Abstract
Cichorium intybus L. is the most economically important species of its genus and among the most important of the Asteraceae family. In chicory, many linkage maps have been produced, several sets of mapped and unmapped markers have been developed, and dozens of genes linked to traits of agronomic interest have been investigated. This treasure trove of information, properly cataloged and organized, is of pivotal importance for the development of superior commercial products with valuable agronomic potential in terms of yield and quality, including reduced bitter taste and increased inulin production, as well as resistance or tolerance to pathogens and resilience to environmental stresses. For this reason, a systematic review was conducted based on the scientific literature published in chicory during 1980–2023. Based on the results obtained from the meta-analysis, we created two consensus maps capable of supporting marker-assisted breeding (MAB) and marker-assisted selection (MAS) programs. By taking advantage of the recently released genome of C. intybus, we built a 639 molecular marker-based consensus map collecting all the available mapped and unmapped SNP and SSR loci available for this species. In the following section, after summarizing and discussing all the genes investigated in chicory and related to traits of interest such as reproductive barriers, sesquiterpene lactone biosynthesis, inulin metabolism and stress response, we produced a second map encompassing 64 loci that could be useful for MAS purposes. With the advent of omics technologies, molecular data chaos (namely, the situation where the amount of molecular data is so complex and unmanageable that their use becomes challenging) is becoming far from a negligible issue. In this review, we have therefore tried to contribute by standardizing and organizing the molecular data produced thus far in chicory to facilitate the work of breeders.
1. Introduction
Chicories (2n = 2x = 18) are economically important dicot species belonging to the Asteraceae family. The Cichorium genus contains six main species, of which four are exclusively wild (Cichorium bottae Deflers., Cichorium spinosum L., Cichorium calvum Sch. Bip. ex Asch., and Cichorium pumilum Jacq.), one is exclusively cultivated (Cichorium endivia L.), and one contains both cultivated and wild individuals (Cichorium intybus L.) [1]. The main botanical variety in terms of economic impact is Cichorium intybus var. foliosum, widely appreciated for its leaves, which are eaten raw or cooked, and characterized by a distinctive bitter taste and crispiness. This variety includes ‘Witloof Chicory’ or ‘Belgian Endive’, commonly known in Europe for its typical etiolated buds named ‘chicon’, and Red Chicory, known as ‘Radicchio’, mostly distributed in northeastern Italy [2,3,4,5]. Apart from leaf chicory, mainly known for its nutritional and health-beneficial properties, an upsurge of interest has been observed in ‘industrial’ or ‘root’ chicory (C. intybus var. sativum), which is mostly used for inulin extraction and as a coffee substitute [6,7,8]. Inulin, as a carbohydrate reserve, accumulates during the first year’s growing season in taproot chicory and is used for food and nonfood applications [9,10].
The other economically relevant species from the same genus is C. endivia, whose curly and smooth leaves (var. crispum and var. latifolium, respectively) are consumed worldwide in fresh salads, with Spain, France, and Italy as major EU producers [9].
From a reproductive point of view, Cichorium intybus is a diploid plant species that is prevalently allogamous due to its efficient sporophytic self-incompatibility system [3,11,12]. Male sterility represents another efficient sexual barrier widely used in chicory to promote outcrossing and to facilitate the exploitation of heterosis through the production of F1 hybrids. In contrast, endive is an autogamous species with a rate of outcrossing of approximately 1% [13]. Chicory and endive, as closely related but distinct species, are completely interfertile and offer a vast genetic pool that, through cross-breeding schemes, might be exploited to obtain progeny with wide genetic diversity [3,4].
One of the main goals in chicory breeding programs is to achieve the best selections with valuable agronomic potential, such as yield, reduced bitter taste, increased inulin production, and resistance to both biotic and abiotic stressors. Molecular markers are fully addressed to assess genetic information on parental genotypes, heterozygosity evaluation and prediction, population uniformity and distinctiveness [13,14]. Moreover, these tools are employed not only in phylogenetic studies and genetic linkage map construction but also for the genetic traceability of the final commercial product [15,16]. As a result, the aim of generating a superior commercial product in accordance with market acceptance could be facilitated through the efficient implementation of marker-assisted breeding (MAB) and marker-assisted selection (MAS) programs [17,18,19].
In this study, after a systematic review of all the scientific literature produced for chicory in the last 40 years, the assembled genome of Cichorium intybus L. by Fan et al. was used to collect and physically map the genetic data available for this species [20]. The main advancements in chicory genetics and how they might be employed in breeding programs are the main topics of this study. To this aim, we developed two user-friendly genomic maps for breeding purposes. The first map contains single-nucleotide polymorphisms (SNPs) as well as simple-sequence repeats (SSRs), which might be helpful in MAB programs. The second map contains all the available gene sequences and marker-related genes, providing up-to-date information for MAS applications. The mapping of molecular markers and genes responsible for relevant agronomic traits has a significant impact on crop productivity and quality, and both maps are designed to act as a starting point for validating markers and genes of interest in chicory.
2. Methods
2.1. Literature Research
The systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. An overall bibliographic analysis for this work was conducted using Scopus and PubMed databases in January 2023. The search was confined to articles published between 1980 and 2023 and written in the English language. Since extensive data availability was expected, we split the research into two main parts representing MAB and MAS. A single list of articles was generated for MAB research, while 10 lists were produced for MAS, corresponding to the most relevant topics considered in chicory (namely, reproductive barriers, sesquiterpene lactone biosynthesis, hydroxycinnamates, inulin metabolism, stress response, blue-lilac color, flowering time, somatic embryogenesis, red discoloration and gene normalization). MAB-related research was conducted using key words as follows: (“Cichorium intybus” OR “chicory”) AND (“SSR” OR “microsatellite” OR “SNP” OR “SNV”). For the MAS part, the 10 most interesting topics were investigated using the specific terms reported in Table 1. After list collection, duplicates (i.e., overlapping results from both databases) were deleted and the eligibility assessment of the remaining articles was manually cured. We first excluded letters, conference papers, notes, articles without full-text availability, short reports and non-suitable articles based on the evaluation of abstracts and keywords. The remaining articles were deeply investigated and further filtered, by removing those either not fully consistent with the topic or lacking molecular data. In addition, we exploited the citations of the selected research papers, which led to an enrichment of the final reference list.

Table 1.
Keyword terms searched within the scientific literature databases. In addition to “Cichorium intybus” OR “chicory”, different keyword terms were applied to the selected databases according to the purpose of the research.
2.2. Data Collection and Maps Drawing
A comprehensive map for marker-assisted breeding purposes was built by using all the SSR- or SNP-containing sequences available in the scientific articles (identified according to the methods described in the previous section). The sequences, according to the indications provided by each article, were retrieved from GenBank and used as a query in the alignment against the C. intybus genome (JAK-NSD01 [20]). A BLASTn search was performed by setting the following parameters for SNPs-containing sequences: E-value, <1 × 10−5; percentage of identity, ≥95%; and minimum query coverage, 95%. For SSR primers, the following parameters were set: E-value, <1 × 10−5; percentage of identity, 100; and query coverage, 100%. The results were then filtered to retain only the five best hits for each query. Each query mapping with the same specificity and percentage of identity in more than one location and/or chromosome was discarded to avoid ambiguities. A consensus map was finally drawn using the ggplot2 (version 3.4.2) used in R environment version 4.2.0.
A second map for marker-assisted selection purposes was built by using all the genes available in the scientific articles (identified in accordance with the methods described in the previous section). The sequences were retrieved from GenBank and used as a query in the alignment against the C. intybus genome (JAK-NSD01 [20]). A BLASTn search was performed by setting the following parameters: E-value, <1 × 10−5; percentage of identity, ≥95; and minimum query coverage, 95%. When available, the closest SNP and SSR (both upstream and downstream) to each gene were selected too. A comprehensive map was finally drawn by using the ggplot2 R package and by plotting the genes along with the above-mentioned associated markers.
3. Results and Discussion
3.1. Screening Literature Results
A preliminary survey of the literature led to the identification of 1441 records (910 from Scopus and 531 from PubMed). Briefly, 314 duplicate articles were removed, whereas 698 records including notes, articles without full-text availability, short surveys, and unrelated articles (based on abstract and keyword screening) were excluded. The remaining 429 results were analyzed based on their full content. From this filter, 361 were excluded because they did not match with the purpose of the study or data availability was limited or lacking. Five additional articles found in the references of the 68 remaining articles were added. Hence, the final reference list comprised 73 studies (Figure 1).
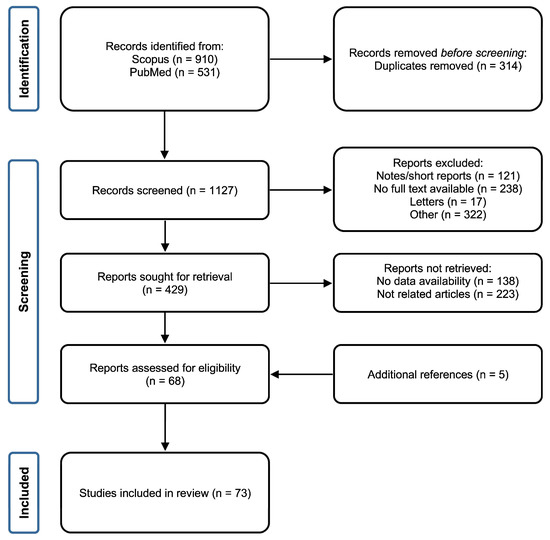
Figure 1.
Flow chart of the screening and selection process followed for the inclusion of the studies in the systematic review (n denotes the number of studies resulting from each filtering step).
