Single-Dose Treatment with Rapamycin Preserves Post-Ischemic Cardiac Function through Attenuation of Fibrosis and Inflammation in Diabetic Rabbit
Abstract
1. Introduction
2. Results
2.1. Rapamycin Improves Post-Ischemic Cardiac Function
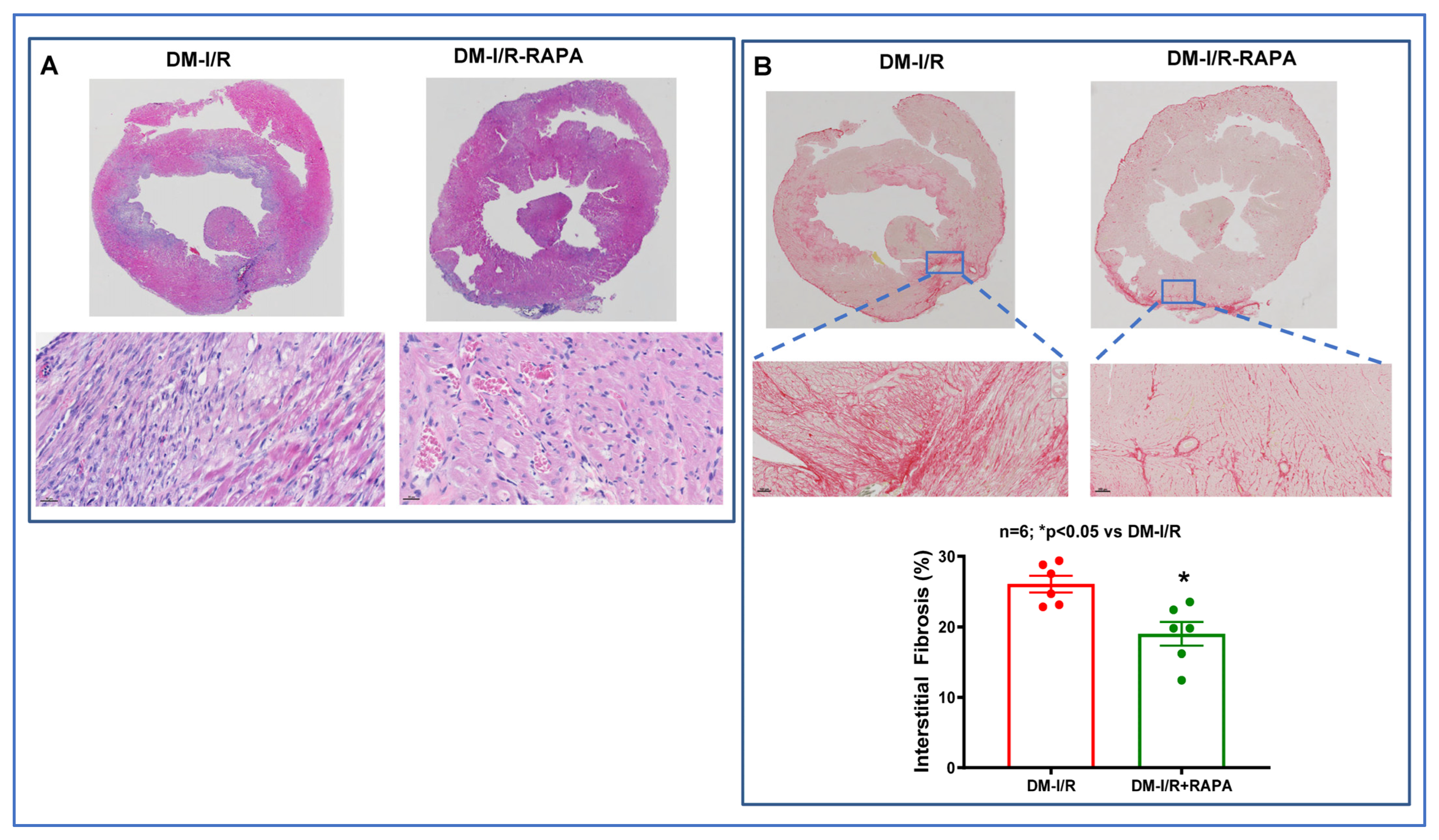
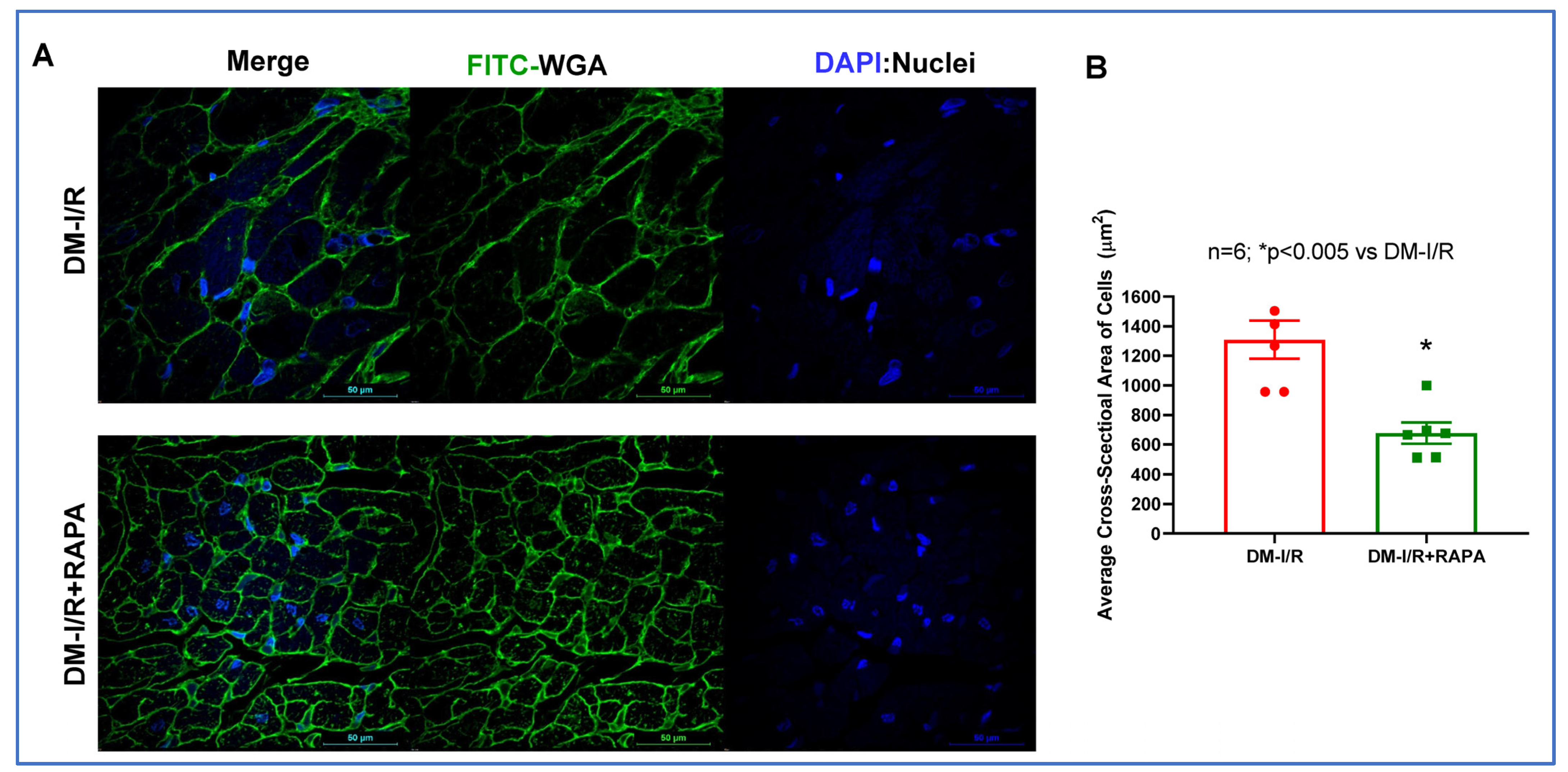
2.2. Rapamycin Reduces Cardiac Fibrosis after I/R Injury
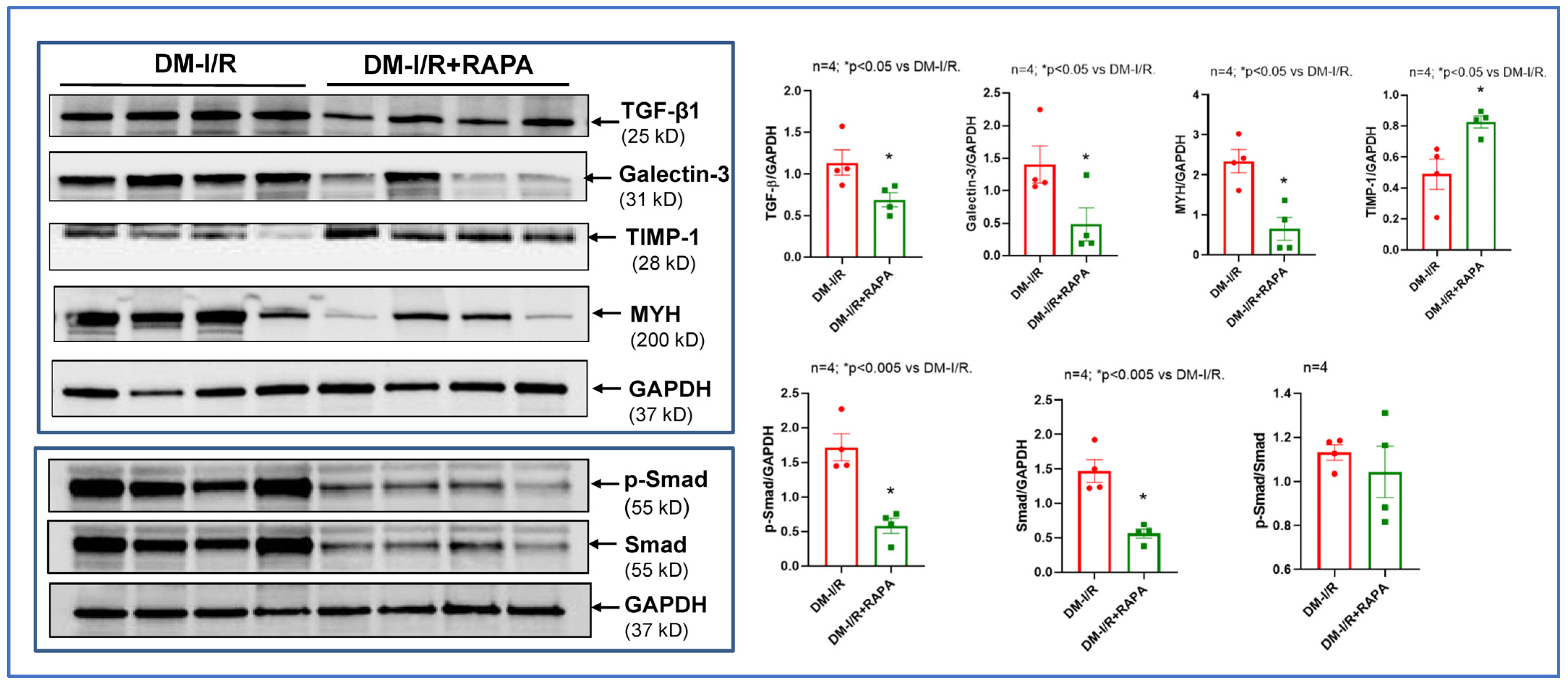
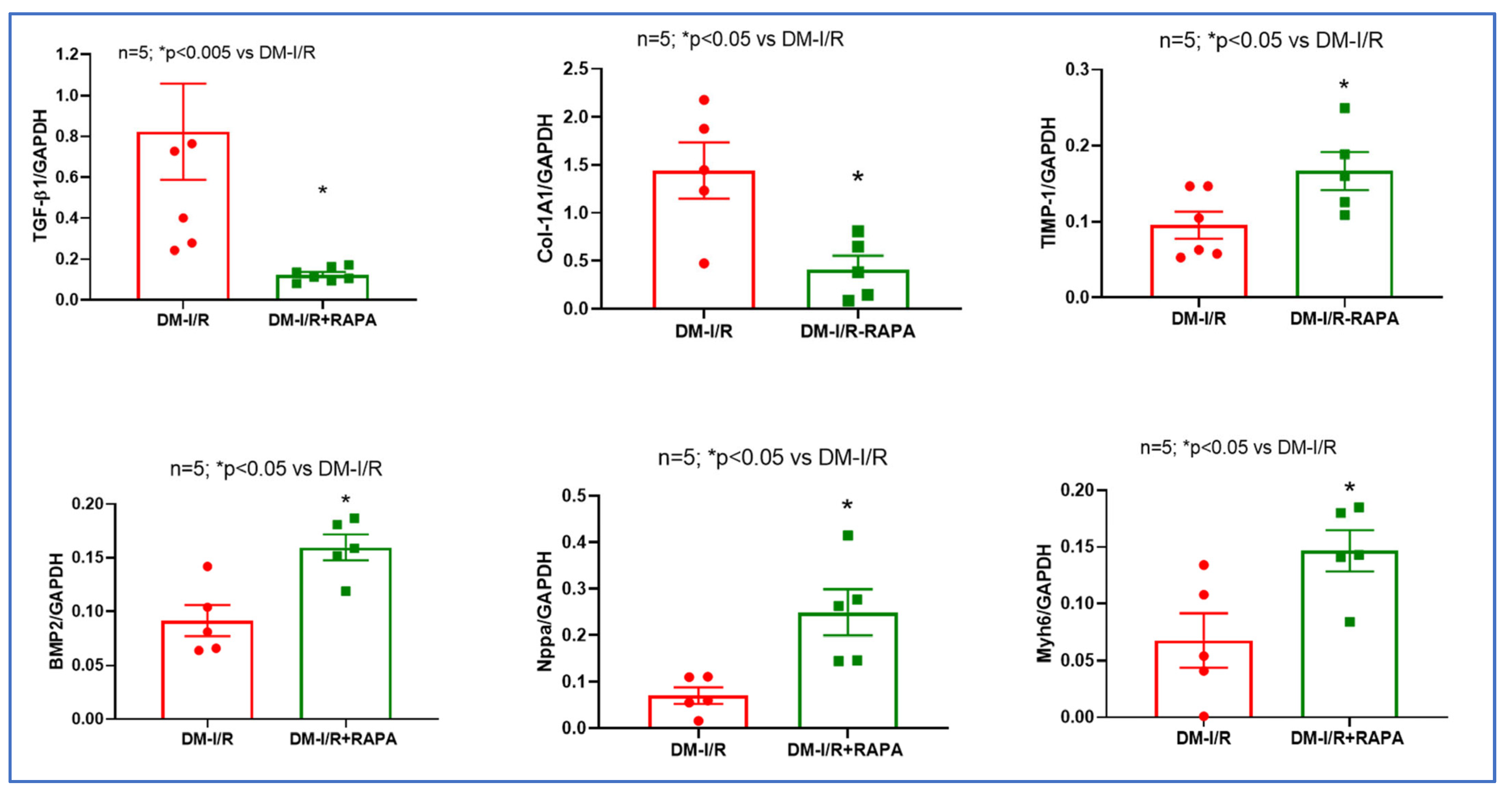
2.3. Rapamycin Alters the Expression of Multiple Fibrosis Markers
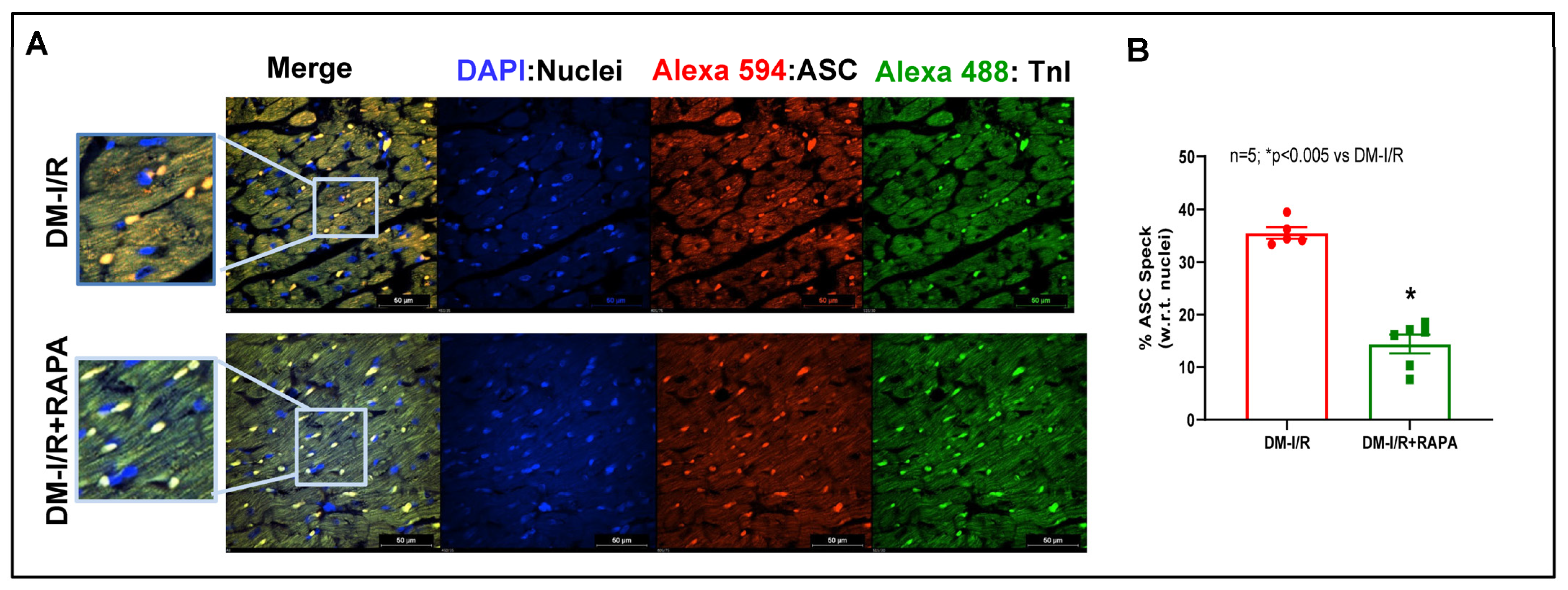
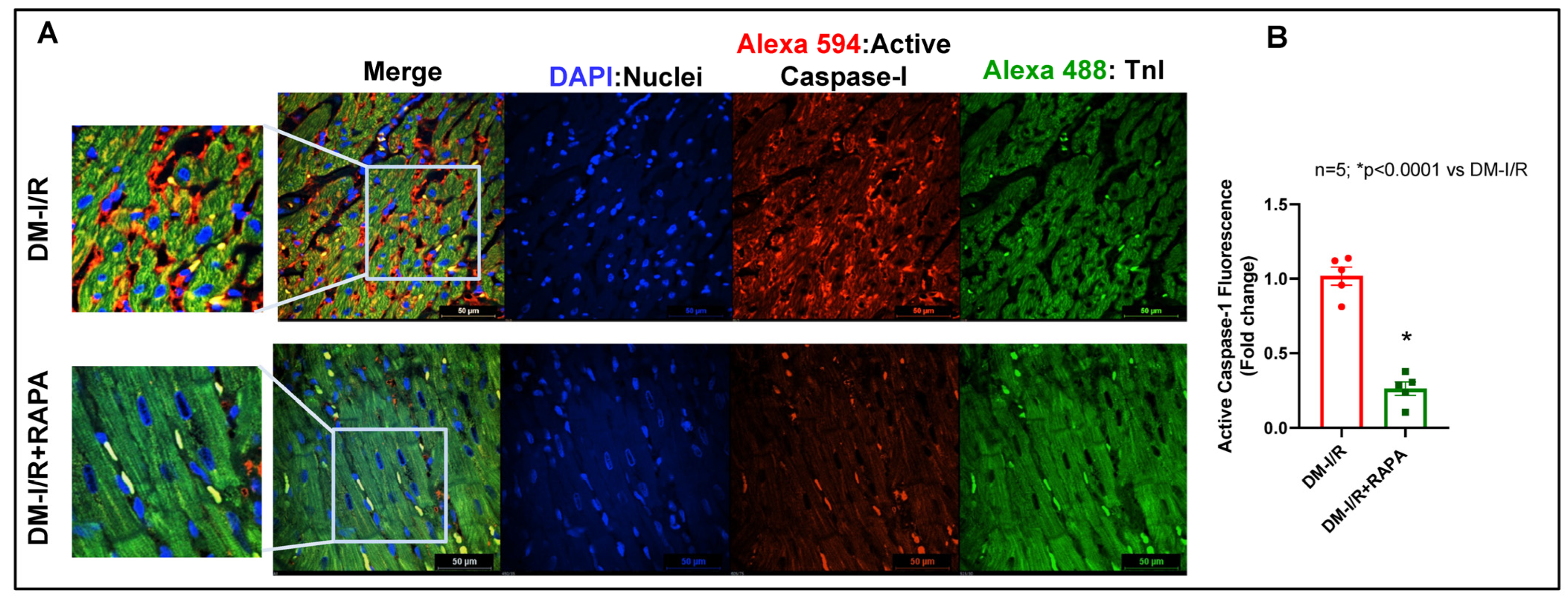
2.4. Rapamycin Attenuates NLRP3-Inflammasome following Ischemia/Reperfusion Injury
3. Discussion
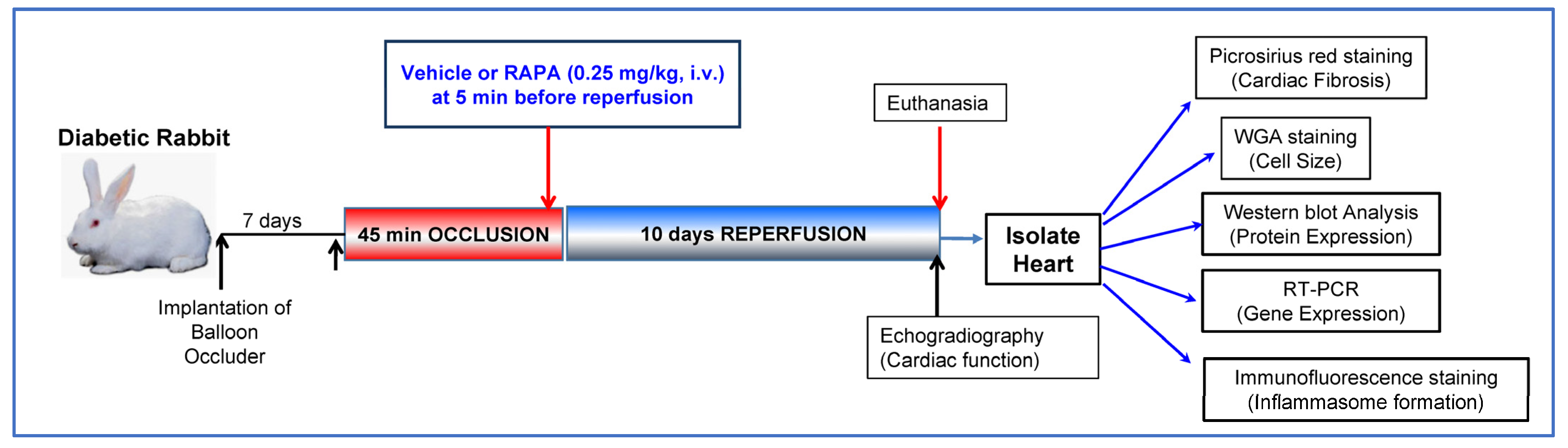
4. Material and Methods
4.1. Induction of Diabetes in Rabbit
4.2. Conscious Ischemia/Reperfusion Injury
4.3. Assessment of Cardiac Function
4.4. Cardiac Fibrosis Measurement
4.5. Wheat Germ Agglutinin (WGA) Staining
4.6. Western Blot Analysis
4.7. RNA Isolation and mRNA Expression
4.8. Immunofluorescence Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gyldenkerne, C.; Olesen, K.K.W.; Madsen, M.; Thim, T.; Jensen, L.O.; Raungaard, B.; Sørensen, H.T.; Bøtker, H.E.; Maeng, M. Extent of coronary artery disease is associated with myocardial infarction and mortality in patients with diabetes mellitus. Clin. Epidemiol. 2019, 11, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Lupón, J.; Barallat, J.; de Antonio, M.; Domingo, M.; Zamora, E.; Moliner, P.; Galán, A.; Santesmases, J.; Pastor, C.; et al. Impact of diabetes on the predictive value of heart failure biomarkers. Cardiovasc. Diabetol. 2016, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Moliner, P.; Mauricio, D. Pathogenesis, Clinical Features and Treatment of Diabetic Cardiomyopathy. Adv. Exp. Med. Biol. 2017, 1067, 197–217. [Google Scholar] [CrossRef]
- Zou, R.; Nie, C.; Pan, S.; Wang, B.; Hong, X.; Xi, S.; Bai, J.; Yu, M.; Liu, J.; Yang, W. Co-administration of hydrogen and metformin exerts cardioprotective effects by inhibiting pyroptosis and fibrosis in diabetic cardiomyopathy. Free. Radic. Biol. Med. 2022, 183, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Salloum, F.; Filippone, S.M.; Durrant, D.E.; Rokosh, G.; Bolli, R.; Kukreja, R.C. Inhibition of mammalian target of rapamycin protects against reperfusion injury in diabetic heart through STAT3 signaling. Basic Res. Cardiol. 2015, 110, 31. [Google Scholar] [CrossRef] [PubMed]
- Samidurai, A.; Roh, S.K.; Prakash, M.; Durrant, D.; Salloum, F.N.; Kukreja, R.C.; Das, A. STAT3-miR-17/20 signalling axis plays a critical role in attenuating myocardial infarction following rapamycin treatment in diabetic mice. Cardiovasc. Res. 2020, 116, 2103–2115. [Google Scholar] [CrossRef]
