2018, 91, e1152–e1165. [Google Scholar] [CrossRef]
- Felgenhauer, K. Protein size and cerebrospinal fluid composition. Klin. Wochenschr. 1974, 52, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Raddant, A.C.; Russo, A.F. Calcitonin gene-related peptide in migraine: Intersection of peripheral inflammation and central modulation. Expert Rev. Mol. Med. 2011, 13, e36. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Sobczuk, A.; Pawlowska, E.; Blasiak, J. Epigenetic Connection of the Calcitonin Gene-Related Peptide and Its Potential in Migraine. Int. J. Mol. Sci. 2022, 23, 6151. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Boucherie, D.M.; Creeney, H.; van Drie, R.W.A.; Farham, F.; Favaretto, S.; Gollion, C.; Grangeon, L.; Lyons, H.; Marschollek, K.; et al. Future targets for migraine treatment beyond CGRP. J Headache Pain. 2023, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Uddman, R.; Edvinsson, L. Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia 2001, 21, 96–101. [Google Scholar] [CrossRef]
- Uddman, R.; Tajti, J.; Hou, M.; Sundler, F.; Edvinsson, L. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia 2002, 22, 112–116. [Google Scholar] [CrossRef]
- Laburthe, M.; Couvineau, A. Molecular pharmacology and structure of VPAC Receptors for VIP and PACAP. Regul. Pept. 2002, 108, 165–173. [Google Scholar] [CrossRef]
- Falktoft, B.; Georg, B.; Fahrenkrug, J. Signaling pathways in PACAP regulation of VIP gene expression in human neuroblastoma cells. Neuropeptides 2009, 43, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Georg, B.; Fahrenkrug, J. Pituitary adelylate cyclase-activating peptide is an activator of vasoactive intestinal polypeptide gene transcription in human neuroblastoma cells. Brain Res. Mol. Brain Res. 2000, 79, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vollesen, A.L.; Hansen, R.D.; Esserlind, A.L.; Amin, F.M.; Christensen, A.F.; Olesen, J.; Ashina, M. Part I: Pituitary adenylate cyclase-activating polypeptide-38 induced migraine-like attacks in patients with and without familial aggregation of migraine. Cephalalgia 2017, 37, 125–135. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Komaki, G.; Arimura, A. Passage of pituitary adenylate cyclase activating polypeptide1-27 and pituitary adenylate cyclase activating polypeptide1-38 across the blood-brain barrier. J. Pharmacol. Exp. Ther. 1993, 267, 690–696. [Google Scholar] [PubMed]
- Onderwater, G.L.J.; Dool, J.; Ferrari, M.D.; Terwindt, G.M. Premonitory symptoms in glyceryl trinitrate triggered migraine attacks: A case-control study. Pain 2020, 161, 2058–2067. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.H.; Mehnert, J.; May, A. Longitudinal Neuroimaging over 30 Days: Temporal Characteristics of Migraine. Ann. Neurol. 2020, 87, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Kuburas, A.; Mason, B.N.; Hing, B.; Wattiez, A.S.; Reis, A.S.; Sowers, L.P.; Moldovan Loomis, C.; Garcia-Martinez, L.F.; Russo, A.F. PACAP Induces Light Aversion in Mice by an Inheritable Mechanism Independent of CGRP. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 4697–4715. [Google Scholar] [CrossRef]