3.2. Toward a Genetic Genomic Map of Chicory Useful for Marker-Assisted Breeding
Linkage maps lay the groundwork for marker-assisted breeding. In chicory, the assembly of the first linkage map based on 371 markers (16 RAPDs, 72 SAMPLs and 283 AFLPs; 1201 cM) was reported in 1997 using an interspecific hybrid cross between C. intybus L. and an inbred line of C. endivia L. [21]. Similarly, Van Stallen et al. built a genetic linkage map based on an intraspecific cross between two inbred lines of witloof chicory (129 RAPD, 609.6 cM) [22]. A further RAPD-based genetic map was focused on the characterization of QTLs for the length and browning of pith [23]. Cassan et al. constructed a RAPD- and SSR-based genetic map (987 cM) to identify QTLs controlling physiological and agronomical traits under two levels of nitrogen fertilization during the vegetative phase of witloof chicory [24]. Unfortunately, all the above-mentioned maps, due to the type of markers (i.e., dominant markers), cannot be used for comparative studies. A turning point was the development by Cadalen et al. of a consensus genetic map from two industrial chicory progenies and one witloof chicory progeny, containing 472 SSR markers and covering 878 cM [25]. This study was further deepened by Gonthier et al. for the identification of markers associated with nuclear male sterility (NMS) and sporophytic self-incompatibility (SSI) loci [12]. Starting from the data produced in the two previous studies, Ghedina et al. developed an efficient multiplex assay for genotyping elite breeding stocks developed from old landraces of Radicchio of Chioggia [26]. This assay, composed of 27 SSR markers selected according to the polymorphism index and distribution, was further integrated with two additional SSR markers and successfully applied by Patella et al., 2019 [13]. Muys et al. produced a genetic map for industrial chicory that included 237 marker loci and spanned a total length of 1208 cM [27]. The map was built combining AFLPs, SSRs, SNPs, and 26 coding sequences. Finally, a high-density linkage map of leaf chicory was constructed using genotyping-by-sequencing (GBS) technology [28]. The map contained 727 SNP markers, covering a total length of 1413 cM. Most importantly, the map was pivotal for the identification of the putative locus responsible for male sterility. All the genetic maps produced in chicory are summarized in Table 2.

Table 2.
Genetic and physical maps developed over the years for Cichorium intybus. When available, for each map, we report the correspondence between each linkage group (LG) and the chromosome (Chr) of the consensus map (from Fan et al., 2022 [20]), the number and type of markers employed, the length of each map, and the taxonomy of the sample used for map construction.
In addition to the aforementioned genetic maps, authors of several studies developed sets of unmapped markers, some of which had applications for a wide range of purposes. Thirty-one EST-SSRs with a high level of transferability potential between Cichorium species were proposed but never validated by Ince [29]. Raulier et al. developed a new set of 15 SSR marker loci to characterize the genetic diversity of the germplasm that originated in the current industrial chicory and to establish the relationships between and within chicory and endive species [1]. However, the sequences of this new set have never been made public by the authors. An additional set of 12 SSR markers was generated by Zavada et al. and used along with chloroplast DNA sequences to assess the temporal genetic changes and diversity in North America and in New England chicory populations [30,31].
Ideally, plant breeders draw on genetic maps and markers to mine information useful for MAB purposes [32]. However, as in the case of chicory, the availability of multiple linkage maps (each based on single and independent populations) and unmapped markers makes the interpretation and exploitation of the data very challenging. This challenge can be overcome through the production of consensus maps. Based on the procedure described in Section 2, we managed to position 639 markers within the physical map from Fan et al. [20], namely 579 SNPs and 60 SSRs derived from the studies of Cadalen et al., Ince, Muys et al., Zavada et al., Patella et al., and Palumbo et al. [13,25,27,28,29,30]. The newly developed consensus map is available in Figure S1.
The observed discrepancy between the number of markers actually used for the consensus map and the total number of markers developed over the years essentially relies on two factors. The first, which is far from negligible, is that in many cases, the authors of the maps have not made (completely or partially) available the markers used. The second is that some of the available markers did not map. This last aspect is not surprising at all considering that almost all the markers were derived from extragenic and therefore less-conserved regions. This observation assumes particular relevance if we take into account that the different genetic maps and marker sets have been developed using interspecific hybrids (C. endivia × C. intybus) or different botanical varieties (C. intybus var. sativum and var. foliosum). The consensus map allowed us to anchor to specific chromosome positions those markers that until now lacked a position (i.e., all the unmapped markers), such as those from Ince and Zavada et al. [29,30]. Furthermore, the consensus map enabled us to establish the correspondences between the linkage groups of the genetic maps produced by Cadalen et al., Muys et al., and Palumbo et al., and the chromosomes assembled by Fan et al. (Table 2) [20,25,27,28]. An example is provided in Figure 2, where chromosome 3 (JAKNSD010000003.1) was found to correspond to LG6 of Cadalen et al. and LG1 of Palumbo et al. [20,25,28]. Within each linkage group/chromosome, most of the markers showed full collinearity. The cases in which collinearity was not observed could be the result of species/variety-specific rearrangements, errors in the construction of the genetic maps, or errors during genome assembly [32]. Finally, it should be noted that, thanks to the integration between the genetic maps and the physical map, it was possible to improve the latter by assigning some of the 199 unassembled contigs (JAKNSD010000010.1-JAKNSD010000208.1) to specific chromosomes. For example, contig 49 (JAKNSD010000049.1) in Figure S1 plausibly represents the terminal portion of chromosome 1 (JAKNSD010000001.1).
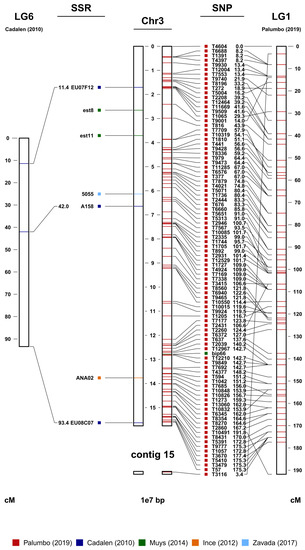
Figure 2.
Chromosome 3 (in the center) from the marker-assisted breeding (MAB) map (the full MAB map is provided as Figure S1). On the left are the SSR sequences and their genetic distances in cM derived from LG6 of Cadalen et al. [25] and other unmapped SSRs from Ince et al. [29] and Zavada et al. [30]. On the right are all the available SNPs and LG1 of Palumbo et al. [28], with the respective genetic distances in cM, and the SNPs deriving from the work of Muys et al. [27]. Contig 15 was associated with chromosome 3 due to the mapping of the SNP marker on the right.
3.3. A Comprehensive Map for Marker-Assisted Selection Purposes
Linkage maps based on molecular markers also have the potential to bridge the gap between a given genotype and the resulting phenotype [33]. The basic principle of MAS is to identify a tight linkage between a marker and a gene controlling a trait of interest (e.g., disease resistance, plant cycle length, flowering time, or the reproductive system). This association can be used for practical purposes, including the preliminary screening of plant materials or to verify the introgression of a given gene. Thus, knowing the association with the gene of interest, traditional breeding methods, such as hybridization, backcrossing, self-pollination, and selection, are facilitated in the constitution of new varieties [34]. In this section, we first reviewed the scientific literature dealing with the identification of genes underlying ten topics of interest in C. intybus, as summarized in Table 3.

Table 3.
The most relevant traits/features investigated in chicory, the responsible genes and/or the associated markers, and the methods used for their identification are reported. The superscript numbers (from 1 to 10) reported for each trait/topic were used to facilitate the correspondence with the genes shown in Figure 3 (for chromosome 6) and Figure S2 (for the entire chromosome set). The GenBank accession numbers of the genes/marker-related genes are reported in Table S1.
According to the literature review described in Section 2, sixty-four sequences with a unique match with as many loci in the genome as possible were retrieved and are graphically represented in Figure S2. Moreover, when available, the closest SNP and SSR (both upstream and downstream) to each gene were mapped too. One of the chromosomes is shown as an example in Figure 3.
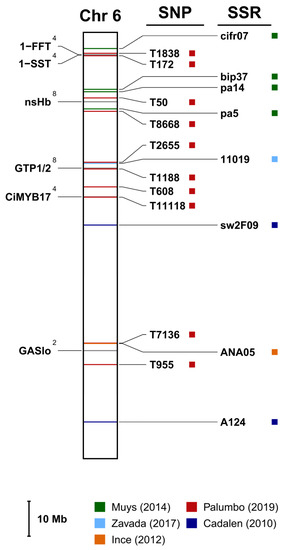
Figure 3.
Chromosome 6 from the marker-assisted selection (MAS) map (the full MAS map is provided as Figure S2). On the left are the genes with their trait identity number (in the form of an apex: 2: sesquiterpene lactone biosynthesis (STL); 4: inulin metabolism; 8: somatic embryogenesis). On the right are reported the markers (SSRs or SNPs) closest to each gene and retrieved from Cadalen et al. [25], Muys et al. [27], Palumbo et al. [28], Ince et al. [29] and Zavada et al. [30]. Each gene is also described in detail in Table 3, whereas Table S1 reports their respective GenBank accession numbers.
3.3.1. Reproductive Barriers
The gene pool controlling the reproductive system of chicory is of great importance for hybrid development [12]. Some of the available genetic maps have been successfully used for the fine mapping of self-incompatibility and male sterility genes [12,25,28,35]. In root chicory, Gonthier et al. mapped the nuclear male sterility 1 (NMS1) locus on LG5 (namely, chromosome 7) [12]. Although the gene responsible for the lack of pollen has not yet been deciphered, the locus was finely confined to a region of 0.8 cM along with thirteen co-segregating AFLP markers. However, only two of them were transformed into SCAR markers named TGGC (1.03 cM) and ATGC (1.29 cM) and deposited in NCBI. In leaf chicory, the study of male sterility is at a more advanced stage. Initially, two SSR markers, M4.12 (acc. number, JF748831) and M4.11b (acc. number, KF880802), originally developed by Cadalen et al. and named EU02C09 and EU03H01, were found to be 5.8 cM and 12.1 cM away from the locus responsible for male sterility (ms1), respectively [25,28]. These two markers were located within LG4 from Cadalen et al., which corresponds to chromosome 9 [25]. Based on a SNP-based linkage map and synteny analysis of Lactuca sativa, it was finally possible to identify a four-nucleotide indel within the second exon of a MYB103-like gene responsible for an anticipated stop codon in male sterile mutants [28]. This gene, encoding a transcription factor involved in the callose dissolution of tetrads and exine development of microspores, was chosen as a candidate for male sterility.