- Samidurai, A.; Salloum, F.N.; Durrant, D.; Chernova, O.B.; Kukreja, R.C.; Das, A. Chronic treatment with novel nanoformulated micelles of rapamycin, Rapatar, protects diabetic heart against ischaemia/reperfusion injury. Br. J. Pharmacol. 2017, 174, 4771–4784. [Google Scholar] [CrossRef]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.-I.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a Binding Partner of Target of Rapamycin (TOR), Mediates TOR Action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef]
- Das, A.; Salloum, F.N.; Durrant, D.; Ockaili, R.; Kukreja, R.C. Rapamycin protects against myocardial ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. J. Mol. Cell. Cardiol. 2012, 53, 858–869. [Google Scholar] [CrossRef]
- Filippone, S.M.; Samidurai, A.; Roh, S.K.; Cain, C.K.; He, J.; Salloum, F.N.; Kukreja, R.C.; Das, A. Reperfusion Therapy with Rapamycin Attenuates Myocardial Infarction through Activation of AKT and ERK. Oxidative Med. Cell. Longev. 2017, 2017, 4619720. [Google Scholar] [CrossRef]
- Khan, S.; Salloum, F.; Das, A.; Xi, L.; Vetrovec, G.W.; Kukreja, R.C. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J. Mol. Cell. Cardiol. 2006, 41, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Völkers, M.; Konstandin, M.H.; Doroudgar, S.; Toko, H.; Quijada, P.; Din, S.; Joyo, A.; Ornelas, L.; Samse, K.; Thuerauf, N.J.; et al. Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation 2013, 128, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Koka, S.; Salloum, F.N.; Xi, L.; Kukreja, R.C. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: Potential role of attenuated oxidative stress and altered contractile protein expression. J. Biol. Chem. 2014, 289, 4145–4160. [Google Scholar] [CrossRef] [PubMed]
- Shum, M.; Bellmann, K.; St-Pierre, P.; Marette, A. Pharmacological inhibition of S6K1 increases glucose metabolism and Akt signalling in vitro and in diet-induced obese mice. Diabetologia 2016, 59, 592–603. [Google Scholar] [CrossRef]
- Völkers, M.; Doroudgar, S.; Nguyen, N.; Konstandin, M.H.; Quijada, P.; Din, S.; Ornelas, L.; Thuerauf, D.J.; Gude, N.; Friedrich, K.; et al. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol. Med. 2013, 6, 57–65. [Google Scholar] [CrossRef]
- Samidurai, A.; Ockaili, R.; Cain, C.; Roh, S.K.; Filippone, S.M.; Kraskauskas, D.; Kukreja, R.C.; Das, A. Differential Regulation of mTOR Complexes with miR-302a Attenuates Myocardial Reperfusion Injury in Diabetes. iScience 2020, 23, 101863. [Google Scholar] [CrossRef]
- Mao, S.; Chen, P.; Pan, W.; Gao, L.; Zhang, M. Exacerbated post-infarct pathological myocardial remodelling in diabetes is associated with impaired autophagy and aggravated NLRP3 inflammasome activation. ESC Heart Fail. 2022, 9, 303–317. [Google Scholar] [CrossRef]