- De Logu, F.; Landini, L.; Janal, M.N.; Li Puma, S.; De Cesaris, F.; Geppetti, P.; Nassini, R. Migraine-provoking substances evoke periorbital allodynia in mice. J. Headache Pain 2019, 20, 18. [Google Scholar] [CrossRef]
- Ernstsen, C.; Christensen, S.L.; Rasmussen, R.H.; Nielsen, B.S.; Jansen-Olesen, I.; Olesen, J.; Kristensen, D.M. The PACAP pathway is independent of CGRP in mouse models of migraine: Possible new drug target? Brain J. Neurol. 2022, 145, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Emery, A.C.; Eiden, L.E. Signaling through the neuropeptide GPCR PAC₁ induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. FASEB J. 2012, 26, 3199–3211. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Zagami, A.S. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain J. Neurol. 1991, 114 Pt 2, 1001–1011. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain J. Neurol. 1994, 117 Pt 3, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Wienholtz, N.K.F.; Christensen, C.E.; Zhang, D.G.; Coskun, H.; Ghanizada, H.; Al-Karagholi, M.A.; Hannibal, J.; Egeberg, A.; Thyssen, J.P.; Ashina, M. Early treatment with sumatriptan prevents PACAP38-induced migraine: A randomised clinical trial. Cephalalgia 2021, 41, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.M.; Asghar, M.S.; Guo, S.; Hougaard, A.; Hansen, A.E.; Schytz, H.W.; van der Geest, R.J.; de Koning, P.J.; Larsson, H.B.; Olesen, J.; et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012, 32, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.M.; Hougaard, A.; Magon, S.; Asghar, M.S.; Ahmad, N.N.; Rostrup, E.; Sprenger, T.; Ashina, M. Change in brain network connectivity during PACAP38-induced migraine attacks: A resting-state functional MRI study. Neurology 2016, 86, 180–187. [Google Scholar] [CrossRef]
- Ghanizada, H.; Al-Karagholi, M.A.; Arngrim, N.; Olesen, J.; Ashina, M. PACAP27 induces migraine-like attacks in migraine patients. Cephalalgia 2020, 40, 57–67. [Google Scholar] [CrossRef]
- Rasmussen, N.B.; Deligianni, C.; Christensen, C.E.; Karlsson, W.K.; Al-Khazali, H.M.; Van de Casteele, T.; Granhall, C.; Amin, F.M.; Ashina, M. The effect of Lu AG09222 on PACAP38- and VIP-induced vasodilation, heart rate increase, and headache in healthy subjects: An interventional, randomized, double-blind, parallel-group, placebo-controlled study. J. Headache Pain 2023, 24, 60. [Google Scholar] [CrossRef]
- Edvinsson, L. Role of VIP/PACAP in primary headaches. Cephalalgia 2013, 33, 1070–1072. [Google Scholar] [CrossRef]
- Goadsby, P.J.; MacDonald, G.J. Extracranial vasodilation mediated by vasoactive intestinal polypeptide (VIP). Brain Res. 1985, 329, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Olesen, I.; Baun, M.; Amrutkar, D.V.; Ramachandran, R.; Christophersen, D.V.; Olesen, J. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides 2014, 48, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Wattiez, A.S.; Balcziak, L.K.; Kuburas, A.; Kutschke, W.J.; Russo, A.F. Vascular actions of peripheral CGRP in migraine-like photophobia in mice. Cephalalgia 2020, 40, 1585–1604. [Google Scholar] [CrossRef]
