Abstract
Maintaining optimal one-carbon metabolism (OCM) is essential for health and pregnancy. In this cross-sectional study, folate status was assessed based on 5-methyltetrahydrofolate (5-MTHF) levels, and the association between 5-MTHF and OCM-related metabolites was investigated in 227 female Japanese university students aged 18–25 years. The participants were divided into high and low 5-MTHF groups based on their folate status. Serum samples of the participants were collected while they were fasting, and 18 OCM-related metabolites were measured using stable-isotope dilution liquid chromatography–electrospray tandem mass spectrometry. The association between serum 5-MTHF and OCM-related metabolite concentrations was assessed using Spearman’s rank correlation coefficient. Serum 5-MTHF concentrations were negatively correlated with total homocysteine (tHcy) concentrations and positively correlated with S-adenosylmethionine (SAM) and total cysteine (tCys) concentrations. Serum 5-MTHF concentrations demonstrated a stronger negative correlation with tHcy/tCys than with tHcy alone. The negative correlation between betaine and tHcy concentrations was stronger in the low 5-MTHF group than in the high 5-MTHF group. The 5-MTHF status could be linked to Hcy flux into the transsulfuration pathway via SAM. Therefore, the tHcy/tCys ratio may be a more sensitive indicator of the 5-MTHF status than tHcy alone. Furthermore, a low 5-MTHF status can enhance Hcy metabolism via betaine.
1. Introduction
One-carbon metabolism (OCM) comprises a folate cycle and choline metabolic pathway linked to a methionine cycle; homocysteine (Hcy) in the methionine cycle is connected to the transsulfuration pathway (Figure 1). OCM is mainly involved in the transfer of one-carbon units required for S-adenosylmethionine (SAM)-dependent methyl transfer reactions [1], nucleic acid synthesis [1,2], and amino acid metabolism, all of which support numerous physiological processes [2,3].
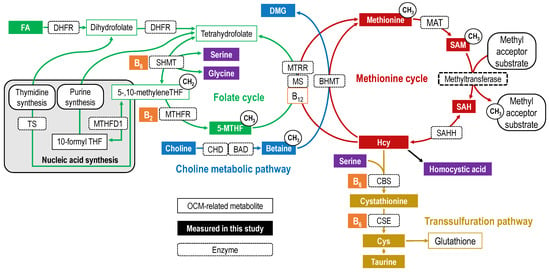
Figure 1.
Overview of one-carbon metabolism. OCM-related metabolites are indicated by rectangular boxes, with the folate cycle in green, choline metabolic pathway in blue, methionine cycle in red, and transsulfuration pathway in yellow. Other vitamins are shown in orange and amino acids and others are shown in purple. The filled rectangular boxes indicate OCM-related metabolites measured in this study. Each arrow represents a biochemical reaction, and the dotted rectangle on the arrow is labeled with the first letter of the enzyme catalyzing the reaction. Abbreviations: 5-MTHF, 5-methyltetrahydrofolate; B12, cobalamin/methylcobalamin; B2, riboflavin; B6, pyridoxal phosphate (pyridoxine/pyridoxal/pyridoxamine); BAD, betaine aldehyde dehydrogenase; BHMT, betaine–homocysteine methyltransferase; CBS, cystationine-β synthase; CHD, choline dehydrogenase; CSE, cystathionine γ-lyase; DMG, dimethylglycine; FA, folic acid; MAT, methionine adenosyltransferase; MS, methionine synthase; MTHFD1, methylenetetrahydrofolate dehydrogenase; MTHFR, methylenetetrahydrofolate reductase; MTRR, methionine synthase reductase; SAH, S-adenosylhomocysteine; SAHH, SAH hydrolase; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate; TS, thymidylate synthase.
Folate mediates the transfer of one-carbon units in OCM [2]. Folic acid (FA) in supplements and fortified foods is reduced to tetrahydrofolate (THF) before entering the folate cycle [4]. THF is primarily metabolized to 5,10-methyleneTHF by obtaining the hydroxymethyl group from serine [5]. Subsequently, 5,10-methyleneTHF is irreversibly reduced to 5-methyltetrahydrofolate (5-MTHF) by vitamin B2-dependent MTHF reductase (MTHFR; EC 1.5.1.20) [6]. The major folate molecular species is 5-MTHF, accounting for 82 to 93% of the total folate in the blood [7,8]. Methionine synthase (EC 2.1.1.13) uses the methyl group of 5-MTHF to remethylate Hcy to methionine, and demethylated THF is recycled to the folate cycle [3,7,8]. Choline is oxidized to betaine primarily in the liver and kidneys [9]. Betaine is catalyzed by betaine–homocysteine S-methyltransferase (BHMT; EC 2.1.1.5), which is expressed primarily in the liver and kidneys and remethylates Hcy to produce methionine and dimethylglycine (DMG) [10,11]. Methionine forms SAM in a reaction catalyzed by methionine adenosyltransferase (EC 2.5.1.6) [12]. SAM is used in methylation reactions that regulate the biological processes of various cellular components and is subsequently metabolized to S-adenosylhomocysteine (SAH) [13]. Methylation reactions include epigenetic modifications of gene expression (e.g., methylation of DNA and histones), biosynthesis of molecules (e.g., protein, phosphatidylcholine, polyamines, creatine, and sarcosine), and oxidation–reduction reactions [14,15]. SAH is reversibly degraded to Hcy and adenosine [16]. Both the folate cycle and choline metabolic pathway can independently supply methyl groups for the remethylation of Hcy to methionine; however, these two pathways are considered to be interrelated [17]. Hcy is also irreversibly metabolized to the sulfur-containing amino acid cysteine (Cys) via the transsulfuration pathway in the liver, pancreas, intestine, kidney, and possibly brain [18,19]. Hcy is first metabolized to cystathionine by the B6-dependent enzyme cystathionine-β-synthase (CBS; EC 4.2.1.22), which condenses serine to Hcy thiol [18,20]. Then, cystathionine is hydrolyzed by the B6-dependent enzyme cystathionine γ-lyase (CSE; EC 4.4.1.1) to Cys [18,21]. Cys is a precursor to sulfur metabolites, such as taurine, glutathione, and hydrogen sulfide [18,19].
OCM metabolic markers have been linked to various diseases. Intracellular Hcy concentrations increase when OCM fails to function adequately [16,22]. The excess Hcy is then excreted from the cells, leading to its increased concentration in the circulation [16,22]. High plasma Hcy concentrations have been linked to cardiovascular diseases [23,24], stroke [25,26], Alzheimer’s disease [27,28], schizophrenia [29,30], macular degeneration [31], diabetes [32], fractures [33], pregnancy complications [34,35,36], small-for-gestational-age offspring [37], and cancer [38]. This suggests that Hcy is an important indicator of health status [39,40]. However, the effects of Hcy-lowering interventions have been modest [41], and whether Hcy is a marker or a causative agent of these diseases remains unclear [39]. Some studies have reported that high plasma SAH concentrations are a considerably more sensitive indicator of cardiovascular diseases than total Hcy (tHcy) concentrations [40]. Homocysteic acid, produced by Hcy and methionine superoxide oxidation, is hypothesized to be an early diagnostic marker for mild cognitive impairment [42]. Low folate status in the blood during early pregnancy increases the risk of fetal neural tube closure defects [43]. In addition, midpregnancy choline status [44] and prepregnancy choline and betaine intake [45] have been linked to the risk of fetal neural tube closure defects. Increased flux in the transsulfuration pathway has been proposed to delay aging and extend life span [46]. During pregnancy, the mother’s OCM status is also important for fetal development and health [47,48]. Therefore, maintaining optimal OCM is essential for health and pregnancy, and thus, a comprehensive understanding of OCM is required.
Previous studies have assessed folate concentrations in circulation using a microbiological assay [49]; however, this assay measures all folate molecular species, including FA and 5-MTHF, and can only assess “total folate” [50,51]. In particular, FA in the blood is biologically inactive and does not accurately reflect its true physiological state, leading to inaccurate and misleading results [52]. Therefore, using liquid chromatography–tandem mass spectrometry is encouraged to quantify individual folate molecular species [49]. Although the total folate concentration in the blood is considered to influence OCM dynamics [53], the association between OCM and 5-MTHF, a major reduced folate, remains unclear. Our study focused on the association between 5-MTHF status and choline metabolism, methionine cycle, and the transsulfuration pathway. A few studies have investigated the metabolic dynamics of OCM [46,54,55,56,57,58,59,60]. However, to the best of our knowledge, no studies have comprehensively investigated the folate cycle, including 5-MTHF, methionine cycle, including SAM and SAH, choline metabolic pathway, or transsulfuration pathway. Furthermore, sex differences in OCM have been reported [54,61,62] because sex hormones, such as estrogen, can upregulate or downregulate various enzymes in OCM [62]. To account for the effects of sex hormones, OCM should be assessed in relation to sex, and women’s characteristics should be further divided into those related to menstruation, pregnancy, and menopause. Therefore, this study aimed to comprehensively measure key OCM-related metabolites in serum collected from young menstruating women and collect basic data regarding the association between 5-MTHF status and OCM.