- Selvarajah, B.; Azuelos, I.; Plate, M.; Guillotin, D.; Forty, E.J.; Contento, G.; Woodcock, H.V.; Redding, M.; Taylor, A.; Brunori, G.; et al. mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-beta(1)-induced collagen biosynthesis. Sci. Signal. 2019, 12, eaav3048. [Google Scholar] [CrossRef]
- Lian, H.; Ma, Y.; Feng, J.; Dong, W.; Yang, Q.; Lü, D.; Zhang, L. Heparin-Binding EGF-Like Growth Factor Induces Heart Interstitial Fibrosis via an Akt/mTor/p70s6k Pathway. PLoS ONE 2012, 7, e44946. [Google Scholar] [CrossRef]
- Buss, S.J.; Muenz, S.; Riffel, J.H.; Malekar, P.; Hagenmueller, M.; Weiss, C.S.; Bea, F.; Bekeredjian, R.; Schinke-Braun, M.; Izumo, S.; et al. Beneficial Effects of Mammalian Target of Rapamycin Inhibition on Left Ventricular Remodeling After Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 54, 2435–2446. [Google Scholar] [CrossRef]
- Debonnaire, P.; Van De Heyning, C.M.; El Haddad, M.; Coussement, P.; Paelinck, B.; de Ceuninck, M.; Timmermans, F.; De Bock, D.; Drieghe, B.; Dujardin, K.; et al. Left Ventricular End-Systolic Dimension and Outcome in Patients With Heart Failure Undergoing Percutaneous MitraClip Valve Repair for Secondary Mitral Regurgitation. Am. J. Cardiol. 2020, 126, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.C.; Riley, L.A.; Mallory, N.T.; Bersi, M.R.; Umbarkar, P.; Gautam, R.; Zhang, Q.; Mahadevan-Jansen, A.; Hatzopoulos, A.K.; Maroteaux, L.; et al. Targeting 5-HT2B Receptor Signaling Prevents Border Zone Expansion and Improves Microstructural Remodeling After Myocardial Infarction. Circulation 2021, 143, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Borne, S.W.M.V.D.; Diez, J.; Blankesteijn, W.M.; Verjans, J.; Hofstra, L.; Narula, J. Myocardial remodeling after infarction: The role of myofibroblasts. Nat. Rev. Cardiol. 2010, 7, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; de Juan Abad, B.L.; Cheng, K. Cardiac fibrosis: Myofibroblast-mediated pathological regulation and drug delivery strategies. Adv. Drug Deliv. Rev. 2021, 173, 504–519. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef]
- Ramachandran, A.; Vizan, P.; Das, D.; Chakravarty, P.; Vogt, J.; Rogers, K.W.; Muller, P.; Hinck, A.P.; Sapkota, G.P.; Hill, C.S. TGF-beta uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. eLife 2018, 7, e31756. [Google Scholar] [CrossRef]
- Bujak, M.; Ren, G.; Kweon, H.J.; Dobaczewski, M.; Reddy, A.; Taffet, G.; Wang, X.F.; Frangogiannis, N.G. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 2007, 116, 2127–2138. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.J.; et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Zhang, W.; Zhang, J.; Yan, Y.; Dong, W.; Liang, E.; Zhang, Y.; Zhang, M. Inhibition of MEF2A prevents hyperglycemia-induced extracellular matrix accumulation by blocking Akt and TGF-beta1/Smad activation in cardiac fibroblasts. Int. J. Biochem. Cell Biol. 2015, 69, 52–61. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; Van Brakel, T.J.; van Berlo, J.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- de Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ramirez, R.; Azpiri-Lopez, J.R.; Gonzalez-Gonzalez, J.G.; Ordaz-Farias, A.; Gonzalez-Carrillo, L.E.; Carrizales-Sepulveda, E.F.; Vera-Pineda, R. Global longitudinal strain as a biomarker in diabetic cardiomyopathy. A comparative study with Gal-3 in patients with preserved ejection fraction. Arch. Cardiol. Mex. 2017, 87, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.; Gonzalez, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; San Jose, G.; Querejeta, R.; Diez, J. Circulating Biomarkers of Myocardial Fibrosis: The Need for a Reappraisal. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, D.A.; Lyasnikova, E.A.; Vasilyeva, E.Y.; Babenko, A.Y.; Shlyakhto, E.V. Type 2 Diabetes Mellitus and Chronic Heart Failure with Midrange and Preserved Ejection Fraction: A Focus on Serum Biomarkers of Fibrosis. J. Diabetes Res. 2020, 2020, 6976153. [Google Scholar] [CrossRef]
- Kremastiotis, G.; Handa, I.; Jackson, C.; George, S.; Johnson, J. Disparate effects of MMP and TIMP modulation on coronary atherosclerosis and associated myocardial fibrosis. Sci. Rep. 2021, 11, 23081. [Google Scholar] [CrossRef]
- Cao, J.-W.; Duan, S.-Y.; Zhang, H.-X.; Chen, Y.; Guo, M. Zinc Deficiency Promoted Fibrosis via ROS and TIMP/MMPs in the Myocardium of Mice. Biol. Trace Element Res. 2020, 196, 145–152. [Google Scholar] [CrossRef]
- Roten, L.; Nemoto, S.; Simsic, J.; Coker, M.L.; Rao, V.; Baicu, S.; Defreyte, G.; Soloway, P.J.; Zile, M.R.; Spinale, F.G. Effects of Gene Deletion of the Tissue Inhibitor of the Matrix Metalloproteinase-type 1 (TIMP-1) on Left Ventricular Geometry and Function in Mice. J. Mol. Cell. Cardiol. 2000, 32, 109–120. [Google Scholar] [CrossRef]
- Ikonomidis, J.S.; Hendrick, J.W.; Parkhurst, A.M.; Herron, A.R.; Escobar, P.G.; Dowdy, K.B.; Stroud, R.E.; Hapke, E.; Zile, M.; Spinale, F.G. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: Effects of exogenous MMP inhibition. Am. J. Physiol. Circ. Physiol. 2005, 288, H149–H158. [Google Scholar] [CrossRef]
- Mercadier, J.J.; Lompré, A.M.; Wisnewsky, C.; Samuel, J.L.; Bercovici, J.; Swynghedauw, B.; Schwartz, K. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ. Res. 1981, 49, 525–532. [Google Scholar] [CrossRef]