- Tore, F.; Korkmaz, O.T.; Dogrukol-Ak, D.; Tunçel, N. The effects of vasoactive intestinal peptide on dura mater nitric oxide levels and vessel-contraction responses in sympathectomized rats. J. Mol. Neurosci. 2010, 41, 288–293. [Google Scholar] [CrossRef]
- Kilinc, E.; Firat, T.; Tore, F.; Kiyan, A.; Kukner, A.; Tunçel, N. Vasoactive Intestinal peptide modulates c-Fos activity in the trigeminal nucleus and dura mater mast cells in sympathectomized rats. J. Neurosci. Res. 2015, 93, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Cernuda-Morollón, E.; Martínez-Camblor, P.; Alvarez, R.; Larrosa, D.; Ramón, C.; Pascual, J. Increased VIP levels in peripheral blood outside migraine attacks as a potential biomarker of cranial parasympathetic activation in chronic migraine. Cephalalgia 2015, 35, 310–316. [Google Scholar] [CrossRef]
- Bellamy, J.L.; Cady, R.K.; Durham, P.L. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache 2006, 46, 24–33. [Google Scholar] [CrossRef]
- Sarchielli, P.; Pini, L.A.; Zanchin, G.; Alberti, A.; Maggioni, F.; Rossi, C.; Floridi, A.; Calabresi, P. Clinical-biochemical correlates of migraine attacks in rizatriptan responders and non-responders. Cephalalgia 2006, 26, 257–265. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Martínez-Camblor, P.; Ramón, C.; Larrosa, D.; Serrano-Pertierra, E.; Pascual, J. CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache 2014, 54, 987–995. [Google Scholar] [CrossRef]
- Hansen, J.M.; Sitarz, J.; Birk, S.; Rahmann, A.M.; Oturai, P.S.; Fahrenkrug, J.; Olesen, J.; Ashina, M. Vasoactive intestinal polypeptide evokes only a minimal headache in healthy volunteers. Cephalalgia 2006, 26, 992–1003. [Google Scholar] [CrossRef]
- Riesco, N.; Cernuda-Morollón, E.; Martínez-Camblor, P.; Pérez-Alvarez, A.I.; Verano, L.; García-Cabo, C.; Serrano-Pertierra, E.; Pascual, J. Relationship between serum levels of VIP, but not of CGRP, and cranial autonomic parasympathetic symptoms: A study in chronic migraine patients. Cephalalgia 2017, 37, 823–827. [Google Scholar] [CrossRef]
- Pellesi, L.; Al-Karagholi, M.A.; Chaudhry, B.A.; Lopez, C.L.; Snellman, J.; Hannibal, J.; Amin, F.M.; Ashina, M. Two-hour infusion of vasoactive intestinal polypeptide induces delayed headache and extracranial vasodilation in healthy volunteers. Cephalalgia 2020, 40, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Karsan, N.; Nagaraj, K.; Goadsby, P.J. Cranial autonomic symptoms: Prevalence, phenotype and laterality in migraine and two potentially new symptoms. J. Headache Pain 2022, 23, 18. [Google Scholar] [CrossRef]
- Mason, B.N.; Kaiser, E.A.; Kuburas, A.; Loomis, M.M.; Latham, J.A.; Garcia-Martinez, L.F.; Russo, A.F. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 204–216. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N. Neuropeptide transmission in brain circuits. Neuron 2012, 76, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Copeland, J.R.; Emson, P.C.; McCulloch, J.; Uddman, R. Nerve Fibers Containing Neuropeptide Y in the Cerebrovascular Bed: Immunocytochemistry, Radioimmunoassay, and Vasomotor Effects. J. Cereb. Blood Flow Metab. 1987, 7, 45–57. [Google Scholar] [CrossRef]