2. Results
2.1. Distribution of Serum 5-MTHF Concentrations in Low and High 5-MTHF Groups
Figure 2 depicts the histograms of serum 5-MTHF concentrations in the low and high 5-MTHF groups. Serum 5-MTHF concentrations exhibited a positively skewed distribution, with the low 5-MTHF group showing a clumped distribution and the high 5-MTHF group showing a scattered distribution.
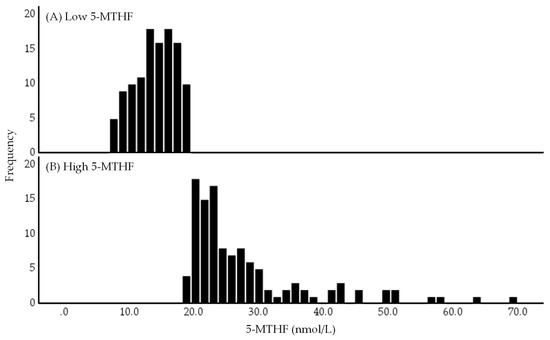
Figure 2.
Histograms of low (A) and high (B) 5-methyltetrahydrofolate (5-MTHF) groups. The low and high 5-MTHF groups were divided based on the median serum 5-MTHF concentration of 19.2 nmol/L. Abbreviations: 5-MTHF, 5-methyltetrahydrofolate.
2.2. Characteristics of the Study Population
The characteristics of the study population are summarized in Table 1. No significant differences in age, height, weight, body mass index (BMI), body fat percentage, or blood pressure were observed between the low and high 5-MTHF groups.

Table 1.
Characteristics of the study population.
2.3. Energy and Nutrient Intakes
Table 2 summarizes the dietary survey results. The high 5-MTHF group had significantly higher energy intake and higher total fiber, potassium, calcium, magnesium, iron, vitamin A, thiamin, vitamin B6, folate, and vitamin C intakes, but significantly lower sodium intake than the low 5-MTHF group.

Table 2.
Energy and nutrient intakes of the subjects.
2.4. Distribution of Serum OCM-Related Metabolite Concentrations
The serum OCM-related metabolite concentrations in the high and low 5-MTHF groups and in the overall study sample are summarized in Table 3. The high 5-MTHF group exhibited significantly higher betaine and total cysteine (tCys) concentrations and betaine/DMG ratios than the low 5-MTHF group. A similar trend was observed for SAM (p = 0.059). However, tHcy and cystathionine concentrations and tCys/tHcy ratios were significantly lower in the high 5-MTHF group than in the low 5-MTHF group. Serum homocysteic acid, pyridoxamine, and pyridoxine concentrations were below the limit of quantification in all samples.

Table 3.
Concentrations of one-carbon metabolism-related metabolites.
2.5. Correlation between Serum OCM-Related Metabolite Concentrations
Supplementary Figure S1 shows correlation matrices, scatter plots, and histograms of serum OCM-related metabolite concentrations. In addition, Figure 3 shows a graphical network depicting their associations. Serum 5-MTHF concentrations exhibited significantly positive correlations with betaine, SAM, and tCys concentrations and significantly negative correlations with tHcy and cystathionine concentrations. Conversely, FA concentrations did not correlate with the serum concentrations of OCM-related metabolites associated with 5-MTHF.
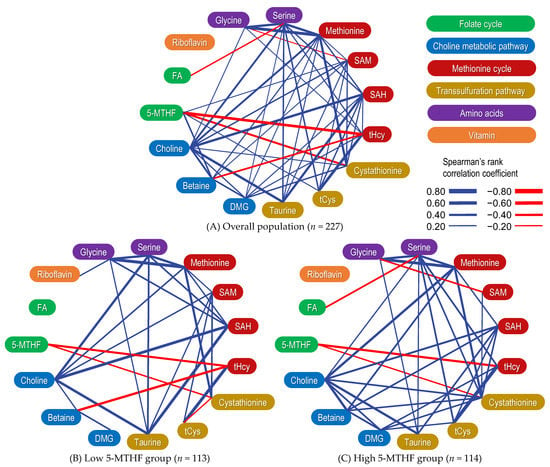
Figure 3.
A graphical network depicting the correlation between one-carbon metabolism-related metabolites. Overall population (A) as well as low (B) and high (C) 5-methyltetrahydrofolate (5-MTHF) groups. Correlations were evaluated using Spearman’s correlation coefficient. Significant positive correlations are indicated by blue lines, whereas significant negative correlations are indicated by red lines. The strength of the correlation is indicated by the thickness of the edges. Abbreviations: 5-MTHF, 5-methyltetrahydrofolate; DMG, dimethylglycine; FA, folic acid; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; tCys, total cysteine; tHcy, total homocysteine.
2.6. Correlation between OCM-Related Metabolites Stratified by 5-MTHF Status
The correlation matrix between serum OCM-related metabolite concentrations when stratified via dichotomous 5-MTHF concentrations is summarized in Supplementary Table S1, and a graphical network depicting their associations is shown in Figure 3. The negative correlation between betaine and tHcy concentrations was stronger in the low 5-MTHF group (ρ = −0.401, p < 0.001) than in the high 5-MTHF group (ρ = −0.097, p = 0.303). The positive correlation between DMG and tHcy concentrations was weaker in the low 5-MTHF group (ρ = 0.058, p = 0.541) than in the high 5-MTHF group (ρ = 0.249, p = 0.007). The correlation matrix between serum OCM-related metabolite concentrations and enzyme activity indices is summarized in Table 4. Similarly, the negative correlation between betaine/DMG ratios and tHcy concentrations was stronger in the low 5-MTHF group (ρ = −0.374, p < 0.001) than in the high 5-MTHF group (ρ = −0.290, p = 0.002). The 5-MTHF concentrations and betaine/DMG ratios showed a significant positive correlation. The significant negative correlation between 5-MTHF concentrations and tHcy/tCys ratios (ρ = −0.602, p < 0.001) was stronger than the correlation between 5-MTHF and tHcy concentrations alone (ρ = −0.456, p < 0.001; Supplementary Figure S1).

Table 4.
Correlation matrices between serum one-carbon metabolite-related metabolite concentrations and enzyme activity indices.
3. Discussion
We measured the serum concentrations of OCM-related metabolites in healthy young women to investigate the association between serum 5-MTHF concentrations and a wide range of OCM-related metabolites. The findings of this study further contribute to preliminary research by demonstrating for the first time that the 5-MTHF state is associated with transsulfuration pathway flux and that betaine-induced Hcy remethylation is enhanced in the low 5-MTHF state.
As expected, the dietary survey results revealed that the high 5-MTHF group exhibited significantly higher folate intake (dietary folate equivalents) than the low 5-MTHF group. On the other hand, the high 5-MTHF group exhibited higher energy and micronutrient intake than the low 5-MTHF group, suggesting a potential dietary influence on OCM. Because food nutrients are complex, it is likely that the intake of folate-rich foods, such as vegetables, altered the intake of other micronutrients. The intake of methionine, cystine, glycine, serine, zinc, riboflavin, and vitamin B12, which are OCM components, did not differ significantly between the high and low 5-MTHF groups. However, the high 5-MTHF group exhibited a significantly higher vitamin B6 intake, an OCM component, than the low 5-MTHF group; this should be considered when interpreting the study results.
Herein, the median serum 5-MTHF concentration in young Japanese women was 19.2 nmol/L, which was comparable to or slightly higher than that reported in previous studies involving healthy adults in countries where FA fortification of grains is not mandated, such as Japan [63,64]. However, it was lower than that reported in young participants in the 2011–2016 National Health and Nutrition Examination Survey in the United States where grains are fortified with FA [65]. The median serum betaine concentration in this study population was 38.7 µmol/L, consistent with other studies on healthy adults [66,67,68]. Serum pyridoxine, pyridoxamine, and homocysteic acid concentrations were at the limit of quantification in this study. However, similar results have been reported in previous studies, implying that their quantification in serum is difficult [69,70].
