Abstract
Huanglongbing, a globally devastating citrus disease, is associated with Candidatus Liberibacter asiaticus (CLas) and is mainly transmitted by Diaphorina citri. Verification of the distribution and dynamics of CLas in D. citri is critical to understanding CLas transmitted by vectors in nature. Here, the distribution and titers of CLas in different sexes and tissues of D. citri adults were investigated by fluorescence in-situ hybridization (FISH) and quantitative real-time PCR (qRT-PCR). Results showed that CLas had widespread distribution in the brain, salivary glands, digestive system, and reproductive system of both females and males, indicating a systemic infection of CLas in D. citri. Moreover, CLas fluorescence intensity and titers were significantly increased in both the digestive system and the female reproductive system with development and there was a marked decreased in both the salivary glands and the male brain, but there was no significant change in the female brain or the male reproductive system. Furthermore, the distribution and dynamics of CLas in embryos and nymphs were investigated. CLas was observed in all laid eggs and subsequent first–second-instar nymphs, indicating that a high percentage of embryos and nymphs resulting from infected D. citri mothers were infected with CLas.
1. Introduction
Huanglongbing (HLB), also called citrus greening disease, is the most fatal disease in citrus varieties worldwide, which results in fruit drop, reduced fruit quality, and twig-and-branch dieback [1,2]. HLB is associated with three known Gram-negative, phloem-limited bacteria: Candidatus Liberibacter africanus (CLaf), Candidatus L. asiaticus (CLas), and Candidatus L. americanus (CLam). Among them, CLas is the most prevalent pathogen and is transmitted by D. citri Kuwayama (Hemiptera: Psyllidae) [3], causing substantial economic losses in citrus-growing regions in Asia, North America, and Brazil in South America [1]. Currently, controlling the insect vector is the most critical step for HLB prevention and management since CLas is non-culturable in vitro.
CLas is transmitted by D. citri in a persistent and propagative manner to complete its horizontal transmission from CLas-positive plants to healthy plants [4]. After ingestion of CLas-positive sap by psyllids, CLas first colonizes and propagates in the digestive tract, then moves through the haemocoel, and eventually reaches the salivary glands of D. citri, where it is inoculated into a new plant during feeding [5,6]. During the circulative journey, CLas breaks multiple barriers, such as the midgut-infection barrier, dissemination barrier, and salivary-escape barrier. Using fluorescence in-situ hybridization (FISH) or quantitative polymerase chain reaction (qPCR), several investigations have been conducted to understand CLas colonizing in most D. citri tissues, such as the filter chamber, midgut, salivary glands, fat body, ovaries, etc. [5,7,8,9,10]. More recently, Chanim et al. [11] used FISH and immunogold labeling to describe CLas accumulation in the cells of psyllid midgut. CLas was observed to be in abundance in tubular and quasispherical forms in various psyllid organs by transmission electron microscopy and immunogold labeling [9]. However, previous studies mainly focused on the detection of CLas at the early stage of D. citri. Little is known about the distribution and titers of CLas in different sexes and tissues of D. citri adults with development.
Transovarial transmission of microbes such as fungi, bacteria, and viruses from mother to offspring is a common phenomenon in nature that has great significance to maintaining a source of infection and its epidemiology [12,13,14,15]. Depending on the transmission mode, microbes are classed into four categories: non-persistent, semi-persistent, circulative–non-propagative, and circulative–propagative. So far, all circulative–propagative viruses, such as reovirus and tenuivirus, and a few circulative–nonpropagative viruses, such as begomoviruses, have been confirmed to transovarially transmit [15,16]. CLas is a typical propagative–circulative bacteria, and a low rate of transovarial transmission by D. citri was shown in previous studies [17,18]. As only 3.6–18% of offspring derived from CLas-positive adults were identified by qPCR, transovarial transmission appears to contribute very little to CLas transmission [17,18]. However, there is no research about the distribution and dynamics of CLas in eggs and, subsequently, nymphs derived from females reared on CLas-positive plants.
In the current study, we combined FISH and quantitative real-time PCR (qRT-PCR) technology to investigate the distribution and titers of CLas in different stages of embryos, nymphs, and adults of D. citri.
2. Results
2.1. Distribution and Relative Titers of CLas in CLas-Positive Adult Males
The distribution and relative titers of CLas in CLas-positive adult males were investigated by FISH and RT-PCR. FISH results showed that CLas was detected in the brain and salivary glands of adult males at 5 days after eclosion (5 DAE), 10 DAE, and 15 DAE, and was not found in the brain or salivary glands of CLas-negative males at 15 DAE (Figure 1A). CLas fluorescence intensity was significantly decreased in the brain (F2,11 = 24.93; p < 0.001) and salivary glands (F2,11 = 18.24; p < 0.001) with development (Figure 1B,C). Moreover, the relative CLas titers showed a gradual decreasing trend in both the brain (F2,11 = 5.54; p < 0.05) and the salivary glands (F2,11 = 49.24; p < 0.001) (Figure 1D,E).
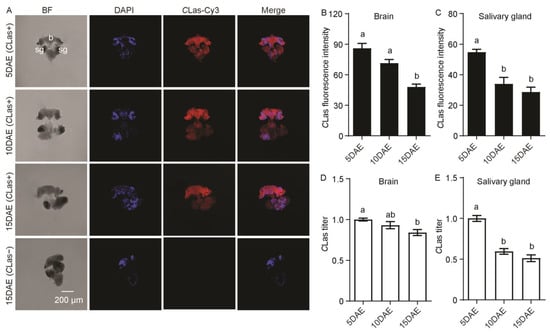
Figure 1.