In C. intybus, self-incompatibility was demonstrated more than 40 years ago [60]. From a molecular point of view, Gonthier et al. assigned the genetic determination of SSI to a single locus located in LG2 (corresponding to chromosome 5) [12]. Moreover, four AFLP-derived SCARs were assigned as TACG, GGAT, TTAA, and AACC due to their close association with the S-locus (the first two markers at 0.51 cM, the third at 0.39 cM and the fourth at 0.52 cM). Within this chromosomal region, Palumbo et al. recently identified MDIS1 INTERACTING RECEPTOR LIKE KINASE 2 (ciMIK2) as the putative female determinant of SSI [36].
The availability of molecular genetic resources for reproductive barriers, such as self-incompatibility and male sterility, in chicory might have a great impact on the implementation of MAS programs to obtain highly heterozygous and phenotypically uniform progeny.
3.3.2. Chicory, the Special Bitter-Taste Vegetable—STL Biosynthesis
Sesquiterpene lactones (STLs) are secondary metabolites responsible for bitterness and have a significant active role in defense against pathogens [61]. From a nutritional standpoint, STLs also possess both beneficial (e.g., anticancer, and antileukemic) and allergenic properties [40,62]. Moreover, a recent study demonstrated that the biosynthesis pathway of sesquiterpene lactone (lactucin) is considered to provide antimalarial activity [63]. Three main enzymes, namely, germacrene A synthase (GAS), germacrene A oxidase (GAO) and costunolide synthase (COS), are involved in STL biosynthesis, as schematically represented in Figure 4A [64].
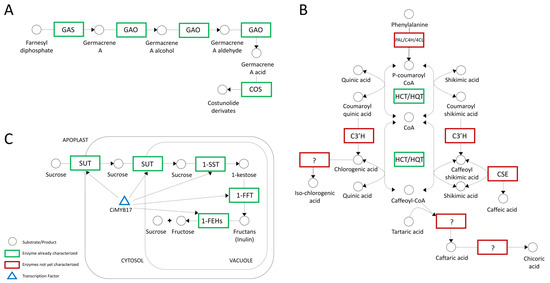
Figure 4.
A schematic representation of three biosynthetic pathways of interest in chicory. Question marks indicate enzymes that have been characterized biochemically but are not supported by molecular data. (A) Sesquiterpene lactone (STL) biosynthetic pathway in chicory based on the studies of De Kraker et al. [64], Cankar et al. [39], Testone et al. [40], and Bogdanovic et al. [65]; (B) putative metabolic pathways involved in hydroxycinnamic acid (HCA) biosynthesis in plants and the two main enzymes HCT and HQT according to Legrand et al. [41]; (C) model of inulin metabolism, proposed by Van Laere and Van Den Ende [66] and Shoorideh et al. [67].
Due to the growing interest in these metabolites, several Asteraceae species have been investigated in this respect [38,39,40,68,69,70,71].
The sequences of three different GAS genes (GASlo, GASsh and GAS1) were retrieved and mapped on different chromosomes and they are represented in Figure S2 [37,40,65]. Initially, two isoenzymes referred to as long (GASlo) and short (GASsh) were isolated and characterized in chicory by Bouwmeester et al. [37]. The short form of the GAS gene was further confirmed via the study by Testone et al., named GASsh2, and published under a different accession number [40]. Indeed, GASsh and GASsh2 mapped to the same position and are indicated as GASsh/2 in Table S1 and Figure S2. GASlo and GASsh genes exhibited a relatively low degree of homology, although their enzymes catalyze the formation of the same product [37]. Based on the expression analysis, the transcripts of both genes were particularly abundant within root and seedling tissues, where the accumulation of bitter sesquiterpene lactones was indeed expected to be at the highest level [37,72]. However, it was reported that the GASsh (chromosome 2) gene was poorly expressed in leaves, while GASlo (chromosome 6) was expressed in both leaf and root tissues [65]. Testone et al. characterized a third gene, named the GAS1 gene, that mapped to chromosome 5 [40]. From these results, the authors suggested the hypothesis of two distinct routes involved in STL synthesis and the involvement of different GAS genes [40].
Similarly, different GAO genes were identified via independent studies and were shown to convert germacrene A to its acid form [38,39,40]. According to Nguyen et al. and Testone et al., two of these sequences, generically named GAO (but deposited under different accession numbers), were mapped to the same position on chromosome 2 [38,40]. A third germacrene A oxidase gene, CYP71AV8, was mapped to chromosome 8 and was found to be involved in STL biosynthesis [39].
In the last step of STL biosynthesis, COS is crucial for the formation of costunolide derivates (Figure 4A). Testone et al., in addition to the GAS and GAO genes, also characterized a COS gene with the main idea of comprehending the regulation of bitter taste in chicories [40].
3.3.3. Hydroxycinnamate Biosynthesis (HCA)
Hydroxycinnamates (HCAs) are secondary plant metabolites with phenylalanine as a precursor and are widely distributed in plants [41,73,74]. In chicory, interest is mostly focused on the biosynthesis of HCAs in the form of chlorogenic acid (CQA), isochlorogenic acid, (diCQA), caftaric acid (CTA), and chicoric acid (diCTA) (Figure 4B). These valuable molecules are responsible for many health benefits and are involved in plant protection against abiotic and biotic stresses [75,76,77]. Legrand et al. shed light on the genetic basis of HCA biosynthesis in C. intybus, isolating, cloning, and biochemically characterizing five full-length cDNA sequences encoding hydroxycinnamoyl-CoA: shikimate/quinate hydroxycinnamoyl transferases HCT1 and HCT2, and hydroxycinnamoyl-CoA/quinate hydroxycinnamoyl transferases HQT1, HQT2, and HQT3 [41]. Similarly, three HQTs and one HCT were discovered in artichoke, indicating the occurrence of several isoforms within these two gene families [41,78].
3.3.4. Inulin Metabolism
Chicory root is one of the major natural sources of inulin, and this water-soluble storage polysaccharide belongs to a group of nondigestible carbohydrates called fructans [79]. Inulin can act as a substitute for fats and sugars and as a texture modifier, and it is becoming increasingly popular as a functional food ingredient [80,81]. The first year of the growing season is a crucial determinant for inulin accumulation in chicory [10]. Inulin metabolism, schematically simplified in Figure 4C, is mediated by fructan-active enzymes known as FAZYs, including sucrose 1-fructosyltransferase (1-SST) and fructan 1-fructosyltransferase (1-FFT), while inulin degradation is catalyzed by several isoforms of fructan 1-exohydrolases (1-FEH Is and 1-FEH IIs), which remove terminal fructose units [42,43,82].
A key factor in inulin accumulation is the allocation of sucrose as a substrate in the taproot [10,83]. In this first step, the main actors are sucrose uptake transporters (SUTs), which transfer sucrose from the source to the sink [10,84,85,86]. Wei et al. observed distinct expression profiles of three SUT genes whose sequences were submitted to GenBank [10]. We localized these sequences to chromosome 5 and 2. A transcription factor named CiMYB17 (chromosome 6) specifically activates the transcription of the 1-SST, 1-FFT, 1-FEH and SUT genes (Figure 4C) [45]. Briefly, 1-SST and 1-FFT were both mapped to chromosome 6. Many independent studies have addressed instead the characterization of fructan 1-exohydrolases (1-FEHs). The cDNA of the fructan 1-exohydrolase I-coding gene (1-FEH I) of chicory (Cichorium intybus L.) was cloned, and its role was confirmed through its heterologous expression in potato [42]. Based on our mapping, 1-FEH I is located on chromosome 9.
Two isoforms were initially thought to be responsible for 1-FEH II production: 1-FEH IIa and 1-FEH IIb [45]. These two sequences are located in LG4 of the Cadalen et al. map at a distance of 1.8 cM from each other and 3.8 cM away from the EU07G10 SSR marker [25]. The same markers resulted in chromosome 9 of the physical map of Fan et al. [20]. In parallel, Michiels et al. cloned and sequenced a cDNA derived from another putative 1-FEH IIa-coding gene (here renamed 1-FEH IIa2 and not to be confused with the original 1-FEH IIa sequence mentioned above) and partially characterized its promoter region in a transient expression assay [44]. Similarly, a sequence analysis of the promoter region of another putative 1-FEH IIb-coding gene (here renamed 1-FEH IIb2 and not to be confused with the original 1-FEH IIb sequence mentioned above) was conducted by Wei et al. [45]. To summarize, 1-FEH IIb was mapped to chromosome 9, whereas the other three 1-FEH II sequences (1-FEH IIa, 1-FEH IIa2, and 1-FEHII b2) retrieved from independent studies was mapped within contig 45 of Fan et al. (JAKNSD010000045.1 [20]) very closely to each other. However, the comparison between the genome assembly [20] and the GBS map by Palumbo et al. [28] supports the hypothesis that contig 45 is actually part of chromosome 9 (Figure S1). Thus, we can finally assume that the four 1-FEH II-related sequences are all located on chromosome 9.
The specific role played by 1-FEH Is and 1-FEH IIs in inulin degradation has not yet been fully elucidated. It was proposed that the induction of chicory 1-FEH Is is mainly dependent on cold treatment, whereas that of 1-FEH IIs seems to be plausibly induced by both cold treatment and defoliation [10,42,43,45,66].