Concentrations of 5-MTHF exhibited a significant positive correlation with SAM and tCys concentrations and a significant negative correlation with tHcy concentrations. Similarly, a previous study involving older adults found a positive correlation between 5-MTHF and SAM concentrations, and FA intervention significantly increased plasma SAM concentrations in the intervention group compared with the control group [55]. In addition, previous in-silico studies using mathematical models reported that SAM concentrations demonstrated a strong linear association with total folate concentrations [71]. In-silico studies have also revealed that 5-MTHF is an allosteric inhibitor of glycine N-methyltransferase (EC 2.1.1.20) [72,73], one of the enzymes mediating the methyl transfer reaction. Thus, it is assumed that an increase in 5-MTHF levels increases SAM by inhibiting glycine N-methyltransferase and suppressing the methylation reaction. The low positive correlation coefficient between serum 5-MTHF and SAM concentrations in this study could be attributed to the ability of SAM to maintain its intracellular concentrations by inhibiting MTHFR [74,75], BHMT [76], and methionine adenosyltransferase [77] and activating CBS [78]. In addition, in-silico studies have suggested that the plasma concentrations of OCM-related metabolites do not accurately reflect their intracellular concentrations [79,80]. The negative correlation between blood 5-MTHF and tHcy concentrations has been reported in pregnant women [81], older people [55], and patients with hypertension [82]. Furthermore, the nonlinear negative relationship in patients with hypertension in whom Hcy concentrations are less likely to be low and approach a plateau as serum 5-MTHF concentrations increase [82] is consistent with the findings of this study (Supplementary Figure S1). Interestingly, the mathematical model of the in-silico study estimated that increased remethylation flux does not reduce Hcy concentrations as it circumnavigates the methionine cycle back to Hcy [62]. Furthermore, the authors of the in-silico study concluded that the increased flux in the transsulfuration pathway is due to CBS activation via SAM and betaine [62]. The present study found that the concentrations of tCys, a transsulfuration pathway metabolite, were positively correlated with the concentrations of 5-MTHF, consistent with the results of the in-silico study [62]. In addition, previous studies have reported a positive correlation between serum total folate and plasma tCys concentrations in healthy adults [83,84]. Previous studies have reported that SAM is an allosteric activator of CBS and regulates the flux of Hcy into the transsulfuration pathway [78,85]. Previous studies have also reported that SAH activates CBS [86]. Herein, SAM and SAH concentrations exhibited significant positive correlations with cystathionine, tCys, and taurine concentrations (Figure 2), implying that SAM and SAH may modulate the flux of the transsulfuration pathway in a concentration-dependent manner. However, the correlation coefficients between SAM or SAH and the transsulfuration pathway metabolites (cystathionine, cysteine, and taurine) varied depending on the 5-MTHF state and require further investigation (Supplementary Table S1). Accordingly, the 5-MTHF state may be associated with Hcy flux into the transsulfuration pathway via SAM.
The negative correlation between serum 5-MTHF concentrations and tHcy/tCys ratios was stronger than that between 5-MTHF and tHcy concentrations alone (Supplementary Figure S1 and Table 4). The tHcy/tCys ratio, a substrate/product ratio, is an indicator of enzyme activity (CBS and CSE) in the transsulfuration pathway. Therefore, higher 5-MTHF concentrations may indicate a lower Hcy and higher Cys association. A previous cross-sectional study involving patients aged 21–88 (median, 62) years who had undergone coronary angiography for suspected coronary artery disease or aortic stenosis revealed a stronger negative association between the plasma total folate concentrations and tCys/tHcy ratios than that between plasma total folate concentrations and plasma tHcy concentrations [59]; these findings were consistent with those of the present study. A case–control study reported comparable results in patients with colorectal cancer and matched controls [87]. Therefore, the tHcy/tCys ratio is a more sensitive indicator of the 5-MTHF status than the tHcy concentration. Further investigation into the clinical implications of increased activation of transsulfuration pathways is warranted. Interestingly, in a previous study, increased Hcy concentrations and decreased Cys concentrations were observed in patients with schizophrenia [88], implying that the transsulfuration pathway plays an important role in the etiology of schizophrenia [88]. This theory suggests that schizophrenia may be characterized by impaired glutathione synthesis and increased susceptibility to oxidative stress. In other words, the pathological hypothesis of schizophrenia is centered on increased oxidative stress and impaired antioxidant function [89]. A previous study also reported that high plasma Hcy concentrations were associated with increased colorectal cancer risk, whereas high Cys concentrations were associated with a lower colorectal cancer risk [89] and that Hcy/Cys ratios were inversely correlated with colorectal cancer risk [87]. Conversely, studies have suggested that high plasma tCys concentrations in healthy women may cause more damage to the vascular endothelium than tHcy [90]. A similar association between betaine and tHcy/tCys ratio has also been shown (Appendix A.1). Therefore, the optimal state of the transsulfuration pathway, including 5-MTHF and betaine, should be investigated to clarify these findings.
Herein, 5-MTHF concentrations exhibited significant positive correlations with betaine concentrations or betaine/DMG ratios (Figure 3 and Table 4). Similarly, previous studies have reported a positive correlation between blood total folate and betaine concentrations [68,91]. According to in-silico studies, SAM concentrations increase with increasing total folate concentrations, SAM inhibits BHMT, and betaine is less likely to be metabolized, resulting in increased betaine concentrations [71]. In addition, previous intervention studies have reported that FA supplementation increases plasma betaine concentrations in both healthy adults [66] and older individuals [92]. Therefore, the findings of this study support those of previous studies that reported an association between total folate concentrations and betaine metabolism. Furthermore, when the cohort was divided into diquantiles based on serum 5-MTHF concentrations, the negative correlation between betaine concentrations or betaine/DMG ratios and tHcy concentrations was stronger in the low 5-MTHF group than in the high 5-MTHF group (Figure 3 and Table 4). Conversely, the positive correlation between tHcy and DMG concentrations was weaker in the low 5-MTHF group than in the high 5-MTHF group. Previous studies have reported that blood betaine concentrations inversely correlate with Hcy concentrations in healthy adults [66,68,93], clinic attendees [94], pregnant women [95], patients with cardiovascular disease [96,97], and patients with chronic renal failure [98], indicating that betaine and folate are important determinants of plasma Hcy concentrations. If the 5-MTHF concentration is low, the supply of methyl groups from 5-MTHF to Hcy may be insufficient for Hcy to remethylate methionine, thus necessitating another Hcy methyl group donation pathway, i.e., betaine to Hcy. In a previous study, when pregnant women were divided into diquantiles based on plasma total folate concentration, the low folate group exhibited higher plasma DMG concentrations and lower plasma betaine concentrations than the high folate group [99]. An interventional study involving healthy men and women reported that an increase in plasma Hcy concentrations following methionine loading was inversely correlated with plasma betaine concentrations and that this association was stronger in patients with low blood folate concentrations than in those with high blood folate concentrations [68,93]. The findings of previous studies evaluating total folate states are consistent with those of the present study [68,93]. As a mechanism, methionine synthase expression has been reported to decrease in the liver of mice fed with a low folate diet [100], and decreased SAM concentrations have been reported to activate BHMT in the rat liver [101]. Certainly, the present study showed a weak positive correlation between SAM and betaine (Appendix A.2). A previous study also estimated a decrease in SAM concentrations and BHMT response activation in the liver using a mathematical model based on known enzyme kinetics, assuming a 50% decrease in folate concentrations [53]. As 5-MTHF can alter the association of tHcy with betaine or DMG, the betaine/DMG ratio, a measure of BHMT activity, may serve as a good indicator of Hcy remethylation via the choline metabolic pathway. Conversely, in-silico studies have reported that the negative association between betaine and tHcy concentrations in women is due to CBS activation based on betaine concentrations rather than a faster BHMT reaction [62]. In other words, CBS activation as a result of high betaine concentrations is less likely to occur when 5-MTHF concentrations are high. Thus, the 5-MTHF status may affect the choline metabolic pathway (Appendix A.3), particularly BHMT or CBS activity, via betaine concentrations in low 5-MTHF conditions, implying that the folate cycle and choline metabolic pathway should be evaluated simultaneously in Hcy studies.
Herein, a negative association between serum 5-MTHF and cystathionine concentrations was observed (Figure 3). A negative correlation between blood total folate and cystathionine concentrations has also been observed in neonates [102] and patients with coronary artery disease [103,104], possibly because Hcy and cystathionine have similar kinetics [104]. This correlation was different than that observed between tHcy and tCys, and the complex transsulfuration pathway needs to be further elucidated.
Serum FA concentrations were not correlated with OCM-related metabolite concentrations associated with 5-MTHF concentrations. This suggests that the concentration of 5-MTHF, a bioactive folate molecule species, as a biomarker may be more useful than that of total folate, which was considered in previous studies [49].