- Parker, R.M.; Herzog, H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999, 11, 1431–1448. [Google Scholar] [CrossRef]
- Stanley, B.G.; Kyrkouli, S.E.; Lampert, S.; Leibowitz, S.F. Neuropeptide Y chronically injected into the hypothalamus: A powerful neurochemical inducer of hyperphagia and obesity. Peptides 1986, 7, 1189–1192. [Google Scholar] [CrossRef]
- Yamanaka, A.; Kunii, K.; Nambu, T.; Tsujino, N.; Sakai, A.; Matsuzaki, I.; Miwa, Y.; Goto, K.; Sakurai, T. Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 2000, 859, 404–409. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, F.; Yang, M.J.; Yu, D.F.; Wu, W.N.; Liu, J.; Ma, L.Q.; Cai, F.; Chen, J.G. Leptin regulated calcium channels of neuropeptide Y and proopiomelanocortin neurons by activation of different signal pathways. Neuroscience 2008, 156, 89–98. [Google Scholar] [CrossRef]
- Sim, L.J.; Joseph, S.A. Arcuate nucleus projections to brainstem regions which modulate nociception. J. Chem. Neuroanat. 1991, 4, 97–109. [Google Scholar] [CrossRef]
- Kuphal, K.E.; Solway, B.; Pedrazzini, T.; Taylor, B.K. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY. Nutrition 2008, 24, 885–891. [Google Scholar] [CrossRef]
- Holland, P.; Goadsby, P.J. The hypothalamic orexinergic system: Pain and primary headaches. Headache 2007, 47, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.R.; Akerman, S.; Goadsby, P.J. Modulation of nociceptive dural input to the trigeminal nucleus caudalis via activation of the orexin 1 receptor in the rat. Eur. J. Neurosci. 2006, 24, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.R.; Akerman, S.; Goadsby, P.J. Orexin 1 receptor activation attenuates neurogenic dural vasodilation in an animal model of trigeminovascular nociception. J. Pharmacol. Exp. Ther. 2005, 315, 1380–1385. [Google Scholar] [CrossRef]
- Bartsch, T.; Levy, M.J.; Knight, Y.E.; Goadsby, P.J. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain 2004, 109, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Supronsinchai, W.; Akerman, S.; Andreou, A.P.; Winrow, C.J.; Renger, J.; Hargreaves, R.; Goadsby, P.J. Evidence for orexinergic mechanisms in migraine. Neurobiol. Dis. 2015, 74, 137–143. [Google Scholar] [CrossRef]
- Martins-Oliveira, M.; Akerman, S.; Tavares, I.; Goadsby, P.J. Neuropeptide Y inhibits the trigeminovascular pathway through NPY Y1 receptor: Implications for migraine. Pain 2016, 157, 1666–1673. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, Y.; An, J.; Qi, Y.; Luo, G. Neuropeptide changes in an improved migraine model with repeat stimulations. Transl. Neurosci. 2021, 12, 523–532. [Google Scholar] [CrossRef]
- Yang, C.; Gong, Z.; Zhang, X.; Miao, S.; Li, B.; Xie, W.; Wang, T.; Han, X.; Wang, L.; Dong, Z.; et al. Neuropeptide Y in the medial habenula alleviates migraine-like behaviors through the Y1 receptor. J. Headache Pain 2023, 24, 61. [Google Scholar] [CrossRef]