3.3.5. Biotic and Abiotic Stresses
A significant number of studies have focused on biotic and abiotic stresses, demonstrating how crucial it is for breeding to understand the underlying mechanisms. In this section, we will discuss some of the actors involved in the stress crosstalk response, including nitrogen metabolism, protoporphyrinogen IX oxidase, dehydrins, dehydration-responsive element-binding protein (DREB), a novel vacuolar Na+/H+ exchanger gene and a corky root-related locus.
Nitrogen metabolism is one of the primary processes for plant growth, productivity, metabolism, and stress tolerance [87,88]. Nitrate reductase is the main actor in the nitrogen assimilatory pathway, catalyzing the two-electron reduction of nitrate to nitrite [89,90,91]. Using whole-mount in situ hybridization, Palms et al. demonstrated that young chicory plants show the spatial regulation of nitrate reductase gene (named nia) in their roots as a function of external nitrate concentration [46]. In this study, the full sequence of nia was isolated and characterized in chicory, and it was further suggested that the chicory genome contains a single nia gene (here mapped to chromosome 2) [46].
Protoporphyrinogen IX oxidase (protox, PPX1), a member of the protoporphyrinogen oxidase (PPO) family, catalyzes the conversion of protoporphyrinogen IX (protogen) into protoporphyrin IX (proto) [92,93]. PPO inhibitors prevent the formation of proto, causing protogen to accumulate in chloroplasts and leak into the cytosol, where it is nonenzymatically oxidized to proto [94,95]. Lipids and proteins are then oxidized, resulting in leaky membranes and the rapid disintegration of organelles and cells [94,96,97]. Adomat et al. isolated and sequenced the cDNA of plastidial PPX1 from chicory [47]. The sequence was later mapped to LG1 in the map of Cadalen et al. [25] and on chromosome 4 in the physical map of Fan et al. [20].
Dehydrins are plant proteins known to be induced in response to environmental stresses (including drought, heat, freezing, metals/metalloids, or salinity), highlighting their potential role in biotechnological strategies to increase resistance in adverse environments [98,99,100,101,102]. Mingeot et al. identified two dehydrin cDNAs (DHN1 and DHN2), both expressed in roots and leaves, with seasonal variations in transcript accumulation [48]. As dehydrins are involved in crosstalk processes, it would be interesting to further characterize and identify correlated genes expressed in response to abiotic stresses.
The DREB1A gene, belonging to the A-1 subtype of the DREB gene subfamily [103,104], has been identified as one of the most significant genes conferring tolerance in crops overcoming stressors [105,106]. To this aim, Lang et al. identified genes involved in abiotic stresses and reported their participation in ABA-independent stress signaling pathways in chicory [49,50]. The results revealed that two genes (DREB1A and DREB2B) were induced by low temperatures and that a novel vacuolar Na+/H+ exchanger gene (CiNHX1) was induced by salt stress [49,50].
Finally, Muys et al. identified the close association between the bip-41 SSR marker and CAld5H (coniferyl alcohol 5-hydroxylase) [27]. In lettuce, the same syntenic region encompassing both bip-41 and CAld5H also contains a recessive gene (cor) conferring resistance to Rhizomonas suberifaciens, the causal agent of corky root [27]. Further analyses are needed to investigate whether or not the same locus is also available in chicory.
3.3.6. Lilac-Blue Color
Flavonoids are a large group of secondary metabolites ubiquitously present in plants [107,108]. They are mostly known for their role as pigments, with studies primarily focusing on the anthocyanin subgroup [8,109,110,111]. In addition, flavonoids are involved in protection against biotic and abiotic stress, in the regulation of developmental processes and in the integrity of the plant structure [8,110] Seitz et al. studied F3′5′H (flavonoid 3′,5′-hydroxylase) evolution from F3′H (flavonoid 3′-hydroxylase) in the Asteraceae family, probably triggered by an amino acid change at one specific position of the substrate recognition site [51]. The attainment of F3′5′H function allows the synthesis of delphinidin-based anthocyanins, which usually provide the basis for lilac to blue flower colors. The two sequences of F3′H and F3′5′H, obtained from this study, were retrieved and mapped nearby on chromosome 9.
3.3.7. Flowering Time
The study of Périlleux et al. focused on the root chicory FL1 gene, which belongs to the FLC/MAF clade of MADS box genes and behaves like the AtFLC gene, the repressor of flowering in Arabidopsis [52]. In this study, it was demonstrated that the FL1 gene is downregulated in response to cold conditions and activated again at devernalizing temperatures. Eventually, the overexpression of FL1 in Arabidopsis caused late flowering, but FL1 repression was unstable when the postvernalization temperature was favorable for flowering and when the plants were devernalized. However, this instability of FL1 repression may be related to the bienniality of root chicory as opposed to Arabidopsis’s annual lifecycle [52]. The FL1 gene was mapped to chromosome 4.
3.3.8. Somatic Embryogenesis (SE)
Somatic embryogenesis (SE) is an asexual propagation pathway requiring a transition of differentiated somatic cells toward embryogenic cells capable of producing embryos in a process resembling zygotic embryogenesis [112]. This mechanism holds great promise as a potential model in studies of early regulatory and morphogenetic events in plant embryogenesis [113]. Studies on gene expression were conducted in chicory comparing a SE-responsive genotype capable of undergoing complete cell reactivation, leading to somatic embryogenesis, with a non-SE-responsive genotype [114,115]. Genes possibly involved in somatic embryogenesis were first investigated through an extensive generation of expressed sequence tags (ESTs), but none of them turned out to be particularly promising [114,115].
Some interesting gene loci expressed during the early stages of somatic embryogenesis were studied in a Cichorium hybrid (C. intybus L., var. sativum × C. endivia L., var. latifolium) after the differential screening of a cDNA library in the leaf tissue [53]. Nonsymbiotic hemoglobin (nsHb) cDNA was isolated via Northern blot analysis. The gene was exclusively expressed under somatic embryogenesis-inducing conditions, excluding the correlation to stress caused by wounding or tissue culture conditions [53]. The sequence of this gene was further integrated into the map of Cadalen et al. (LG9 [25]) and on chromosome 6 (Figure S2).
Furthermore, β-1,3-glucanases, glutathione S-transferases and GTP binding proteins were investigated in independent studies as putative additional genes involved in SE mechanisms. Grimault et al. showed that during SE, callose β-1,3-glucanases were localized in the cell walls of embryogenic cells and embryos, suggesting a possible role in callose degradation [116]. After SE induction, Helleboid et al. isolated three different and possibly paralogous CG (callose glucanase) genes, all encoding β-1,3-glucanases [55]. The CG2 and CG3 cDNA sequences showed very high identity (98.5%), whereas they shared only 70% identity with CG1. In the map built and provided in Figure S2, these three genes were localized on chromosome 5, with CG2 and CG3 mapping in the same position (and referred to as CG2/3).
A cDNA encoding a glutathione S-transferase, chi-GST1, was isolated during the early stages of SE via a differential display in leaf tissues of chicory [54]. This led to the hypothesis that the transcript accumulation of chi-GST1 was specific to the developing leaf of the SE cultivar, whereas no expression was observed in the leaf tissue of the non-SE-responsive cultivar [54]. Moreover, it was shown that GST genes were involved in a variety of processes, such as the detoxification of xenobiotic molecules, protection against the damaging effects of oxidative compounds resulting from cellular metabolism (such as lipid peroxidation), and the intracellular transport of nonsubstrate molecules [54,117,118].
Similarly, transcripts from leaf tissue explants of a SE-responsive chicory and a non-SE counterpart were compared to identify genes expressed during the early stages of SE [56]. By using the mRNA differential display method, two full-length GTP-binding protein cDNAs were expressed exclusively in the leaf tissue of the SE-responsive genotype. The two full-length GTP1 and GTP2 cDNAs differed by only 10 nucleotides, and the deduced proteins diverged by three amino acids. The two sequences were mapped to the same position on the physical map (Figure 3, chromosome 6, GTP1/2). GTP1 and GTP2 sequences might represent two alleles of the same gene, as suggested by the authors [56].
3.3.9. Red Discoloration
Discoloration is a key postcutting trait that causes a loss of quality and consumer rejection [57,119,120,121,122]. In response to cutting, chicory gradually turns red, since tissue wounding induces the de novo synthesis of phenylalanine ammonia-lyase (PAL) and the activation of the phenylpropanoid pathway. The expression patterns of the genes encoding two phenylalanine ammonia-lyase (PAL) proteins (PAL1 and PAL2) were analyzed in postcut chicon tissues in response to heat treatment and controlled atmosphere storage [57]. PAL1 and PAL2 were strongly expressed in unheated cut tissues, whereas heat shock was found to reduce the level of PAL1 transcripts in sliced endive, preventing discoloration. Transcript sequences of PAL1 and PAL2 were therefore included in the map reported in Figure S2, specifically in chromosomes 4 (PAL1) and 1 (PAL2) [57]. Comparable experiments in lettuce demonstrated that heat shock reduces the accumulation of PAL mRNAs and hence inhibits tissue browning in leaves [123].
3.3.10. Gene Normalization
A critical step in the design of qRT—PCR experiments is the identification of reference genes. They are essential for data normalization and responsible for the accuracy of the data. Most importantly, the expression level of optimal reference genes should be comparable to that of the target genes, and expression should be stable under the chosen experimental conditions [58,59,124,125]. In chicory, Maroufi et al. identified seven candidate reference genes, namely, nicotinamide adenine dinucleotide dehydrogenase (NADHD), actin (ACT), β-tubulin (TUB), glyceraldehyde-3-phosphate-dehydrogenase (GADPH), histone H3 (H3), elongation factor 1-alpha (EF) and 18S rRNA (rRNA) [59]. Six of the seven sequences were available and are included in Figure S2. Similarly, Delporte et al., in a thorough investigation, analyzed 12 reference genes for data normalization, suitable for both cell cultures and seedlings [58]. Ten out of twelve were unequivocally matched with as many loci of the physical map of Fan et al. as possible [20].