This study has several strengths. Early morning fasting blood samples were collected, and standardized blood collection protocols were used to minimize the following effects on OCM-related metabolites: changes in the blood concentration of the measured substance due to eating immediately before the blood sample is collected; errors related to human activities at the time of blood collection; and variation in the deterioration of the substance being measured. Furthermore, the serum concentrations of OCM-related metabolites were measured using the stable-isotope dilution mass spectrometry method with internal standards for all measured components, yielding high-quality quantitative results [105].
However, this study has several limitations. First, given the cross-sectional survey design, it is impossible to establish a causal association between OCM metabolic dynamics. Further intervention studies on FA or 5-MTHF are required. Second, serum OCM-related metabolites do not always directly reflect OCM dynamics in organ cells. Third, 5-MTHF-dependent Hcy remethylation is a vitamin B12-dependent reaction, and vitamin B12 is an important determinant of Hcy [106,107]; however, it was not measured in this study. Fourth, recruitment and regional influences, such as only including women from one university who majored in nutrition, might have introduced a sampling bias. Furthermore, FA-fortified rice was routinely served in the university cafeteria. Therefore, generalizing the findings to the Japanese population should be considered with caution. Fifth, Hcy is influenced by numerous factors, including lifestyle [106]; however, confounding factors influencing blood Hcy concentrations were left unadjusted. Sixth, while postprandial OCM kinetic variability was minimized by fasting, the potential impact of habitual diet cannot be ruled out [108,109]. In particular, vitamin B6 intake may have promoted transsulfuration pathway flux. Finally, single-nucleotide polymorphisms may have an impact on OCM [56]; however, they have not been considered.
4. Materials and Methods
4.1. Study Design
This study was conducted at Kagawa Nutrition University, Saitama, Japan, between October and December 2018. This was a cross-sectional study designed to investigate the association between diet and blood components such as biochemical test values, OCM-related metabolites, fatty acids, and antioxidants; we reported associations between serum OCM-related metabolites.
4.2. Participants
Healthy female university students aged 18–25 years majoring in nutrition were included. The following students were excluded: (1) those with health conditions that may affect the biomarker concentration; (2) those with a history of, or who have serious hepatic, renal, cardiac, pulmonary, gastrointestinal (including gastrectomy), or organ disorders; diabetes; food allergies; or other serious diseases; (3) those receiving medicines that can affect lipid metabolism or FA metabolism or anti-inflammatory and antioxidant drugs that may affect the measured values in this study; (4) those pregnant and lactating or planning for pregnancy and lactation; (5) those with systolic blood pressure <90 mmHg; (6) those who had previously donated large amounts of blood; (7) those participating in other clinical trials or studies or within 4 weeks of the end of those studies; (8) those with BMI >25 because BMI may affect blood SAM and SAH levels [110,111]; and (9) those judged by the principal investigator to be ineligible for this study. The participants were recruited via a university bulletin board.
A total of 258 eligible participants consented to the study, including proxy consent for minors. After the study began, 31 participants dropped out owing to consent withdrawals (n = 9), lost contact (n = 6), health problems (n = 1), busyness (n = 4), and withdrawals (n = 11), leaving 227 participants. The participants completed a lifestyle questionnaire and underwent physical measurements, and their fasting blood samples were collected after at least 10 h of fasting.
4.3. Ethics
The study protocol was approved by the Ethics Committee of Kagawa Nutrition University (protocol code no. 204) and was conducted in accordance with the Helsinki Declaration. All participants provided written informed consent before participating in the study.
4.4. Sample Collection and Processing
To minimize dietary effects [63,67,112], the study participants were instructed to stop eating and drinking by 10 pm and consume nothing but water until blood collection. The participants visited the laboratory in the morning, where 15 mL of venous blood was collected in the supine position and delivered into a serum separation tube. The blood was allowed to clot at room temperature for 30 min, centrifuged at 4 °C for 10 min at 2000× g, and the serum was then separated within an hour and frozen at −80 °C. Under these storage conditions, the concentrations of OCM-related metabolites measured in this study have been reported to be generally stable [51,113,114].
4.5. Measurement of Serum OCM-Related Metabolites
We previously described the method for measuring OCM-related metabolites [81]. After combining the methods of Guiraud et al. [70] and Zheng et al. [115], we set up multiple reaction monitoring transitions for 18 OCM-related metabolites and their corresponding 18 internal standards (m/z 460.2→313.2 (5-MTHF), m/z 465.2→313.2 (5-MTHF−13C5), m/z 442.2→295.2 (FA), m/z 447.2→295.2 (FA−13C5), m/z 104.0→60.1 (choline), m/z 113.0→69.1 (choline-d9), m/z 118.1→58.0 (betaine), m/z 129.0→66.1 (betaine-d11), m/z 104.1→58.1 (DMG), m/z 110.1→64.0 (DMG-d6), m/z 150.1→104.0 (methionine), m/z 153.0→107.0 (methionine-d3), m/z 399.1→250.3 (SAM), m/z 402.2→250.2 (SAM-d3), m/z 385.2→134 (SAH), m/z 389.2→136.2 (SAH-d4), m/z 136.0→90.0 (Hcy), m/z 140.0→93.9 (Hcy-d4), m/z 184.1→138.3 (homocysteic acid), m/z 188.1→142.0 (homocysteic acid-d4), m/z 223.2→134.1 (cystathionine), m/z 227.1→138.1 (cystathionine-d4), m/z 122.1→59.1 (Cys), m/z 124.2→61.0 (Cys-d2), m/z 126.1→107.8 (taurine), m/z 128.1→110.2 (taurine−13C2), m/z 106.1→60.1 (serine), m/z 109.1→63.1 (serine-d3), m/z 76.0→30.0 (glycine), m/z 78.0→31.9 (glycine-d2), m/z 377.1→243.1 (riboflavin), m/z 383.2→249.1 (riboflavin−13C415N2), m/z 169.2→152.2 (pyridoxamine), m/z 172.1→155.1 (pyridoxamine-d3), m/z 170.1→134.1 (pyridoxine), and m/z 172.1→136.0 (pyridoxine-d2)) [105]. Hcy and Cys were detected in reduced forms as tHcy and tCys, respectively, under the influence of reducing agents. For liquid chromatography, an Agilent 1200 Series (Agilent Technologies, Tokyo, Japan) was used, and the ion source was a Turbo Ion Spray (Applied Biosystems SCIEX, Tokyo, Japan). The triple quadrupole mass spectrometer was a 4000 QTRAP System (Applied Biosystems SCIEX). The measurement time was 13 min, and the mobile phase flow rate was 500 μL/min, with “A” being a 5 mmol/L perfluoroheptanoic acid solution, “B” being an acetonitrile gradient, and the separation column being an XSelect HSS T3 Column, 2.5 μm, 100 × 2.1 μm (Nihon Waters, Tokyo, Japan). The measurements were taken twice, and the mean value was used. For precision control, eight-point calibration curves were created every 24 h, and quality control measurements were taken every 12 h. The intra-assay and interassay coefficients of variation were 0.3% to 9.1% and 0.5 to 13.5%, respectively. When measuring, control sera were under low-concentration conditions in a preliminary validity test [116]. Analyst 1.6.3 software was employed to process and quantify the data. The concentration value was 0 if the peak could not be detected or the signal-to-noise ratio was <10 as the quantification limit.
4.6. Attribute Data
Data regarding the participants’ ages were collected using a self-administered questionnaire.
4.7. Nutrient Intake
Dietary data were collected for 7 days before blood collection using a continuous, non-weighted dietary record in conjunction with digital images captured using a digital camera or smartphone according to a validated dietary survey method [117,118,119]. In brief, the participants were instructed to photograph all their meals except water while writing the menu name, ingredients used, and portion size on a self-administered food record form. The photographs were captured before and after the meal, and the participants were asked to place a designated card that would serve as a scale to measure the food. If they ate out or consumed products, they were asked to record the restaurant and brand names of the food. Trained nutrition students and researchers used digital images and dietary records to infer the type and weight of individual food items consumed by the participants based on the standardized Dietary Survey Manual [120] and obtained additional information from the participants when information was unclear. Individual food items were coded based on Japan’s Standard Tables of Food Composition, 2015 [121], and food codes that best approximated the state of the food at the time of eating were selected. Estimated energy and nutrient intakes were calculated using Excel eiyoukun, Version 8.2 (Kenpakusha, Tokyo, Japan). Nutrient intakes were averaged over 7 days, and energy was adjusted for each nutrient intake using the density method.