- Razeghi, P.; Essop, M.F.; Huss, J.M.; Abbasi, S.; Manga, N.; Taegtmeyer, H. Hypoxia-induced switches of myosin heavy chain iso-gene expression in rat heart. Biochem. Biophys. Res. Commun. 2003, 303, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Carniel, E.; Taylor, M.R.; Sinagra, G.; Di Lenarda, A.; Ku, L.; Fain, P.R.; Boucek, M.M.; Cavanaugh, J.; Miocic, S.; Slavov, D.; et al. Alpha-myosin heavy chain: A sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation 2005, 112, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Pope, B.; Hoh, J.; Weeds, A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980, 118, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Schunkert, H.; Isoyama, S.; Wei, J.; Nadal-Ginard, B.; Grossman, W.; Izumo, S. Age-related differences in the expression of proto-oncogene and contractile protein genes in response to pressure overload in the rat myocardium. J. Clin. Investig. 1992, 89, 939–946. [Google Scholar] [CrossRef]
- Buttrick, P.; Perla, C.; Malhotra, A.; Geenen, D.; Lahorra, M.; Scheuer, J. Effects of chronic dobutamine on cardiac mechanics and biochemistry after myocardial infarction in rats. Am. J. Physiol. Content 1991, 260, H473–H479. [Google Scholar] [CrossRef]
- Reiser, P.J.; Portman, M.A.; Ning, X.-H.; Moravec, C.S. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. Circ. Physiol. 2001, 280, H1814–H1820. [Google Scholar] [CrossRef]
- Forte, M.; Marchitti, S.; Di Nonno, F.; Stanzione, R.; Schirone, L.; Cotugno, M.; Bianchi, F.; Schiavon, S.; Raffa, S.; Ranieri, D.; et al. NPPA/atrial natriuretic peptide is an extracellular modulator of autophagy in the heart. Autophagy 2023, 19, 1087–1099. [Google Scholar] [CrossRef]
- Feng, J.A.; Perry, G.; Mori, T.; Hayashi, T.; Oparil, S.; Chen, Y.-F. Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin. Exp. Pharmacol. Physiol. 2003, 30, 343–349. [Google Scholar] [CrossRef]
- Mori, T.; Chen, Y.-F.; Feng, J.A.; Hayashi, T.; Oparil, S.; Perry, G.J. Volume overload results in exaggerated cardiac hypertrophy in the atrial natriuretic peptide knockout mouse. Cardiovasc. Res. 2004, 61, 771–779. [Google Scholar] [CrossRef]
- Oliver, P.M.; Fox, J.E.; Kim, R.; Rockman, H.A.; Kim, H.-S.; Reddick, R.L.; Pandey, K.N.; Milgram, S.L.; Smithies, O.; Maeda, N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc. Natl. Acad. Sci. USA 1997, 94, 14730–14735. [Google Scholar] [CrossRef]
- Knowles, J.W.; Esposito, G.; Mao, L.; Hagaman, J.R.; Fox, J.E.; Smithies, O.; Rockman, H.A.; Maeda, N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A–deficient mice. J. Clin. Investig. 2001, 107, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Kuga, H.; Ogawa, K.; Oida, A.; Taguchi, I.; Nakatsugawa, M.; Hoshi, T.; Sugimura, H.; Abe, S.; Kaneko, N. Administration of Atrial Natriuretic Peptide Attenuates Reperfusion Phenomena and Preserves Left Ventricular Regional Wall Motion After Direct Coronary Angioplasty for Acute Myocardial Infarction. Circ. J. 2003, 67, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kasama, S.; Furuya, M.; Toyama, T.; Ichikawa, S.; Kurabayashi, M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur. Heart J. 2008, 29, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Tsutamoto, T.; Wada, A.; Maeda, K.; Mabuchi, N.; Tsutsui, T.; Horie, H.; Ohnishi, M.; Kinoshita, M. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 37, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- A Schleutermann, D.; Murdoch, J.L.; A Walker, B.; Bias, W.B.; A Chase, G.; Freidhoff, L.B.; A McKusick, V. A linkage study of the Marfan syndrome. Clin. Genet. 1976, 10, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Even, P.; Bloch-Faure, M.; Guerre-Millo, M.; Moustaid-Moussa, N.; Ferre, P.; Quignard-Boulange, A. Deletion of the Angiotensin Type 2 Receptor (AT2R) Reduces Adipose Cell Size and Protects From Diet-Induced Obesity and Insulin Resistance. Diabetes 2005, 54, 991–999. [Google Scholar] [CrossRef]
- Uehlinger, D.E.; Weidmann, P.; Gnädinger, M.P.; Hasler, L.; Bachmann, C.; Shaw, S.; Hellmüller, B.; Lang, R.E. Increase in Circulating Insulin Induced by Atrial Natriuretic Peptide in Normal Humans. J. Cardiovasc. Pharmacol. 1986, 8, 1122–1129. [Google Scholar] [CrossRef]
- Perera, N.; Ritchie, R.H.; Tate, M. The Role of Bone Morphogenetic Proteins in Diabetic Complications. ACS Pharmacol. Transl. Sci. 2020, 3, 11–20. [Google Scholar] [CrossRef]
- Schreiber, I.; Dorpholz, G.; Ott, C.E.; Kragesteen, B.; Schanze, N.; Lee, C.T.; Kohrle, J.; Mundlos, S.; Ruschke, K.; Knaus, P. BMPs as new insulin sensitizers: Enhanced glucose uptake in mature 3T3-L1 adipocytes via PPARgamma and GLUT4 upregulation. Sci. Rep. 2017, 7, 17192. [Google Scholar] [CrossRef]
- Wang, H.T.; Liu, C.F.; Tsai, T.H.; Chen, Y.L.; Chang, H.W.; Tsai, C.Y.; Leu, S.; Zhen, Y.Y.; Chai, H.T.; Chung, S.Y.; et al. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J. Transl. Med. 2012, 10, 145. [Google Scholar] [CrossRef]
- Che, H.; Wang, Y.; Li, H.; Li, Y.; Sahil, A.; Lv, J.; Liu, Y.; Yang, Z.; Dong, R.; Xue, H.; et al. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-beta1/Smads signaling in diabetic cardiomyopathy. FASEB J. 2020, 34, 5282–5298. [Google Scholar] [CrossRef] [PubMed]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gardner, S.E.; Clarke, M. Cell Death, Damage-Associated Molecular Patterns, and Sterile Inflammation in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2011, 31, 2781–2786. [Google Scholar] [CrossRef]
- Ansley, D.M.; Wang, B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013, 229, 232–241. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Dang, S.P.; Li, S.S.; Liu, Y.; Qi, M.M.; Wang, N.; Miao, L.F.; Wu, Y.; Li, X.Y.; Wang, C.X.; et al. Glucose Fluctuations Aggravate Myocardial Fibrosis via the Nuclear Factor-kappaB-Mediated Nucleotide-Binding Oligomerization Domain-Like Receptor Protein 3 Inflammasome Activation. Front. Cardiovasc. Med. 2022, 9, 748183. [Google Scholar] [CrossRef]