4. Conclusions
Molecular advances in chicory represent priceless information that deserves to be properly collected, organized and stored for practical applications in breeding programs. Molecular marker technologies have been widely and successfully employed in many other horticultural crops and are considered extremely helpful for anticipating the selection process for plants with desirable traits. The reported results aimed to provide researchers with a simple mapping report that includes details on the genetic and physical locations of markers as well as additional results from other datasets that include genes and molecular markers. This led to the production of two easy-to-access consensus maps, one consisting of 639 molecular markers, useful for MAB applications, and a second reporting 64 sequences of genes or marker-related genes, useful for MAS purposes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241411663/s1.
Author Contributions
G.B. and F.P.: conceptualization. S.D. and G.G.: methodology. S.D. formal analysis. G.G.: data analysis. S.D. and F.P.: writing—original draft preparation. S.D., G.G., F.P. and G.B.: writing—review and editing. G.B. and F.P.: supervision. G.B.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed within the Agritech National Research Center and received funding from the European Union Next-Generation EU (Piano Nazionale di Ripresa e Resilienza (PNRR)—Missione 4 Componente 2, Investimento 1.4—D.D. 1032 17 June 2022, CN00000022. Our study represents an original paper related to both Spoke 1 “Plant, animal and microbial genetic resources and adaptation to climate changes” and Spoke 4 “Multifunctional and resilient agriculture and forestry systems for the mitigation of climate change risks”. In particular, it is a baseline for the fulfilment of milestones within Task 1.3.5 titled “Genome-wide strategies for fast-forward molecular breeding aimed at the assessment of genetic distinctiveness, uniformity and stability (DUS) and identity of pre-commercial varieties” and Task 4.1.1 titled “Next-generation genotyping and -omics technologies for the molecular prediction of multiple resilient traits in crop plants”. This manuscript reflects only the authors’ views and opinions, and neither the European Union nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raulier, P.; Maudoux, O.; Notté, C.; Draye, X.; Bertin, P. Exploration of Genetic Diversity within Cichorium endivia and Cichorium intybus with Focus on the Gene Pool of Industrial Chicory. Genet. Resour. Crop Evol. 2015, 63, 243–259. [Google Scholar] [CrossRef]
- Stephens, J.M. Chicory-Cichorium intybus L.; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 1994. [Google Scholar]
- Lucchin, M.; Varotto, S.; Barcaccia, G.; Parrini, P. Chicory and Endive. In Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 3–48. ISBN 978-0-387-30443-4. [Google Scholar]
- Barcaccia, G.; Ghedina, A.; Lucchin, M. Current Advances in Genomics and Breeding of Leaf Chicory (Cichorium intybus L.). Agriculture 2016, 6, 50. [Google Scholar] [CrossRef]
- Galla, G.; Ghedina, A.; Tiozzo, S.C.; Barcaccia, G. Toward a First High-Quality Genome Draft for Marker-Assisted Breeding in Leaf Chicory, Radicchio (Cichorium intybus L.). In Plant Genomics; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Bahmani, M. Overview of Medicinal Plants Used for Cardiovascular System Disorders and Diseases in Ethnobotany of Different Areas in Iran. J. Herbmed. Pharmacol. 2016, 5, 39–44. [Google Scholar]
- Al-Snafi, A.E. Medical Importance of Cichorium intybus. IOSR J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a Food Ingredient—Nutritional Composition, Bioactivity, Safety, and Health Claims: A Review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Testone, G.; Sobolev, A.P.; Mele, G.; Nicolodi, C.; Gonnella, M.; Arnesi, G.; Biancari, T.; Giannino, D. Leaf Nutrient Content and Transcriptomic Analyses of Endive (Cichorium endivia) Stressed by Downpour-Induced Waterlog Reveal a Gene Network Regulating Kestose and Inulin Contents. Hortic. Res. 2021, 8, 92. [Google Scholar] [CrossRef]
- Wei, H.; Bausewein, A.; Steininger, H.; Su, T.; Zhao, H.; Harms, K.; Greine, S.; Rausch, T. Linking Expression of Fructan Active Enzymes, Cell Wall Invertases and Sucrose Transporters with Fructan Profiles in Growing Taproot of Chicory (Cichorium intybus): Impact of Hormonal and Environmental Cues. Front. Plant Sci. 2016, 7, 1806. [Google Scholar] [CrossRef]
- Barcaccia, G.; Pal Lo Ttin, I.L.; Soattin, M.; Lazzarin, R.; Parrini, P.; Lucchin, M.; Weber, W.E. Genomic DNA Fingerprints as a Tool for Identifying Cultivated Types of Radicchio (Cichorium intybus L.) from Veneto, Italy. Plant Breed. 2002, 122, 178–183. [Google Scholar] [CrossRef]
- Gonthier, L.; Blassiau, C.; Mörchen, M.; Cadalen, T.; Poiret, M.; Hendriks, T.; Quillet, M.C. High-Density Genetic Maps for Loci Involved in Nuclear Male Sterility (NMS1) and Sporophytic Self-Incompatibility (S-Locus) in Chicory (Cichorium intybus L., Asteraceae). Theor. Appl. Genet. 2013, 126, 2103–2121. [Google Scholar] [CrossRef]
- Patella, A.; Scariolo, F.; Palumbo, F.; Barcaccia, G. Genetic Structure of Cultivated Varieties of Radicchio (Cichorium intybus L.): A Comparison between F1 Hybrids and Synthetics. Plants 2019, 8, 213. [Google Scholar] [CrossRef]
- Scariolo, F.; Palumbo, F.; Barcaccia, G. Molecular Characterization and Genetic Structure Evaluation of Breeding Populations of Fennel (Foeniculum vulgare Mill.). Agronomy 2022, 12, 542. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Das Kumar, B. Molecular Markers: It’s Application in Crop Improvement. J. Crop Sci. Biotechnol. 2009, 12, 169–181. [Google Scholar] [CrossRef]
- Sabev, P.; Valkova, N.; Todorovska, E.G. Molecular Markers and Their Application in Cotton Breeding: Progress and Future Perspectives. Bulg. J. Agric. Sci. 2020, 26, 816–828. [Google Scholar]
- Semagn, K.; Bjørnstad, Å.; Ndjiondjop, M.N. Progress and Prospects of Marker Assisted Backcrossing as a Tool in Crop Breeding Programs. Afr. J. Biotechnol. 2006, 5, 2588–2603. [Google Scholar]
- Palumbo, F.; Galla, G.; Vitulo, N.; Barcaccia, G. First Draft Genome Sequencing of Fennel (Foeniculum vulgare Mill.): Identification of Simple Sequence Repeats and Their Application in Marker-Assisted Breeding. Mol. Breed. 2018, 38, 122. [Google Scholar] [CrossRef]
- Barcaccia, G.; Palumbo, F.; Scariolo, F.; Vannozzi, A.; Borin, M.; Bona, S. Potentials and Challenges of Genomics for Breeding Cannabis Cultivars. Front. Plant Sci. 2020, 11, 573299. [Google Scholar] [CrossRef]
- Fan, W.; Wang, S.; Wang, H.; Wang, A.; Jiang, F.; Liu, H.; Zhao, H.; Xu, D.; Zhang, Y. The Genomes of Chicory, Endive, Great Burdock and Yacon Provide Insights into Asteraceae Palaeo-Polyploidization History and Plant Inulin Production. Mol. Ecol. Resour. 2022, 22, 3124–3140. [Google Scholar] [CrossRef]
- De Simone, M.; Morgante, M.; Lucchin, M.; Parrini, P.; Marocco, A. A First Linkage Map of Cichorium intybus L. Using a One-Way Pseudo-Testcross and PCR-Derived Markers. Mol. Breed. 1997, 3, 415–425. [Google Scholar] [CrossRef]
- Van Stallen, N.; Vandenbussche, B.; Verdoodt, V.; De Proft, M. Construction of a Genetic Linkage Map for Witloof (Cichorium L. var foliosum Hegi). Plant Breed. 2003, 122, 521–525. [Google Scholar] [CrossRef]
- Van Stallen, N.; Vandenbussche, B.; Londers, E.; Noten, V.; De Proft, M. QTL Analysis of Important Pith Characteristics in a Cross between Two Inbred lines of Chicory (Cichorium intybus var foliosum): A Preliminary Study. Plant Breed. 2005, 124, 54–58. [Google Scholar]
- Cassan, L.; Moreau, L.; Segouin, S.; Bellamy, A.; Falque, M.; Limami, A.M. Genetic Map Construction and Quantitative Trait Loci (QTL) Mapping for Nitrogen Use Efficiency and Its Relationship with Productivity and Quality of the Biennial Crop Belgian Endive (Cichorium intybus L.). J. Plant Physiol. 2010, 167, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Cadalen, T.; Mörchen, M.; Blassiau, C.; Clabaut, A.; Scheer, I.; Hilbert, J.L.; Hendriks, T.; Quillet, M.C. Development of SSR Markers and Construction of a Consensus Genetic Map for Chicory (Cichorium intybus L.). Mol. Breed. 2010, 25, 699–722. [Google Scholar] [CrossRef]
- Ghedina, A.; Galla, G.; Cadalen, T.; Hilbert, J.L.; Caenazzo, S.T.; Barcaccia, G. A Method for Genotyping Elite Breeding Stocks of Leaf Chicory (Cichorium intybus L.) by Assaying Mapped Microsatellite Marker Loci Genetics. BMC Res. Notes 2015, 8, 831. [Google Scholar] [CrossRef] [PubMed]
- Muys, C.; Thienpont, C.N.; Dauchot, N.; Maudoux, O.; Draye, X.; Cutsem, P. Van Integration of AFLPs, SSRs and SNPs Markers into a New Genetic Map of Industrial Chicory (Cichorium intybus L. var Sativum). Plant Breed. 2014, 133, 130–137. [Google Scholar] [CrossRef]
- Palumbo, F.; Qi, P.; Pinto, V.B.; Devos, K.M.; Barcaccia, G. Construction of the First Snp-Based Linkage Map Using Genotyping-by-Sequencing and Mapping of the Male-Sterility Gene in Leaf Chicory. Front. Plant Sci. 2019, 10, 276. [Google Scholar] [CrossRef]
- Ince, G.A. A Contig-Based Microsatellite Marker Approach and Its Application in Cichorium ESTs. Rom. Biotechnol. Lett. 2012, 17, 7033. [Google Scholar]
- Závada, T.; Malik, R.J.; Kesseli, R.V. Population Structure in Chicory (Cichorium intybus): A Successful U.S. Weed since the American Revolutionary War. Ecol. Evol. 2017, 7, 4209–4219. [Google Scholar] [CrossRef]
- Závada, T.; Malik, R.J.; Mazumder, L.; Kesseli, R.V. Radical Shift in the Genetic Composition of New England Chicory Populations. J. Ecol. 2023, 111, 391–399. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Boudiar, R.; Casas, A.M.; Igartua, E.; Contreras-Moreira, B. BARLEYMAP: Physical and Genetic Mapping of Nucleotide Sequences and Annotation of Surrounding Loci in Barley. Mol. Breed. 2015, 35, 13. [Google Scholar] [CrossRef]
- Rana, M.; Sood, A.; Hussain, W.; Kaldate, R.; Sharma, T.R.; Gill, R.K.; Kumar, S.; Singh, S. Gene Pyramiding and Multiple Character Breeding. In Lentils; Singh, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 83–124. ISBN 978-0-12-813522-8. [Google Scholar]
- Kushanov, F.N.; Turaev, O.S.; Ernazarova, D.K.; Gapparov, B.M.; Oripova, B.B.; Kudratova, M.K.; Rafieva, F.U.; Khalikov, K.K.; Erjigitov, D.S.; Khidirov, M.T.; et al. Genetic Diversity, QTL Mapping, and Marker-Assisted Selection Technology in Cotton (Gossypium spp.). Front. Plant Sci. 2021, 12, 779386. [Google Scholar] [CrossRef]
- Barcaccia, G.; Tiozzo Caenazzo, S. New Male Sterile Mutant of Leaf Chicory, Including Radicchio, Used to Produce Chicory Plants and Seeds with Traits Such as Male Sterility Exhibiting Cytological Phenotype with Shapeless, Small and Shrunken Microspores in Dehiscent Anthers. European Patent EP2713705A1, 5 January 2014. Available online: https://patents.google.com/patent/EP2713705B1 (accessed on 12 January 2023).