4.8. Blood Pressure Measurement
Blood pressure was measured using a P2000 Electronic Blood Pressure Monitor (TERUMO, Yamanashi, Japan). The mean systolic and diastolic blood pressure was measured twice in the resting state according to the Japanese guidelines for managing hypertension [122].
4.9. Anthropometric Data
The participants’ anthropometric measurements were collected using standardized methods during laboratory visits for blood sampling. Body weight and body fat percentage were measured using TBF-110 (TANITA, Nagano, Japan).
4.10. Statistical Analysis
The distribution of subject characteristics data, nutrient intake, and serum concentrations of OCM-related metabolites used in the analysis were mostly non-normal and continuous variables were expressed as medians (25–75th percentile values). Since there are no thresholds established for serum 5-MTHF concentrations such as deficiency, insufficiency, sufficiency, or excess, based on a previous study [99], a median serum 5-MTHF concentration of 19.2 nmol/L was used to stratify the participants into high and low 5-MTHF groups, and the Mann–Whitney U test was used to compare these groups. The enzyme activity indices included the betaine/DMG ratio [98] for BHMT activity, SAM/SAH ratio [54] for methyltransferase activity, and tCys/tHcy ratio [59] for enzyme activity in the transsulfuration pathway (CBS and CSE). The significance level was set at a two-tailed p-value of <0.05. IBM Statistical Package for the Social Sciences Statistics, Version 28 (IBM, Japan) was used for statistical analysis.
5. Conclusions
Previous studies assessed folate status without considering folate molecular species. Thus, we extended the findings of previous studies by comprehensively measuring OCM-related metabolites, including 5-MTHF, a major reduced folate. FA showed no association with important OCM-related metabolites, indicating that blood 5-MTHF levels could be useful for evaluating folate status. The 5-MTHF state could be linked to the Hcy flux into the transsulfuration pathway via SAM. Therefore, the tHcy/tCys ratio may be a more sensitive indicator of the 5-MTHF status than tHcy concentrations alone. A low 5-MTHF status may affect the choline metabolic pathway, particularly BHMT or CBS activity via betaine, implying that the folate cycle and choline metabolic pathway should be evaluated simultaneously in Hcy studies. Further intervention studies on FA or 5-MTHF are warranted to determine the causal association between the abovementioned findings.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241310993/s1.
Author Contributions
Conceptualization, Y.K. (Yoshinori Kubo); methodology, Y.K. (Yoshinori Kubo), T.K., K.S., A.T., S.H., H.F. and Y.K. (Yasuo Kagawa); validation, Y.K. (Yoshinori Kubo); formal analysis, Y.K. (Yoshinori Kubo); investigation, Y.K. (Yoshinori Kubo), T.K., K.S., S.H., A.T. and Y.K. (Yasuo Kagawa); resources, T.K. and M.N.; data curation, T.K. and K.S.; writing—original draft preparation, Y.K. (Yoshinori Kubo); writing—review and editing, Y.K. (Yoshinori Kubo), T.K., K.S. and H.F.; visualization, Y.K. (Yoshinori Kubo); supervision, T.K.; project administration, T.K.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
The study was conducted in accordance with the Helsinki Declaration and was approved by the Ethics Committee of Kagawa Nutrition University (protocol code no. 204; 16 November 2018).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The dataset used in this study is available upon reasonable request.
Acknowledgments
We would like to thank all the participants in this study and all those who assisted in the research.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5-MTHF | 5-methyltetrahydrofolate |
| BHMT | Betaine–homocysteine S-methyltransferase |
| BMI | Body mass index |
| CBS | Cystathionine β-synthase |
| CSE | Cystathionine γ-lyase |
| Cys | Cysteine |
| DMG | Dimethylglycine |
| FA | Folic acid |
| Hcy | Homocysteine |
| MAT | Methionine adenosyltransferase |
| MTHFR | Methylenetetrahydrofolate reductase |
| OCM | One-carbon metabolism |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| tCys | Total cysteine |
| tHcy | Total homocysteine |
| THF | Tetrahydrofolate |
Appendix A
Appendix A.1. Association between Betaine Concentrations and tHcy/tCys Ratio
Similar to the correlation between 5-MTHF concentrations and tHcy/tCys ratios, the correlation between betaine concentrations and tHcy/tCys ratios (ρ = −0.324, Table 3) was stronger than that between betaine and tHcy concentrations alone (ρ = −0.292, Supplementary Table S1). This was also reported in a previous study [59], and the tHcy/tCys ratio could serve as a sensitive indicator of the betaine status and a simple indicator of the complex folate cycle and choline metabolic pathway.
Appendix A.2. Association between Betaine and SAM Concentrations
Similar to 5-MTHF, betaine concentrations that remethylate Hcy had a significant positive correlation with SAM concentrations. A previous study also reported that betaine concentrations positively correlated with SAM concentrations in healthy men and women [123], indicating the significance of considering betaine status in addition to 5-MTHF status in OCM methyl group metabolism. However, the association between betaine and SAM could be indirect, since 5-MTHF and betaine are positively correlated.
Appendix A.3. 5-MTHF Status and its Association with Choline Metabolic Pathways
Herein, significant correlations were detected between choline and betaine, choline and DMG, and betaine and DMG concentrations, with a stronger correlation in the high 5-MTHF group than in the low 5-MTHF group (Supplementary Table S1). These associations have also been reported in previous studies involving pregnant women and healthy adults [67,68,93], and they may be explained by the fact that choline is a precursor to betaine and DMG [68]. The findings of the present study imply that the association between these choline metabolic pathways may be influenced by the 5-MTHF status.
References
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Stover, P.J.; Field, M.S. Trafficking of intracellular folates. Adv. Nutr. 2011, 2, 325–331. [Google Scholar] [CrossRef]
- Shane, B. Folate and vitamin B12 metabolism: Overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food Nutr. Bull. 2008, 29, S5–S16. discussion S17–S19. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.R.; Dyson, H.J.; Wright, P.E. Structure, dynamics, and catalytic function of dihydrofolate reductase. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A.; Ambili, M.; Jala, V.R.; Subramanya, H.S.; Savithri, H.S. Structure-function relationship in serine hydroxymethyltransferase. Biochim. Biophys. Acta 2003, 1647, 24–29. [Google Scholar] [CrossRef]
- Födinger, M.; Hörl, W.H.; Sunder-Plassmann, G. Molecular biology of 5,10-methylenetetrahydrofolate reductase. J. Nephrol. 2000, 13, 20–33. [Google Scholar]
- Pfeiffer, C.M.; Fazili, Z.; McCoy, L.; Zhang, M.; Gunter, E.W. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin. Chem. 2004, 50, 423–432. [Google Scholar] [CrossRef]
- Kirsch, S.H.; Knapp, J.P.; Herrmann, W.; Obeid, R. Quantification of key folate forms in serum using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 68–75. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Sunden, S.L.; Renduchintala, M.S.; Park, E.I.; Miklasz, S.D.; Garrow, T.A. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys. 1997, 345, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Harris, B.J.; Kyle, W.E. Methionine metabolism in mammals: Kinetic study of betaine-homocysteine methyltransferase. Arch. Biochem. Biophys. 1972, 153, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.M.; Hoffman, J.L. Fractionation and kinetic properties of rat liver and kidney methionine adenosyltransferase isozymes. Biochemistry 1983, 22, 1636–1641. [Google Scholar] [CrossRef]