- Kushi, A.; Sasai, H.; Koizumi, H.; Takeda, N.; Yokoyama, M.; Nakamura, M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15659–15664. [Google Scholar] [CrossRef] [PubMed]
- Martins-Oliveira, M.; Akerman, S.; Holland, P.R.; Hoffmann, J.R.; Tavares, I.; Goadsby, P.J. Neuroendocrine signaling modulates specific neural networks relevant to migraine. Neurobiol. Dis. 2017, 101, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Szentirmai, E.; Krueger, J.M. Central administration of neuropeptide Y induces wakefulness in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R473–R480. [Google Scholar] [CrossRef]
- Tóth, A.; Hajnik, T.; Záborszky, L.; Détári, L. Effect of basal forebrain neuropeptide Y administration on sleep and spontaneous behavior in freely moving rats. Brain Res. Bull. 2007, 72, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M. The NPY system in stress, anxiety and depression. Neuropeptides 2004, 38, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Gallai, V.; Sarchielli, P.; Trequattrini, A.; Paciaroni, M.; Usai, F.; Palumbo, R. Neuropeptide Y in juvenile migraine and tension-type headache. Headache 1994, 34, 35–40. [Google Scholar] [CrossRef]
- Caproni, S.; Corbelli, I.; Pini, L.A.; Cupini, M.L.; Calabresi, P.; Sarchielli, P. Migraine preventive drug-induced weight gain may be mediated by effects on hypothalamic peptides: The results of a pilot study. Cephalalgia 2011, 31, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, S.D.; Bekker-Nielsen Dunbar, M.; Snoer, A.H.; Deen, M.; Edvinsson, L. Serotonin and Neuropeptides in Blood From Episodic and Chronic Migraine and Cluster Headache Patients in Case-Control and Case-Crossover Settings: A Systematic Review and Meta-Analysis. Headache 2020, 60, 1132–1164. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Widerlöv, E.; Ekman, R.; Kovács, K.; Jelencsik, I.; Bozsik, G.; Kapócs, G. Suboccipital cerebrospinal fluid and plasma concentrations of somatostatin, neuropeptide Y and beta-endorphin in patients with common migraine. Neuropeptides 1992, 22, 111–116. [Google Scholar] [CrossRef]
- Valenzuela, R.F.; Donoso, M.V.; Mellado, P.A.; Huidobro-Toro, J.P. Migraine, but not subarachnoid hemorrhage, is associated with differentially increased NPY-like immunoreactivity in the CSF. J. Neurol. Sci. 2000, 173, 140–146. [Google Scholar] [CrossRef]
- Chabi, A.; Zhang, Y.; Jackson, S.; Cady, R.; Lines, C.; Herring, W.J.; Connor, K.M.; Michelson, D. Randomized controlled trial of the orexin receptor antagonist filorexant for migraine prophylaxis. Cephalalgia 2015, 35, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Karsan, N.; Bose, P.; Newman, J.; Goadsby, P.J. Are some patient-perceived migraine triggers simply early manifestations of the attack? J. Neurol. 2021, 268, 1885–1893. [Google Scholar] [CrossRef]
- Winter, A.C.; Berger, K.; Buring, J.E.; Kurth, T. Body mass index, migraine, migraine frequency and migraine features in women. Cephalalgia 2009, 29, 269–278. [Google Scholar] [CrossRef]