- Palumbo, F.; Draga, S.; Magon, G.; Gabelli, G.; Vannozzi, A.; Farinati, S.; Scariolo, F.; Lucchin, M.; Barcaccia, G. MIK2 Is a Candidate Gene of the S-Locus for Sporophytic Self-Incompatibility (SSI) in Chicory (Cichorium intybus, Asteraceae). Front. Plant Sci. 2023, 14, 1204538. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Kodde, J.; Verstappen, F.W.A.; Altug, I.G.; De Kraker, J.W.; Wallaart, T.E. Isolation and Characterization of Two Germacrene A Synthase CDNA Clones from Chicory. Plant Physiol. 2002, 129, 134–144. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Göpfert, J.C.; Ikezawa, N.; MacNevin, G.; Kathiresan, M.; Conrad, J.; Spring, O.; Ro, D.K. Biochemical Conservation and Evolution of Germacrene A Oxidase in Asteraceae. J. Biol. Chem. 2010, 285, 16588–16598. [Google Scholar] [CrossRef]
- Cankar, K.; Van Houwelingen, A.; Bosch, D.; Sonke, T.; Bouwmeester, H.; Beekwilder, J. A Chicory Cytochrome P450 Mono-Oxygenase CYP71AV8 for the Oxidation of (+)-Valencene. FEBS Lett. 2011, 585, 178–182. [Google Scholar] [CrossRef]
- Testone, G.; Mele, G.; Di Giacomo, E.; Gonnella, M.; Renna, M.; Tenore, G.C.; Nicolodi, C.; Frugis, G.; Iannelli, M.A.; Arnesi, G.; et al. Insights into the Sesquiterpenoid Pathway by Metabolic Profiling and de Novo Transcriptome Assembly of Stem-Chicory (Cichorium intybus Cultigroup “Catalogna”). Front. Plant Sci. 2016, 7, 1676. [Google Scholar] [CrossRef]
- Legrand, G.; Delporte, M.; Khelifi, C.; Harant, A.; Vuylsteker, C.; Mörchen, M.; Hance, P.; Hilbert, J.L.; Gagneul, D. Identification and Characterization of Five BAHD Acyltransferases Involved in Hydroxycinnamoyl Ester Metabolism in Chicory. Front. Plant Sci. 2016, 7, 741. [Google Scholar] [CrossRef]
- Van Den Ende, W.; Lammens, W.; Van Laere, A.; Schroeven, L.; Le Roy, K. Donor and Acceptor Substrate Selectivity among Plant Glycoside Hydrolase Family 32 Enzymes. FEBS J. 2009, 276, 5788–5798. [Google Scholar] [CrossRef]
- Van Den Ende, W.; Michiels, A.; Wonterghem, V.; Clerens, S.P.; Laere, A.J. Van Defoliation Induces Fructan 1-Exohydrolase II in Witloof Chicory Roots. Cloning and Purification of Two Isoforms, Fructan 1-Exohydrolase IIa and Fructan 1-Exohydrolase IIb. Mass Fingerprint of the Fructan 1-Exohydrolase II Enzymes 1. Plant Physiol. 2001, 126, 1186–1195. [Google Scholar] [CrossRef]
- Michiels, A.; Van Laere, A.; Van Den Ende, W.; Tucker, M. Expression Analysis of a Chicory Fructan 1-Exohydrolase Gene Reveals Complex Regulation by Cold. J. Exp. Bot. 2004, 55, 1325–1333. [Google Scholar] [CrossRef]
- Wei, H.; Bausewein, A.; Greiner, S.; Dauchot, N.; Harms, K.; Rausch, T. CiMYB17, a Stress-Induced Chicory R2R3-MYB Transcription Factor, Activates Promoters of Genes Involved in Fructan Synthesis and Degradation. New Phytol. 2017, 215, 281–298. [Google Scholar] [CrossRef]
- Palms, B.; Goupil, P.; De Almeida Engler, J.; Van Der Straeten, D.; Van Montagu, M.; Rambour, S. Evidence for the Nitrate-Dependent Spatial Regulation of the Nitrate Reductase Gene in Chicory Roots. Planta 1996, 200, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Adomat, C.; Böger, P. Cloning, Sequence, Expression, and Characterization of Protoporphyrinogen IX Oxidase from Chicory. Pestic. Biochem. Physiol. 2000, 66, 49–62. [Google Scholar] [CrossRef]
- Mingeot, D.; Dauchot, N.; Van Cutsem, P.; Watillon, B. Characterisation of Two Cold Induced Dehydrin Genes from Cichorium intybus L. Mol. Biol. Rep. 2009, 36, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lin, M.; Lin, Z.; Zhao, L.; Zhao, G.; Li, Q.; Yin, X. Identification, Functional Characterization, and Expression Pattern of a NaCl-Inducible Vacuolar Na+/H+ Antiporter in Chicory (Cichorium intybus L.). Plant Growth Regul. 2015, 75, 605–614. [Google Scholar] [CrossRef]
- Liang, M.; Chen, D.; Lin, M.; Zheng, Q.; Huang, Z.; Lin, Z.; Zhao, G. Isolation and Characterization of Two DREB1 Genes Encoding Dehydration-Responsive Element Binding Proteins in Chicory (Cichorium intybus). Plant Growth Regul. 2014, 73, 45–55. [Google Scholar] [CrossRef]
- Seitz, C.; Ameres, S.; Schlangen, K.; Forkmann, G.; Halbwirth, H. Multiple Evolution of Flavonoid 3′,5′-Hydroxylase. Planta 2015, 242, 561–573. [Google Scholar] [CrossRef]
- Périlleux, C.; Pieltain, A.; Jacquemin, G.; Bouché, F.; Detry, N.; D’Aloia, M.; Thiry, L.; Aljochim, P.; Delansnay, M.; Mathieu, A.S.; et al. A Root Chicory MADS Box Sequence and the Arabidopsis Flowering Repressor FLC Share Common Features That Suggest Conserved Function in Vernalization and De-Vernalization Responses. Plant J. 2013, 75, 390–402. [Google Scholar] [CrossRef]
- Hendriks, T.; Scheer, I.; Quillet, M.-C.; Randoux, B.; Delbreil, B.; Vasseur, J.; Hilbert, J.-L. A Nonsymbiotic Hemoglobin Gene Is Expressed during Somatic Embryogenesis in Cichorium. Biochim. Biophys. Acta BBA 1998, 1443, 193–197. [Google Scholar] [CrossRef]
- Galland, R.; Randoux, B.; Vasseur, J.; Hilbert, J.-L. A Glutathione S-Transferase CDNA Identified by MRNA Differential Display Is Upregulated during Somatic Embryogenesis in Cichorium. Biochim. Biophys. Acta BBA Gene Struct. Expr. 2001, 1522, 212–216. [Google Scholar] [CrossRef]
- Helleboid, S.; Chapman, A.; Hendriks, T.; Inzé, D.; Vasseur, J.; Hilbert, J.-L. Cloning of β-1,3-Glucanases Expressed during Cichorium Somatic Embryogenesis. Plant Mol. Biol. 2000, 42, 377–386. [Google Scholar] [CrossRef]
- Randoux, B.; Quillet, M.-C.; Rambaud, C.; Vasseur, J.; Hilbert, J.-L. Characterisation of CDNAs Homologous to Rab5-GTP Binding Protein Expressed during Early Somatic Embryogenesis in Chicory. Plant Sci. 2002, 162, 413–422. [Google Scholar] [CrossRef]
- Salman, A.; Goupil, P.; Filgueiras, H.; Charles, F.; Ledoigt, G.; Sallanon, H. Controlled Atmosphere and Heat Shock Affect PAL1 and HSP90 MRNA Accumulation in Fresh-Cut Endive (Cichorium intybus L.). Eur. Food Res. Technol. 2008, 227, 721–726. [Google Scholar] [CrossRef]
- Delporte, M.; Legrand, G.; Hilbert, J.L.; Gagneul, D. Selection and Validation of Reference Genes for Quantitative Real-Time PCR Analysis of Gene Expression in Cichorium intybus. Front. Plant Sci. 2015, 6, 651. [Google Scholar] [CrossRef]
- Maroufi, A.; Van Bockstaele, E.; De Loose, M. Validation of Reference Genes for Gene Expression Analysis in Chicory (Cichorium intybus) Using Quantitative Real-Time PCR. BMC Mol. Biol. 2010, 11, 15. [Google Scholar] [CrossRef]
- Eenink, A.H. Compatibility and Incompatibility in Witloof-Chicory (Cichorium intybus L.). 2. The Incompatibility System. Euphytica 1981, 30, 77–85. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L.; López-Muñoz, R.; Williams, A.R. Antiparasitic Activity of Chicory (Cichorium intybus) and Its Natural Bioactive Compounds in Livestock: A Review; BioMed Central Ltd.: London, UK, 2018; Volume 11. [Google Scholar]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Channotiya, J.; Tiwari, A.; Taj, G.; Verma, A.K.; Dubey, A. In-Silico and Molecular Docking Studies on Germacrene A Synthase Enzyme and Sesuiterpene Lactone (Lactucin) Involved in Antimalarial Activity of Cichorium intybus. Netw. Model. Anal. Health Inform. Bioinform. 2021, 10, 24. [Google Scholar] [CrossRef]