- Chiang, P.K.; Gordon, R.K.; Tal, J.; Zeng, G.C.; Doctor, B.P.; Pardhasaradhi, K.; McCann, P.P. S-Adenosylmethionine and methylation. FASEB J. 1996, 10, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Lucock, M. Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell. Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Finkelstein, J.D. The metabolism of homocysteine: Pathways and regulation. Eur. J. Pediatr. 1998, 157 (Suppl. S2), S40–S44. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.; Bailey, L. Folate and choline interrelationships: Metabolic and potential health implications. In Folate in Health and Disease, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Oxford, UK, 2009; pp. 449–465. [Google Scholar]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Banerjee, R.; Zou, C.G. Redox regulation and reaction mechanism of human cystathionine-β-synthase: A PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef]
- Jurkowska, H.; Kaczor-Kaminska, M.; Bronowicka-Adamska, P.; Wrobel, M. Cystathionine γ-lyase. Postepy Hig. Med. Dosw. Online 2014, 68, 1–9. [Google Scholar] [CrossRef]
- Guttormsen, A.B.; Schneede, J.; Ueland, P.M.; Refsum, H. Kinetics of total plasma homocysteine in subjects with hyperhomocysteinemia due to folate or cobalamin deficiency. Am. J. Clin. Nutr. 1996, 63, 194–202. [Google Scholar] [CrossRef]
- Peng, H.Y.; Man, C.F.; Xu, J.; Fan, Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: A meta-analysis of prospective studies. J. Zhejiang Univ. Sci. B 2015, 16, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine level and coronary heart disease incidence: A systematic review and meta-analysis. Mayo Clin. Proc. 2008, 83, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Q.; Chen, Q.; Li, Q.; Guo, C.; Tian, G.; Qie, R.; Han, M.; Huang, S.; Li, Y.; et al. Association of homocysteine level with risk of stroke: A dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, N.N.; Telles, J.P.M.; Pipek, L.Z.; Nascimento, R.F.V.; Gusmao, R.C.; Teixeira, M.J.; Figueiredo, E.G. Homocysteine is associated with higher risks of ischemic stroke: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0276087. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.C.; Cheung, M.W.; Fu, E.; Win, H.H.; Zaw, M.H.; Ng, A.; Mak, A. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am. J. Geriatr. Psychiatry 2011, 19, 607–617. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Chang, H.; Liu, X.; Zhu, R. Homocysteine and Folic Acid: Risk Factors for Alzheimer’s Disease-An Updated Meta—Analysis. Front. Aging Neurosci. 2021, 13, 665114. [Google Scholar] [CrossRef]
- Muntjewerff, J.W.; Kahn, R.S.; Blom, H.J.; den Heijer, M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: A meta-analysis. Mol. Psychiatry 2006, 11, 143–149. [Google Scholar] [CrossRef]
- Numata, S.; Kinoshita, M.; Tajima, A.; Nishi, A.; Imoto, I.; Ohmori, T. Evaluation of an association between plasma total homocysteine and schizophrenia by a Mendelian randomization analysis. BMC Med. Genet. 2015, 16, 54. [Google Scholar] [CrossRef]
- Huang, P.; Wang, F.; Sah, B.K.; Jiang, J.; Ni, Z.; Wang, J.; Sun, X. Homocysteine and the risk of age-related macular degeneration: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 10585. [Google Scholar] [CrossRef]
- Lei, X.; Zeng, G.; Zhang, Y.; Li, Q.; Zhang, J.; Bai, Z.; Yang, K. Association between homocysteine level and the risk of diabetic retinopathy: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 61. [Google Scholar] [CrossRef]
- He, T.; Jin, X.; Koh, Y.S.; Zhang, Q.; Zhang, C.; Liu, F. The association of homocysteine, folate, vitamin B12, and vitamin B6 with fracture incidence in older adults: A systematic review and meta-analysis. Ann. Transl. Med. 2021, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, H.Y.; Qiao, Z.Y.; Gong, F.X. Homocysteine level and gestational diabetes mellitus: A systematic review and meta-analysis. Gynecol. Endocrinol. 2021, 37, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, J.; Wang, X.; Gu, H. High level of homocysteine is associated with pre-eclampsia risk in pregnant woman: A meta-analysis. Gynecol. Endocrinol. 2022, 38, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X. A Novel Review of Homocysteine and Pregnancy Complications. Biomed. Res. Int. 2021, 2021, 6652231. [Google Scholar] [CrossRef]
- Hogeveen, M.; Blom, H.J.; den Heijer, M. Maternal homocysteine and small-for-gestational-age offspring: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 130–136. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, X.; Wu, W.; Guo, Y.; Cui, W. Elevated homocysteine level and folate deficiency associated with increased overall risk of carcinogenesis: Meta-analysis of 83 case-control studies involving 35,758 individuals. PLoS ONE 2015, 10, e0123423. [Google Scholar] [CrossRef] [PubMed]
- Skovierova, H.; Vidomanova, E.; Mahmood, S.; Sopkova, J.; Drgova, A.; Cervenova, T.; Halasova, E.; Lehotsky, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Su, X.; Huang, W.; Zhang, J.; Peng, C.; Huang, H.; Wu, X.; Huang, H.; Xia, M.; Ling, W. Role of S-adenosylhomocysteine in cardiovascular disease and its potential epigenetic mechanism. Int. J. Biochem. Cell Biol. 2015, 67, 158–166. [Google Scholar] [CrossRef]
- Marti-Carvajal, A.J.; Sola, I.; Lathyris, D.; Dayer, M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 8, CD006612. [Google Scholar] [CrossRef]
- Boldyrev, A.A. Molecular mechanisms of homocysteine toxicity. Biochemistry 2009, 74, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Devine, O.; Hao, L.; Dowling, N.F.; Li, S.; Molloy, A.M.; Li, Z.; Zhu, J.; Berry, R.J. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 2014, 349, g4554. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Finnell, R.H.; Blom, H.J.; Carmichael, S.L.; Vollset, S.E.; Yang, W.; Ueland, P.M. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology 2009, 20, 714–719. [Google Scholar] [CrossRef]
- Shaw, G.M.; Carmichael, S.L.; Yang, W.; Selvin, S.; Schaffer, D.M. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 2004, 160, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mota-Martorell, N.; Jove, M.; Borras, C.; Berdun, R.; Obis, E.; Sol, J.; Cabre, R.; Pradas, I.; Galo-Licona, J.D.; Puig, J.; et al. Methionine transsulfuration pathway is upregulated in long-lived humans. Free Radic. Biol. Med. 2021, 162, 38–52. [Google Scholar] [CrossRef]
- Cochrane, K.M.; Williams, B.A.; Elango, R.; Barr, S.I.; Karakochuk, C.D. Pregnancy-induced alterations of 1-carbon metabolism and significance for maternal nutrition requirements. Nutr. Rev. 2022, 80, 1985–2001. [Google Scholar] [CrossRef]
- Kalhan, S.C. One carbon metabolism in pregnancy: Impact on maternal, fetal and neonatal health. Mol. Cell. Endocrinol. 2016, 435, 48–60. [Google Scholar] [CrossRef]
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Fazili, Z.; Lacher, D.A.; Bailey, R.L.; Blackmore, S.; Bock, J.L.; Brody, L.C.; Carmel, R.; et al. Biomarkers of folate status in NHANES: A roundtable summary. Am. J. Clin. Nutr. 2011, 94, 303S–312S. [Google Scholar] [CrossRef]
- Sobczynska-Malefora, A.; Harrington, D.J. Laboratory assessment of folate (vitamin B(9)) status. J. Clin. Pathol. 2018, 71, 949–956. [Google Scholar] [CrossRef]
- Verstraete, J.; Kiekens, F.; Strobbe, S.; De Steur, H.; Gellynck, X.; Van Der Straeten, D.; Stove, C.P. Clinical determination of folates: Recent analytical strategies and challenges. Anal. Bioanal. Chem. 2019, 411, 4383–4399. [Google Scholar] [CrossRef]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F.; Reed, M.C.; Lam, S.L.; Shane, B.; Gregory, J.F., 3rd; Ulrich, C.M. In silico experimentation with a model of hepatic mitochondrial folate metabolism. Theor. Biol. Med. Model. 2006, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- King, W.D.; Ho, V.; Dodds, L.; Perkins, S.L.; Casson, R.I.; Massey, T.E. Relationships among biomarkers of one-carbon metabolism. Mol. Biol. Rep. 2012, 39, 7805–7812. [Google Scholar] [CrossRef]
- Obeid, R.; Kirsch, S.H.; Kasoha, M.; Eckert, R.; Herrmann, W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism 2011, 60, 673–680. [Google Scholar] [CrossRef]
- Fredriksen, A.; Meyer, K.; Ueland, P.M.; Vollset, S.E.; Grotmol, T.; Schneede, J. Large-scale population-based metabolic phenotyping of thirteen genetic polymorphisms related to one-carbon metabolism. Hum. Mutat. 2007, 28, 856–865. [Google Scholar] [CrossRef]