- Martins-Oliveira, M.; Tavares, I.; Goadsby, P.J. Was it something I ate? Understanding the bidirectional interaction of migraine and appetite neural circuits. Brain Res. 2021, 1770, 147629. [Google Scholar] [CrossRef] [PubMed]
- Siva, Z.O.; Uluduz, D.; Keskin, F.E.; Erenler, F.; Balcı, H.; Uygunoğlu, U.; Saip, S.; Göksan, B.; Siva, A. Determinants of glucose metabolism and the role of NPY in the progression of insulin resistance in chronic migraine. Cephalalgia 2018, 38, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Antonijevic, I.; Murck, H.; Kuenzel, H.; Steiger, A. Neuropeptide Y (NPY) shortens sleep latency but does not suppress ACTH and cortisol in depressed patients and normal controls. Psychoneuroendocrinology 2006, 31, 100–107. [Google Scholar] [CrossRef]
- Karsan, N.; Goadsby, P.J. Migraine Is More Than Just Headache: Is the Link to Chronic Fatigue and Mood Disorders Simply Due to Shared Biological Systems? Front. Hum. Neurosci. 2021, 15, 646692. [Google Scholar] [CrossRef]
- Meye, F.J.; Adan, R.A. Feelings about food: The ventral tegmental area in food reward and emotional eating. Trends Pharmacol. Sci. 2014, 35, 31–40. [Google Scholar] [CrossRef]
- Martins-Oliveira, M.; Akerman, S.; Holland, P.R.; Tavares, I.; Goadsby, P.J. Pharmacological modulation of ventral tegmental area neurons elicits changes in trigeminovascular sensory processing and is accompanied by glycemic changes: Implications for migraine. Cephalalgia 2022, 42, 1359–1374. [Google Scholar] [CrossRef]
- Bernecker, C.; Pailer, S.; Kieslinger, P.; Horejsi, R.; Möller, R.; Lechner, A.; Wallner-Blazek, M.; Weiss, S.; Fazekas, F.; Truschnig-Wilders, M.; et al. GLP-2 and leptin are associated with hyperinsulinemia in non-obese female migraineurs. Cephalalgia 2010, 30, 1366–1374. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Offen, W.W.; Klein, E.G.; Phebus, L.A.; Hipskind, P.; Johnson, K.W.; Ryan, R.E., Jr. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia 2001, 21, 102–106. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Wang, O.; Saper, J.R.; Stoltz, R.; Silberstein, S.D.; Mathew, N.T. Ineffectiveness of neurokinin-1 antagonist in acute migraine: A crossover study. Cephalalgia 1997, 17, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Szok, D.; Majlath, Z.; Tuka, B.; Csati, A.; Vecsei, L. Migraine and neuropeptides. Neuropeptides 2015, 52, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Snoer, A.; Lund, N.; Beske, R.; Hagedorn, A.; Jensen, R.H.; Barloese, M. Cluster headache beyond the pain phase: A prospective study of 500 attacks. Neurology 2018, 91, e822–e831. [Google Scholar] [CrossRef] [PubMed]
- Snoer, A.; Lund, N.; Beske, R.; Jensen, R.; Barloese, M. Pre-attack signs and symptoms in cluster headache: Characteristics and time profile. Cephalalgia 2018, 38, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
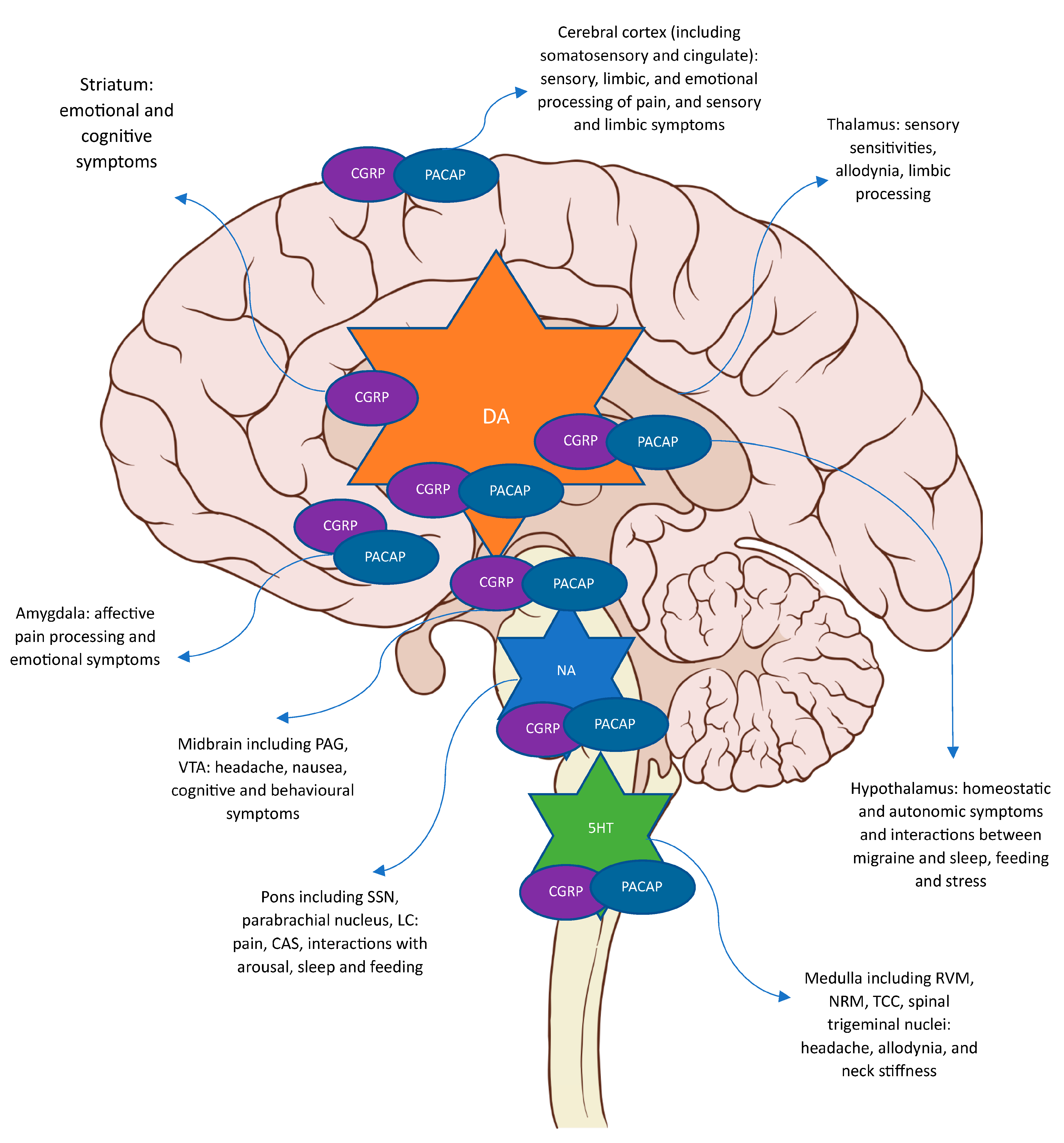
| Neuropeptide | Role in Migraine | Role in Migraine Therapeutics |
|---|---|---|