- De Kraker, J.-W.; Franssen, M.C.R.; Dalm, M.C.F.; De Groot, A.; Bouwmeester, H.J. Biosynthesis of Germacrene A Carboxylic Acid in Chicory Roots. Demonstration of a Cytochrome P450 (+)-Germacrene A Hydroxylase and NADP-Dependent Sesquiterpenoid Dehydrogenase(s) Involved in Sesquiterpene Lactone Biosynthesis. Plant Physiol. 2001, 125, 1930–1940. [Google Scholar] [CrossRef]
- Bogdanović, M.; Cankar, K.; Todorović, S.; Dragicević, M.; Simonović, A.; van Houwelingen, A.; Schijlen, E.; Schipper, B.; Gagneul, D.; Hendriks, T.; et al. Tissue Specific Expression and Genomic Organization of Bitter Sesquiterpene Lactone Biosynthesis in Cichorium intybus L. (Asteraceae). Ind. Crops Prod. 2019, 129, 253–260. [Google Scholar] [CrossRef]
- Van Laere, A.; Van Den Ende, W. Inulin Metabolism in Dicots: Chicory as a Model System. Plant Cell Environ. 2002, 25, 803–813. [Google Scholar] [CrossRef]
- Shoorideh, H.; Peighambari, S.A.; Omidi, M.; Naghavi, M.R.; Maroufi, A. Spatial Expression of Genes in Inulin Biosynthesis Pathway in Wild and Root Type Chicory. J. Agric. Sci. Technol. 2018, 20, 1049–1058. [Google Scholar]
- Ikezawa, N.; Göpfert, J.C.; Nguyen, D.T.; Kim, S.U.; O’Maille, P.E.; Spring, O.; Ro, D.K. Lettuce Costunolide Synthase (CYP71BL2) and Its Homolog (CYP71BL1) from Sunflower Catalyze Distinct Regio- and Stereoselective Hydroxylations in Sesquiterpene Lactone Metabolism. J. Biol. Chem. 2011, 286, 21601–21611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.A.; de Vos, R.C.H.; Beekwilder, J.; van der Krol, S.; Bouwmeester, H.J. Reconstitution of the Costunolide Biosynthetic Pathway in Yeast and Nicotiana Benthamiana. PLoS ONE 2011, 6, e23255. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Saillard, N.; Yang, T.; Franssen, M.C.R.; Bouwmeester, H.J.; Jongsma, M.A. Biosynthesis of Sesquiterpene Lactones in Pyrethrum (Tanacetum cinerariifolium). PLoS ONE 2013, 8, e65030. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Han, X.; Liu, X.; Wang, Q.; Zhang, J.; Zhao, H.; Tang, J.; Luo, K.; Zhai, Z.; et al. The Chromosome-Scale Assembly of Endive (Cichorium endivia) Genome Provides Insights into the Sesquiterpenoid Biosynthesis. Genomics 2022, 114, 110400. [Google Scholar] [CrossRef]
- Rees, S.; Harborne, J. The Role of Sesquiterpene Lactones and Phenolics in the Chemical Defence of the Chicory Plant. Phytochemistry 1985, 24, 2225–2231. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Siquet, C.; Orrù, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic Phenolic Antioxidants: Correlation between Antioxidant Profile, Partition Coefficients and Redox Properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Lallemand, L.A.; Zubieta, C.; Lee, S.G.; Wang, Y.; Acajjaoui, S.; Timmins, J.; McSweeney, S.; Jez, J.M.; McCarthy, J.G.; McCarthy, A.A. A Structural Basis for the Biosynthesis of the Major Chlorogenic Acids Found in Coffee. Plant Physiol. 2012, 160, 249–260. [Google Scholar] [CrossRef]
- Bahri, M.; Hance, P.; Grec, S.; Quillet, M.C.; Trotin, F.; Hilbert, J.L.; Hendriks, T. A “Novel” Protocol for the Analysis of Hydroxycinnamic Acids in Leaf Tissue of Chicory (Cichorium intybus L., Asteraceae). Sci. World J. 2012, 2012, 142983. [Google Scholar] [CrossRef]
- Kandeler, R.; Ullrich, W.R. Symbolism of Plants: Examples from European-Mediterranean Culture Presented with Biology and History of Art. J. Exp. Bot. 2009, 60, 3297–3299. [Google Scholar] [CrossRef]
- Sonnante, G.; D’Amore, R.; Blanco, E.; Pierri, C.L.; de Palma, M.; Luo, J.; Tucci, M.; Martin, C. Novel Hydroxycinnamoyl-Coenzyme a Quinate Transferase Genes from Artichoke Are Involved in the Synthesis of Chlorogenic Acid. Plant Physiol. 2010, 153, 1224–1238. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, Health Benefits and Food Applications; Elsevier: Amsterdam, The Netherlands, 2016; Volume 147. [Google Scholar]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary Fiber Type Reflects Physiological Functionality: Comparison of Grain Fiber, Inulin, and Polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef]
- Stoyanova, S.; Geuns, J.; Hideg, É.; Van Den Ende, W. The Food Additives Inulin and Stevioside Counteract Oxidative Stress. Int. J. Food Sci. Nutr. 2011, 62, 207–214. [Google Scholar] [CrossRef]
- Kusch, U.; Greiner, S.; Steininger, H.; Meyer, A.D.; Corbière-Divialle, H.; Harms, K.; Rausch, T. Dissecting the Regulation of Fructan Metabolism in Chicory (Cichorium intybus) Hairy Roots. New Phytol. 2009, 184, 127–140. [Google Scholar] [CrossRef]
- van Arkel, J.; Vergauwen, R.; Sévenier, R.; Hakkert, J.C.; van Laere, A.; Bouwmeester, H.J.; Koops, A.J.; van der Meer, I.M. Sink Filling, Inulin Metabolizing Enzymes and Carbohydrate Status in Field Grown Chicory (Cichorium intybus L.). J. Plant Physiol. 2012, 169, 1520–1529. [Google Scholar] [CrossRef]
- Jian, H.; Lu, K.; Yang, B.; Wang, T.; Zhang, L.; Zhang, A.; Wang, J.; Liu, L.; Qu, C.; Li, J. Genome-Wide Analysis and Expression Profiling of the SUC and SWEET Gene Families of Sucrose Transporters in Oilseed Rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1464. [Google Scholar] [CrossRef]
- Péron, T.; Candat, A.; Montiel, G.; Veronesi, C.; Macherel, D.; Delavault, P.; Simier, P. New Insights into Phloem Unloading and Expression of Sucrose Transporters in Vegetative Sinks of the Parasitic Plant Phelipanche ramosa L. (Pomel). Front. Plant Sci. 2017, 7, 2048. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Alves, H.L.S.; Matiolli, C.C.; Soares, R.C.; Almadanim, M.C.; Oliveira, M.M.; Abreu, I.A. Carbon/Nitrogen Metabolism and Stress Response Networks—Calcium-Dependent Protein Kinases as the Missing Link? J. Exp. Bot. 2021, 72, 4190–4201. [Google Scholar] [CrossRef]
- Di Martino, C.; Torino, V.; Minotti, P.; Pietrantonio, L.; Del Grosso, C.; Palmieri, D.; Palumbo, G.; Crawford, T.W.; Carfagna, S. Mycorrhized Wheat Plants and Nitrogen Assimilation in Coexistence and Antagonism with Spontaneous Colonization of Pathogenic and Saprophytic Fungi in a Soil of Low Fertility. Plants 2022, 11, 924. [Google Scholar] [CrossRef]
- Kelly, S. The Amount of Nitrogen Used for Photosynthesis Modulates Molecular Evolution in Plants. Mol. Biol. Evol. 2018, 35, 1616–1625. [Google Scholar] [CrossRef]
- Hoff, J.E.; Wilcox, G.E.; Jones, C.M. The Effect of Nitrate and Ammonium Nitrogen on the Free Amino Acid Composition of Tomato Plants and Tomato Fruit. J. Am. Soc. Hortic. Sci. 1994, 99, 27–30. [Google Scholar] [CrossRef]
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a Better Understanding of the Genetic and Physiological Basis for Nitrogen Use Efficiency in Maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef]
- Koch, M.; Breithaupt, C.; Kiefersauer, R.; Freigang, J.; Huber, R.; Messerschmidt, A. Crystal Structure of Protoporphyrinogen IX Oxidase: A Key Enzyme in Haem and Chlorophyll Biosynthesis. EMBO J. 2004, 23, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Barker, A.; Tranel, P.J. Origins and Structure of Chloroplastic and Mitochondrial Plant Protoporphyrinogen Oxidases: Implications for the Evolution of Herbicide Resistance. Pest. Manag. Sci. 2018, 74, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ahn, Y.O.; Nam, J.-W.; Hong, M.-K.; Song, N.; Kim, T.; Yu, G.-H.; Sung, S.-K. Biochemical and Physiological Mode of Action of Tiafenacil, a New Protoporphyrinogen IX Oxidase-Inhibiting Herbicide. Pestic. Biochem. Physiol. 2018, 152, 38–44. [Google Scholar] [CrossRef]