- Rooney, M.; Bottiglieri, T.; Wasek-Patterson, B.; McMahon, A.; Hughes, C.F.; McCann, A.; Horigan, G.; Strain, J.J.; McNulty, H.; Ward, M. Impact of the MTHFR C677T polymorphism on one-carbon metabolites: Evidence from a randomised trial of riboflavin supplementation. Biochimie 2020, 173, 91–99. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Luo, G.A.; Liang, Q.L.; Wang, Y.; Yang, H.H.; Wang, Y.M.; Zheng, X.Y.; Song, X.M.; Chen, G.; Zhang, T.; et al. Neural tube defects and disturbed maternal folate- and homocysteine-mediated one-carbon metabolism. Exp. Neurol. 2008, 212, 515–521. [Google Scholar] [CrossRef]
- Ulvik, A.; Hustad, S.; McCann, A.; Midttun, O.; Nygard, O.K.; Ueland, P.M. Ratios of One-Carbon Metabolites Are Functional Markers of B-Vitamin Status in a Norwegian Coronary Angiography Screening Cohort. J. Nutr. 2017, 147, 1167–1173. [Google Scholar] [CrossRef]
- Kvestad, I.; McCann, A.; Chandyo, R.K.; Giil, L.M.; Shrestha, M.; Ulak, M.; Hysing, M.; Ueland, P.M.; Strand, T.A. One-Carbon Metabolism in Nepalese Infant-Mother Pairs and Child Cognition at 5 Years Old. J. Nutr. 2021, 151, 883–891. [Google Scholar] [CrossRef]
- Eussen, S.J.; Nilsen, R.M.; Midttun, O.; Hustad, S.; Ijssennagger, N.; Meyer, K.; Fredriksen, A.; Ulvik, A.; Ueland, P.M.; Brennan, P.; et al. North-south gradients in plasma concentrations of B-vitamins and other components of one-carbon metabolism in Western Europe: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2013, 110, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Sadre-Marandi, F.; Dahdoul, T.; Reed, M.C.; Nijhout, H.F. Sex differences in hepatic one-carbon metabolism. BMC Syst. Biol. 2018, 12, 89. [Google Scholar] [CrossRef]
- Obeid, R.; Schon, C.; Pietrzik, K.; Menzel, D.; Wilhelm, M.; Smulders, Y.; Knapp, J.P.; Bohni, R. Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts. Nutrients 2020, 12, 3623. [Google Scholar] [CrossRef]
- Hannisdal, R.; Ueland, P.M.; Svardal, A. Liquid chromatography-tandem mass spectrometry analysis of folate and folate catabolites in human serum. Clin. Chem. 2009, 55, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Fazili, Z.; Sternberg, M.R.; Potischman, N.; Wang, C.Y.; Storandt, R.J.; Yeung, L.; Yamini, S.; Gahche, J.J.; Juan, W.; Qi, Y.P.; et al. Demographic, Physiologic, and Lifestyle Characteristics Observed with Serum Total Folate Differ among Folate Forms: Cross-Sectional Data from Fasting Samples in the NHANES 2011–2016. J. Nutr. 2020, 150, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Melse-Boonstra, A.; Holm, P.I.; Ueland, P.M.; Olthof, M.; Clarke, R.; Verhoef, P. Betaine concentration as a determinant of fasting total homocysteine concentrations and the effect of folic acid supplementation on betaine concentrations. Am. J. Clin. Nutr. 2005, 81, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.I.; Ueland, P.M.; Vollset, S.E.; Midttun, O.; Blom, H.J.; Keijzer, M.B.; den Heijer, M. Betaine and folate status as cooperative determinants of plasma homocysteine in humans. Arter. Thromb. Vasc. Biol. 2005, 25, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Midttun, O.; Hustad, S.; Schneede, J.; Vollset, S.E.; Ueland, P.M. Plasma vitamin B-6 forms and their relation to transsulfuration metabolites in a large, population-based study. Am. J. Clin. Nutr. 2007, 86, 131–138. [Google Scholar] [CrossRef]
- Guiraud, S.P.; Montoliu, I.; Da Silva, L.; Dayon, L.; Galindo, A.N.; Corthesy, J.; Kussmann, M.; Martin, F.P. High-throughput and simultaneous quantitative analysis of homocysteine-methionine cycle metabolites and co-factors in blood plasma and cerebrospinal fluid by isotope dilution LC-MS/MS. Anal. Bioanal. Chem. 2017, 409, 295–305. [Google Scholar] [CrossRef]
- Reed, M.C.; Nijhout, H.F.; Neuhouser, M.L.; Gregory, J.F., 3rd; Shane, B.; James, S.J.; Boynton, A.; Ulrich, C.M. A mathematical model gives insights into nutritional and genetic aspects of folate-mediated one-carbon metabolism. J. Nutr. 2006, 136, 2653–2661. [Google Scholar] [CrossRef]
- Wagner, C.; Briggs, W.T.; Cook, R.J. Inhibition of glycine N-methyltransferase activity by folate derivatives: Implications for regulation of methyl group metabolism. Biochem. Biophys. Res. Commun. 1985, 127, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.C.; Gamble, M.V.; Hall, M.N.; Nijhout, H.F. Mathematical analysis of the regulation of competing methyltransferases. BMC Syst. Biol. 2015, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Jencks, D.A.; Mathews, R.G. Allosteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine. Effects of adenosylmethionine and NADPH on the equilibrium between active and inactive forms of the enzyme and on the kinetics of approach to equilibrium. J. Biol. Chem. 1987, 262, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Kutzbach, C.; Stokstad, E.L. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim. Biophys. Acta 1971, 250, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Martin, J.J. Inactivation of betaine-homocysteine methyltransferase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 1984, 118, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Trevijano, E.R.; Latasa, M.U.; Carretero, M.V.; Berasain, C.; Mato, J.M.; Avila, M.A. S-adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: A new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000, 14, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Kyle, W.E.; Martin, J.L.; Pick, A.M. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 1975, 66, 81–87. [Google Scholar] [CrossRef]
- Duncan, T.M.; Reed, M.C.; Nijhout, H.F. A population model of folate-mediated one-carbon metabolism. Nutrients 2013, 5, 2457–2474. [Google Scholar] [CrossRef]
- Duncan, T.M.; Reed, M.C.; Nijhout, H.F. The relationship between intracellular and plasma levels of folate and metabolites in the methionine cycle: A model. Mol. Nutr. Food Res. 2013, 57, 628–636. [Google Scholar] [CrossRef]
- Kubo, Y.; Fukuoka, H.; Kawabata, T.; Shoji, K.; Mori, C.; Sakurai, K.; Nishikawa, M.; Ohkubo, T.; Oshida, K.; Yanagisawa, N.; et al. Distribution of 5-Methyltetrahydrofolate and Folic Acid Levels in Maternal and Cord Blood Serum: Longitudinal Evaluation of Japanese Pregnant Women. Nutrients 2020, 12, 1633. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, S.; Chen, D.; Yang, Y.; Liang, Q.; Huo, Y.; Zhou, Z.; Zhang, N.; Wang, Z.; Liu, L.; et al. Association between serum 5-methyltetrahydrofolate and homocysteine in Chinese hypertensive participants with different MTHFR C677T polymorphisms: A cross-sectional study. Nutr. J. 2022, 21, 29. [Google Scholar] [CrossRef]
- Lima, A.; Ferin, R.; Bourbon, M.; Baptista, J.; Pavao, M.L. Hypercysteinemia, A Potential Risk Factor for Central Obesity and Related Disorders in Azores, Portugal. J. Nutr. Metab. 2019, 2019, 1826780. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.A.; Hervig, T.; Stakkestad, J.A.; Drablos, P.A.; Apeland, T.; Wentzel-Larsen, T.; Bates, C.J. Serum folate is significantly correlated with plasma cysteine concentrations in healthy industry workers. Ann. Nutr. Metab. 2011, 58, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin. Chem. Lab. Med. 2007, 45, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Kyle, W.E.; Harris, B.J. Methionine metabolism in mammals: Regulatory effects of S-adenosylhomocysteine. Arch. Biochem. Biophys. 1974, 165, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Gylling, B.; Myte, R.; Ulvik, A.; Ueland, P.M.; Midttun, O.; Schneede, J.; Hallmans, G.; Haggstrom, J.; Johansson, I.; Van Guelpen, B.; et al. One-carbon metabolite ratios as functional B-vitamin markers and in relation to colorectal cancer risk. Int. J. Cancer 2019, 144, 947–956. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Chen, C.; Pan, Y.; Cui, X.; Sun, J.; Zhao, F.; Cao, Y. Simultaneous determination of serum homocysteine, cysteine, and methionine in patients with schizophrenia by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2022, 36, e5366. [Google Scholar] [CrossRef]