- Stienstra, R.; van Diepen, J.A.; Tack, C.J.; Zaki, M.H.; van de Veerdonk, F.L.; Perera, D.; Neale, G.A.; Hooiveld, G.J.; Hijmans, A.; Vroegrijk, I.; et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Qiu, Z.; Lei, S.; Zhao, B.; Wu, Y.; Su, W.; Liu, M.; Meng, Q.; Zhou, B.; Leng, Y.; Xia, Z.-Y. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxidative Med. Cell. Longev. 2017, 2017, 9743280. [Google Scholar] [CrossRef]
- Yu, Y.W.; Que, J.Q.; Liu, S.; Huang, K.Y.; Qian, L.; Weng, Y.B.; Rong, F.N.; Wang, L.; Zhou, Y.Y.; Xue, Y.J.; et al. Sodium-Glucose Co-transporter-2 Inhibitor of Dapagliflozin Attenuates Myocardial Ischemia/Reperfusion Injury by Limiting NLRP3 Inflammasome Activation and Modulating Autophagy. Front. Cardiovasc. Med. 2021, 8, 768214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, R.; Mo, Y.; Zhang, Q.; Sherwood, L.C.; Chien, S. Creating a Long-Term Diabetic Rabbit Model. Exp. Diabetes Res. 2010, 2010, 289614. [Google Scholar] [CrossRef] [PubMed]
- Klocke, R.; Tian, W.; Kuhlmann, M.T.; Nikol, S. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc. Res. 2007, 74, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-M.; Dart, A.M.; Dewar, E.; Jennings, G.; Du, X.-J. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc. Res. 2000, 45, 330–338. [Google Scholar] [CrossRef]
- Saleh, M.G.; Sharp, S.-K.; Alhamud, A.; Spottiswoode, B.S.; van der Kouwe, A.J.W.; Davies, N.H.; Franz, T.; Meintjes, E.M. Long-Term Left Ventricular Remodelling in Rat Model of Nonreperfused Myocardial Infarction: Sequential MR Imaging Using a 3T Clinical Scanner. J. Biomed. Biotechnol. 2012, 2012, 504037. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, Y.; Wang, H.; Chen, F.; Ye, Y.; Jin, L.; Marchal, G.; Ni, Y. A modified rabbit model of reperfused myocardial infarction for cardiac MR imaging research. Int. J. Cardiovasc. Imaging 2009, 25, 289–298. [Google Scholar] [CrossRef]
- Feng, Y.; Bogaert, J.; Oyen, R.; Ni, Y. An overview on development and application of an experimental platform for quantitative cardiac imaging research in rabbit models of myocardial infarction. Quant. Imaging Med. Surg. 2014, 4, 358–375. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, F.; Ma, Z.; Dekeyzer, F.; Yu, J.; Xie, Y.; Cona, M.M.; Oyen, R.; Ni, Y. Towards Stratifying Ischemic Components by Cardiac MRI and Multifunctional Stainings in a Rabbit Model of Myocardial Infarction. Theranostics 2013, 4, 24–35. [Google Scholar] [CrossRef]