| CGRP | Sensitisation, vasodilatation, mast cell degranulation, CSD, circadian variability to migraine, photophobia, allodynia, and interactions with NOS | Small molecule CGRP antagonists (gepants) for attack abortion and rimegepant for both acute and preventive effects (oral and nasal formulations) Monoclonal antibodies against CGRP ligand or canonical receptor for migraine prevention (subcutaneous and intravenous formulations) |
| PACAP | Vasodilatation, photophobia, allodynia, sensitisation, and CASs | PAC1 and PACAP receptor antibodies hold potential |
| VIP | Vasodilatation, CASs, mast cell regulation, and interaction between sensory and parasympathetic systems | VPAC1 and VPAC2 receptors may hold potential in the future |
| Neuropeptide Y | Regulation of sleep, promotion of feeding, stress responses, anxiety, allodynia, and interaction between pain and physiological processes | NPY Y1 agonism may hold potential in the future |
| Agouti-related peptide and proopiomelanocortin (POMC) and cocaine and amphetamine-related transcript (CART) | Regulation of feeding, with opposing signalling effects in response to leptin, hypothalamic projections regulating energy balance homeostasis, and links between migraine and feeding and obesity and diabetes | Nil as yet, but these peptides alter appetite and may be implicated in the links between migraine and disordered feeding (and there may be a role of the ventral tegmental area in both migraine headache and modulating food craving) [260,261] |
| Orexins A and B | Sleep regulation, glucose metabolism, and possible preclinical role in headache [236,237] | Specific Ox1 antagonism may hold therapeutic promise in the future [253] |
| Leptin | Appetite suppression and possible link to raised BMI in migraine [262], preclinical evidence for a possible role in headache [244] | Targeting the interactions between migraine and impaired metabolic homeostasis may hold future therapeutic promise |
| Substance P and neurokinin A | Vasodilatation, plasma protein extravasation, mast cell degranulation, and platelet aggregation | Neurokinin 1 inhibition and plasma protein extravasation inhibitors have failed in clinical trials of migraine [263,264] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karsan, N.; Gosalia, H.; Goadsby, P.J. Molecular Mechanisms of Migraine: Nitric Oxide Synthase and Neuropeptides. Int. J. Mol. Sci. 2023, 24, 11993. https://doi.org/10.3390/ijms241511993
Karsan N, Gosalia H, Goadsby PJ. Molecular Mechanisms of Migraine: Nitric Oxide Synthase and Neuropeptides. International Journal of Molecular Sciences. 2023; 24(15):11993. https://doi.org/10.3390/ijms241511993
Chicago/Turabian StyleKarsan, Nazia, Helin Gosalia, and Peter J. Goadsby. 2023. "Molecular Mechanisms of Migraine: Nitric Oxide Synthase and Neuropeptides" International Journal of Molecular Sciences 24, no. 15: 11993. https://doi.org/10.3390/ijms241511993
APA StyleKarsan, N., Gosalia, H., & Goadsby, P. J. (2023). Molecular Mechanisms of Migraine: Nitric Oxide Synthase and Neuropeptides. International Journal of Molecular Sciences, 24(15), 11993. https://doi.org/10.3390/ijms241511993