- Miller, J.W.; Beresford, S.A.; Neuhouser, M.L.; Cheng, T.Y.; Song, X.; Brown, E.C.; Zheng, Y.; Rodriguez, B.; Green, R.; Ulrich, C.M. Homocysteine, cysteine, and risk of incident colorectal cancer in the Women’s Health Initiative observational cohort. Am. J. Clin. Nutr. 2013, 97, 827–834. [Google Scholar] [CrossRef]
- Keller, A.C.; Klawitter, J.; Hildreth, K.L.; Christians, U.; Putnam, K.; Kohrt, W.M.; Reusch, J.E.B.; Moreau, K.L. Elevated plasma homocysteine and cysteine are associated with endothelial dysfunction across menopausal stages in healthy women. J. Appl. Physiol. 2019, 126, 1533–1540. [Google Scholar] [CrossRef]
- Imbard, A.; Smulders, Y.M.; Barto, R.; Smith, D.E.; Kok, R.M.; Jakobs, C.; Blom, H.J. Plasma choline and betaine correlate with serum folate, plasma S-adenosyl-methionine and S-adenosyl-homocysteine in healthy volunteers. Clin. Chem. Lab. Med. 2013, 51, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.; Ueland, P.M.; Clarke, R.; Blom, H.J.; Hoefnagels, W.H.; van Staveren, W.A.; de Groot, L.C. The association of betaine, homocysteine and related metabolites with cognitive function in Dutch elderly people. Br. J. Nutr. 2007, 98, 960–968. [Google Scholar] [CrossRef]
- Holm, P.I.; Hustad, S.; Ueland, P.M.; Vollset, S.E.; Grotmol, T.; Schneede, J. Modulation of the homocysteine-betaine relationship by methylenetetrahydrofolate reductase 677 C->t genotypes and B-vitamin status in a large-scale epidemiological study. J. Clin. Endocrinol. Metab. 2007, 92, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; George, P.M.; Dellow, W.J.; Scott, R.S.; Chambers, S.T. Homocysteine, glycine betaine, and N,N-dimethylglycine in patients attending a lipid clinic. Metabolism 2005, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Velzing-Aarts, F.V.; Holm, P.I.; Fokkema, M.R.; van der Dijs, F.P.; Ueland, P.M.; Muskiet, F.A. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am. J. Clin. Nutr. 2005, 81, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, B.C.; Chen, Z.; Laryea, M.D.; Wendel, U.; Lussier-Cacan, S.; Genest, J., Jr.; Mar, M.H.; Zeisel, S.H.; Castro, C.; Garrow, T.; et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003, 17, 512–514. [Google Scholar] [CrossRef]
- Holm, P.I.; Bleie, O.; Ueland, P.M.; Lien, E.A.; Refsum, H.; Nordrehaug, J.E.; Nygard, O. Betaine as a determinant of postmethionine load total plasma homocysteine before and after B-vitamin supplementation. Arter. Thromb. Vasc. Biol. 2004, 24, 301–307. [Google Scholar] [CrossRef]
- McGregor, D.O.; Dellow, W.J.; Lever, M.; George, P.M.; Robson, R.A.; Chambers, S.T. Dimethylglycine accumulates in uremia and predicts elevated plasma homocysteine concentrations. Kidney Int. 2001, 59, 2267–2272. [Google Scholar] [CrossRef]
- Fernandez-Roig, S.; Cavalle-Busquets, P.; Fernandez-Ballart, J.D.; Ballesteros, M.; Berrocal-Zaragoza, M.I.; Salat-Batlle, J.; Ueland, P.M.; Murphy, M.M. Low folate status enhances pregnancy changes in plasma betaine and dimethylglycine concentrations and the association between betaine and homocysteine. Am. J. Clin. Nutr. 2013, 97, 1252–1259. [Google Scholar] [CrossRef]
- Christensen, K.E.; Wu, Q.; Wang, X.; Deng, L.; Caudill, M.A.; Rozen, R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J. Nutr. 2010, 140, 1736–1741. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J. Biol. Chem. 1984, 259, 9508–9513. [Google Scholar] [CrossRef]
- Refsum, H.; Grindflek, A.W.; Ueland, P.M.; Fredriksen, A.; Meyer, K.; Ulvik, A.; Guttormsen, A.B.; Iversen, O.E.; Schneede, J.; Kase, B.F. Screening for serum total homocysteine in newborn children. Clin. Chem. 2004, 50, 1769–1784. [Google Scholar] [CrossRef]
- DeRatt, B.N.; Ralat, M.A.; Lysne, V.; Tayyari, F.; Dhar, I.; Edison, A.S.; Garrett, T.J.; Midttun, O.; Ueland, P.M.; Nygard, O.K.; et al. Metabolomic Evaluation of the Consequences of Plasma Cystathionine Elevation in Adults with Stable Angina Pectoris. J. Nutr. 2017, 147, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- MacCoss, M.J.; Fukagawa, N.K.; Matthews, D.E. Measurement of intracellular sulfur amino acid metabolism in humans. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E947–E955. [Google Scholar] [CrossRef]
- Ciccimaro, E.; Blair, I.A. Stable-isotope dilution LC-MS for quantitative biomarker analysis. Bioanalysis 2010, 2, 311–341. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Dominguez-Salas, P.; Moore, S.E.; Cole, D.; da Costa, K.A.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Innis, S.M.; Waterland, R.A.; Zeisel, S.H.; et al. DNA methylation potential: Dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am. J. Clin. Nutr. 2013, 97, 1217–1227. [Google Scholar] [CrossRef]
- Lind, M.V.; Lauritzen, L.; Pedersen, O.; Vestergaard, H.; Stark, K.D.; Hansen, T.; Ross, A.B.; Kristensen, M. Higher intake of fish and fat is associated with lower plasma s-adenosylhomocysteine: A cross-sectional study. Nutr. Res. 2017, 46, 78–87. [Google Scholar] [CrossRef]
- Lind, M.V.; Lauritzen, L.; Vestergaard, H.; Hansen, T.; Pedersen, O.; Kristensen, M.; Ross, A.B. One-carbon metabolism markers are associated with cardiometabolic risk factors. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 402–410. [Google Scholar] [CrossRef]
- van Driel, L.M.; Eijkemans, M.J.; de Jonge, R.; de Vries, J.H.; van Meurs, J.B.; Steegers, E.A.; Steegers-Theunissen, R.P. Body mass index is an important determinant of methylation biomarkers in women of reproductive ages. J. Nutr. 2009, 139, 2315–2321. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Yetley, E.A.; Lacher, D.A.; Bailey, R.L.; Johnson, C.L. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J. Nutr. 2015, 145, 520–531. [Google Scholar] [CrossRef]
- Hustad, S.; Eussen, S.; Midttun, O.; Ulvik, A.; van de Kant, P.M.; Morkrid, L.; Gislefoss, R.; Ueland, P.M. Kinetic modeling of storage effects on biomarkers related to B vitamin status and one-carbon metabolism. Clin. Chem. 2012, 58, 402–410. [Google Scholar] [CrossRef]
- Stabler, S.P.; Allen, R.H. Quantification of serum and urinary S-adenosylmethionine and S-adenosylhomocysteine by stable-isotope-dilution liquid chromatography-mass spectrometry. Clin. Chem. 2004, 50, 365–372. [Google Scholar] [CrossRef]
- Zheng, X.H.; Jiang, L.Y.; Zhao, L.T.; Zhang, Q.Y.; Ding, L. Simultaneous quantitation of folic acid and 5-methyltetrahydrofolic acid in human plasma by HPLC-MS/MS and its application to a pharmacokinetic study. J. Pharm. Anal. 2015, 5, 269–275. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; CDER; CVM. Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 15 March 2023).
- Wang, D.H.; Kogashiwa, M.; Ohta, S.; Kira, S. Validity and reliability of a dietary assessment method: The application of a digital camera with a mobile phone card attachment. J. Nutr. Sci. Vitaminol. 2002, 48, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.; Eriksen, R.; Lamb, K.; McMeel, Y.; Vergnaud, A.C.; Spear, J.; Aresu, M.; Chan, Q.; Elliott, P.; Frost, G. Dietary assessment of British police force employees: A description of diet record coding procedures and cross-sectional evaluation of dietary energy intake reporting (The Airwave Health Monitoring Study). BMJ Open 2017, 7, e012927. [Google Scholar] [CrossRef]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31 (Suppl. S3), 38–45. [Google Scholar] [CrossRef]
- Date, C.; Tokudome, Y.; Yoshiike, N. The Manual for Dietary Survey, 3rd ed.; Nanzando Co., Ltd.: Tokyo, Japen, 2016. [Google Scholar]
- Council for Science and Technology; Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard Tables of Food Composition in Japan 2015, 7th ed.; Official Gazette Co-Operation of Japan: Tokyo, Japan, 2015. (In Japanese)
- Shimamoto, K.; Ando, K.; Fujita, T.; Hasebe, N.; Higaki, J.; Horiuchi, M.; Imai, Y.; Imaizumi, T.; Ishimitsu, T.; Ito, M.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens. Res. 2014, 37, 253–390. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Nelson, H.H.; Robien, K.; Arning, E.; Bottiglieri, T.; Koh, W.P.; Yuan, J.M. One-carbon metabolism nutrient status and plasma S-adenosylmethionine concentrations in middle-aged and older Chinese in Singapore. Int. J. Mol. Epidemiol. Genet. 2012, 3, 160–173. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).