- Feng, Y.; Hemmeryckx, B.; Frederix, L.; Lox, M.; Wu, J.; Heggermont, W.; Lu, H.R.; Gallacher, D.; Oyen, R.; Lijnen, H.R.; et al. Monitoring reperfused myocardial infarction with delayed left ventricular systolic dysfunction in rabbits by longitudinal imaging. Quant. Imaging Med. Surg. 2018, 8, 754–769. [Google Scholar] [CrossRef]
- Samidurai, A.; Ockaili, R.; Cain, C.; Roh, S.K.; Filippone, S.M.; Kraskauskas, D.; Kukreja, R.C.; Das, A. Preclinical model of type 1 diabetes and myocardial ischemia/reperfusion injury in conscious rabbits—Demonstration of cardioprotection with rapamycin. STAR Protoc. 2021, 2, 100772. [Google Scholar] [CrossRef]
- Jones, S.P.; Tang, X.L.; Guo, Y.; Steenbergen, C.; Lefer, D.J.; Kukreja, R.C.; Kong, M.; Li, Q.; Bhushan, S.; Zhu, X.; et al. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): A new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ. Res. 2015, 116, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Cain, C.; Mauro, A.G.; Romeo, F.; Ockaili, R.; Chau, V.Q.; Nestler, J.A.; Devarakonda, T.; Ghosh, S.; Das, A.; et al. Sacubitril/Valsartan Averts Adverse Post-Infarction Ventricular Remodeling and Preserves Systolic Function in Rabbits. J. Am. Coll. Cardiol. 2018, 72, 2342–2356. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samidurai, A.; Saravanan, M.; Ockaili, R.; Kraskauskas, D.; Lau, S.Y.V.; Kodali, V.; Ramasamy, S.; Bhoopathi, K.; Nair, M.; Roh, S.K.; et al. Single-Dose Treatment with Rapamycin Preserves Post-Ischemic Cardiac Function through Attenuation of Fibrosis and Inflammation in Diabetic Rabbit. Int. J. Mol. Sci. 2023, 24, 8998. https://doi.org/10.3390/ijms24108998
Samidurai A, Saravanan M, Ockaili R, Kraskauskas D, Lau SYV, Kodali V, Ramasamy S, Bhoopathi K, Nair M, Roh SK, et al. Single-Dose Treatment with Rapamycin Preserves Post-Ischemic Cardiac Function through Attenuation of Fibrosis and Inflammation in Diabetic Rabbit. International Journal of Molecular Sciences. 2023; 24(10):8998. https://doi.org/10.3390/ijms24108998
Chicago/Turabian StyleSamidurai, Arun, Manu Saravanan, Ramzi Ockaili, Donatas Kraskauskas, Suet Ying Valerie Lau, Varun Kodali, Shakthi Ramasamy, Karthikeya Bhoopathi, Megha Nair, Sean K. Roh, and et al. 2023. "Single-Dose Treatment with Rapamycin Preserves Post-Ischemic Cardiac Function through Attenuation of Fibrosis and Inflammation in Diabetic Rabbit" International Journal of Molecular Sciences 24, no. 10: 8998. https://doi.org/10.3390/ijms24108998
APA StyleSamidurai, A., Saravanan, M., Ockaili, R., Kraskauskas, D., Lau, S. Y. V., Kodali, V., Ramasamy, S., Bhoopathi, K., Nair, M., Roh, S. K., Kukreja, R. C., & Das, A. (2023). Single-Dose Treatment with Rapamycin Preserves Post-Ischemic Cardiac Function through Attenuation of Fibrosis and Inflammation in Diabetic Rabbit. International Journal of Molecular Sciences, 24(10), 8998. https://doi.org/10.3390/ijms24108998