- Park, M.; Randel, W.J.; Kinnison, D.E.; Bourassa, A.E.; Degenstein, D.A.; Roth, C.Z.; McLinden, C.A.; Sioris, C.E.; Livesey, N.J.; Santee, M.L. Variability of Stratospheric Reactive Nitrogen and Ozone Related to the QBO. J. Geophys. Res. Atmos. 2017, 122, 10103–10118. [Google Scholar] [CrossRef]
- Duke, S.O.; Rebeiz, C.A. Porphyrin Biosynthesis as a Tool in Pest Management, an Overview. In Porphyric Pesticides; ACS Symposium Series; ACS: Washington, DC, USA, 1994. [Google Scholar]
- Hallahan, B.J.; Camilleri, P.; Smith, A.; Bowyer, J.R. Mode of Action Studies on a Chiral Diphenyl Ether Peroxidizing Herbicide: Correlation between Differential Inhibition of Protoporphyrinogen IX Oxidase Activity and Induction of Tetrapyrrole Accumulation by the Enantiomers. Plant Physiol. 1992, 100, 1211–1216. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: A Commonalty in the Response of Plants to Dehydration and Low Temperature. Physiol. Plant 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Puhakainen, T.; Hess, M.W.; Mäkelä, P.; Svensson, J.; Heino, P.; Palva, E.T. Overexpression of Multiple Dehydrin Genes Enhances Tolerance to Freezing Stress in Arabidopsis. Plant Mol. Biol. 2004, 54, 743–753. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) Proteins and Their Encoding Genes in Arabidopsis Thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Tiwari, P.; Chakrabarty, D. Dehydrin in the Past Four Decades: From Chaperones to Transcription Co-Regulators in Regulating Abiotic Stress Response. Curr. Res. Biotechnol. 2021, 3, 249–259. [Google Scholar] [CrossRef]
- Abdul Aziz, M.; Sabeem, M.; Mullath, S.K.; Brini, F.; Masmoudi, K. Plant Group II LEA Proteins: Intrinsically Disordered Structure for Multiple Functions in Response to Environmental Stresses. Biomolecules 2021, 11, 1662. [Google Scholar] [CrossRef]
- Kohan-Baghkheirati, E.; Bagherieh-Najjar, M.; Abdolzadeh, A.; Geisler-Lee, J. Altered DREB1A Gene Expression in Arabidopsis Thaliana Leads to Change in Root Growth, Antioxidant Enzymes Activity, and Response to Salinity but Not to Cold. J. Genet. Resour. 2018, 4, 90–104. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Ardebili, Z.O.; Ebadi, M. Nitric Oxide and Selenium Nanoparticles Confer Changes in Growth, Metabolism, Antioxidant Machinery, Gene Expression, and Flowering in Chicory (Cichorium intybus L.): Potential Benefits and Risk Assessment. Environ. Sci. Pollut. Res. 2021, 28, 3136–3148. [Google Scholar] [CrossRef]
- Donde, R.; Gupta, M.K.; Gouda, G.; Kumar, J.; Vadde, R.; Sahoo, K.K.; Dash, S.K.; Behera, L. Computational Characterization of Structural and Functional Roles of DREB1A, DREB1B and DREB1C in Enhancing Cold Tolerance in Rice Plant. Amino Acids 2019, 51, 839–853. [Google Scholar] [CrossRef]
- Kidokoro, S.; Watanabe, K.; Ohori, T.; Moriwaki, T.; Maruyama, K.; Mizoi, J.; Myint Phyu Sin Htwe, N.; Fujita, Y.; Sekita, S.; Shinozaki, K.; et al. Soybean DREB1/CBF-Type Transcription Factors Function in Heat and Drought as Well as Cold Stress-Responsive Gene Expression. Plant J. 2015, 81, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Katan, M.B. Absorption, Metabolism and Health Effects of Dietary Flavonoids in Man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Verpoorte, R.; van der Heijden, R.; Memelink, J. Engineering the Plant Cell Factory for Secondary Metabolite Production. Transgenic Res. 2000, 9, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Amalesh, S.; Gouranga, D.; Sanjoy Kumar, D. Roles of Flavonoids in Plants. Int. J. Pharm. Sci. Technol. 2011, 6, 12–35. [Google Scholar]
- Karami, O.; Aghavaisi, B.; Mahmoudi Pour, A. Molecular Aspects of Somatic-to-Embryogenic Transition in Plants. J. Chem. Biol. 2009, 2, 177–190. [Google Scholar] [CrossRef]
- Zimmerman, J.L. Somatic Embryogenesis: A Model for Early Development in Higher Plants. Plant Cell 1993, 5, 1411–1423. [Google Scholar] [CrossRef]
- Legrand, S.; Hendriks, T.; Hubert, J.L.; Quillet, M.C. Characterization of Expressed Sequence Tags Obtained by SSH during Somatic Embryogenesis in Cichorium intybus L. BMC Plant Biol. 2007, 7, 27. [Google Scholar] [CrossRef]
- Lucau-Danila, A.; Laborde, L.; Legrand, S.; Huot, L.; Hot, D.; Lemoine, Y.; Hilbert, J.L.; Hawkins, S.; Quillet, M.C.; Hendriks, T.; et al. Identification of Novel Genes Potentially Involved in Somatic Embryogenesis in Chicory (Cichorium intybus L.). BMC Plant Biol. 2010, 10, 27. [Google Scholar] [CrossRef]
- Grimault, V.; Helleboid, S.; Vasseur, J.; Hilbert, J.-L. Co-Localization of ß-1,3-Glucanases and Callose during Somatic Embryogenesis in Cichorium. Plant Signal. Behav. 2007, 2, 455–461. [Google Scholar] [CrossRef]
- Islam, S.; Rahman, I.A.; Islam, T.; Ghosh, A. Genome-Wide Identification and Expression Analysis of Glutathione S-Transferase Gene Family in Tomato: Gaining an Insight to Their Physiological and Stress-Specific Roles. PLoS ONE 2017, 12, e0187504. [Google Scholar] [CrossRef]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions. Front. Plant Sci. 2018, 871, 1836. [Google Scholar] [CrossRef]
- Kays, S.J. Preharvest Factors Affecting Appearance. Postharvest Biol. Technol. 1999, 15, 233–247. [Google Scholar] [CrossRef]
- Hilton, H.W.; Clifford, S.C.; Wurr, D.C.E.; Burton, K.S. The Influence of Agronomic Factors on the Visual Quality of Field-Grown, Minimally-Processed Lettuce. J. Hortic. Sci. Biotechnol. 2009, 84, 193–198. [Google Scholar] [CrossRef]
- Hunter, P.J.; Chadwick, M.; Graceson, A.; Hambidge, A.; Hand, P.; Heath, J.; Lignou, S.; Oruna-Concha, M.J.; Pink, D.; Rada, B.; et al. Elucidation of the Biochemical Pathways Involved in Two Distinct Cut-Surface Discolouration Phenotypes of Lettuce. Postharvest Biol. Technol. 2022, 183, 111753. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.N. Application of Processing and Packaging Hurdles for Fresh-Cut Fruits and Vegetables Preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Belen Martin-Diana, A.; Rico, D.; Barry-Ryan, C.; Mulcahy, J.; Frias, J.; Henehan, G.T.M. Effect of Heat Shock on Browning-Related Enzymes in Minimally Processed Iceberg Lettuce and Crude Extracts. Biosci. Biotechnol. Biochem. 2005, 69, 1677–1685. [Google Scholar] [CrossRef]
- Gimeno, J.; Eattock, N.; Van Deynze, A.; Blumwald, E. Selection and Validation of Reference Genes for Gene Expression Analysis in Switchgrass (Panicum virgatum) Using Quantitative Real-Time RT-PCR. PLoS ONE 2014, 9, e91474. [Google Scholar] [CrossRef]
- Huang, L.; Yan, H.; Jiang, X.; Zhang, X.; Zhang, Y.; Huang, X.; Zhang, Y.; Miao, J.; Xu, B.; Frazier, T.; et al. Evaluation of Candidate Reference Genes for Normalization of Quantitative RT-PCR in Switchgrass Under Various Abiotic Stress Conditions. Bioenergy Res. 2014, 7, 1201–1211. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).