Analysis of distribution and relative titers of CLas in the brain and salivary glands of CLas-positive adult males by FISH and RT-PCR. (A) Representative confocal images of CLas distribution in the brain and salivary glands of CLas-positive adult males at 5, 10, and 15 DAE and CLas-negative adult males at 15 DAE. b: brain, sg: salivary gland. DAE: days after eclosion, CLas-: CLas-negative, CLas+: CLas-positive. Scale bar = 200 μm, BF: imaging of bright field allowed for identification of regions in different tissues; DAPI: the cell nuclei was stained with DAPI and visualized in blue. CLas-Cy3: The CLas signal was marked by Cy3 and visualized in red. Merge: merged imaging of co-localization of cell nuclei and CLas. (B) CLas fluorescence intensity in male brain. (C) CLas fluorescence intensity in male salivary glands. (D) Relative CLas titer in male brain. (E) Relative CLas titer in male salivary glands. The different letters at the top of the columns in (B–E) figures indicate statistically significant differences (in all cases p < 0.05).
In addition, CLas had a widespread distribution in the male gut and Malphigian tubules (Figure 2A). CLas was not detected in the gut or Malphigian tubules of CLas-negative males at 15 DAE (Figure 2A). Along with the development of males, CLas fluorescence intensity was significantly increased in the Malphigian tubules (F2,11 = 14.89; p < 0.01), but there was no significant change in the gut (F2,11 = 3.22; p > 0.05) (Figure 2B,C). Moreover, the relative CLas titer in the digestive system obviously increased with development (F2,11 = 99.86; p < 0.001) (Figure 2D).
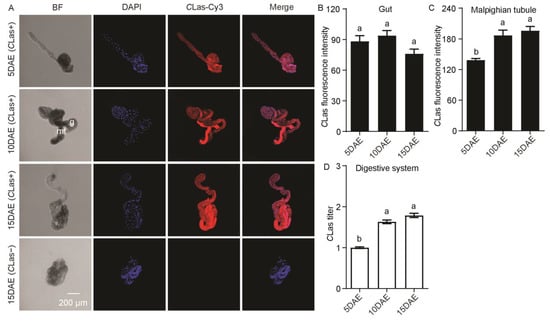
Figure 2.
Analysis of distribution and relative titer of CLas in CLas-positive adult-male digestive system by FISH and RT-PCR. (A) Representative confocal images of CLas distribution in the digestive system of CLas-positive males at 5 DAE, 10 DAE, and 15 DAE and CLas-negative adult males at 15 DAE. DAE: days after eclosion. g: gut, mt: Malpighian tubule. CLas-: CLas-negative, CLas+: CLas-positive. Scale bar is 200 μm. (B) CLas fluorescence intensity in male gut. (C) CLas fluorescence intensity in male Malpighian tubules. (D) Relative CLas titer in male digestive system. The different letters at the top of the columns in (B–D) figures indicate statistically significant differences (in all cases p < 0.05).
In Figure 3A, CLas was detected in the male reproductive system, including the testis and sperm sac. However, no CLas signal was detected in the testis and sperm sac of CLas-negative males at 15 DAE. For CLas fluorescence intensity in the male testis, there was no significant difference during development (F2,11 = 4.19; p > 0.05) (Figure 3B), but the fluorescence intensity of CLas in sperm sacs at 15 DAE was obviously weaker than those at 5 DAE and 10 DAE (F2,11 = 6.76; p < 0.05) (Figure 3C). As for the relative CLas titer, there was no significant difference in the male reproductive system with development (F2,11 = 4.01; p > 0.05) (Figure 3D).
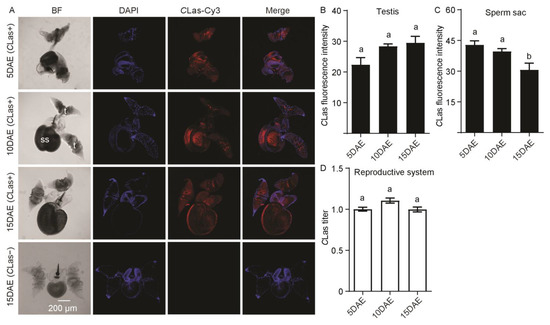
Figure 3.
Analysis of distribution and relative titer of CLas in CLas-positive male reproductive system by FISH and RT-PCR. (A) Representative confocal images of CLas distribution in the reproductive system of CLas-positive males at 5 DAE, 10 DAE, and 15 DAE and CLas-negative adult males at 15 DAE. DAE: days after eclosion. t: testis, ss: sperm sac. CLas-: CLas-negative, CLas+: CLas-positive. Scale bar is 200 μm. (B) CLas fluorescence intensity in male testis. (C) CLas fluorescence intensity in male sperm sac. (D) Relative CLas titer in male reproductive system. The different letters at the top of the columns in (B–D) figures indicate statistically significant differences (in all cases p < 0.05).
Taken together, CLas fluorescence intensity was highest in the Malpighian tubules, followed by the gut, brain, and sperm sac, and lowest in the salivary glands and testes of adult males (5 day-old males: F5,23 = 94.53, p < 0.001; 10 day-old males: F5,23 = 122.4, p < 0.001; 15 day-old males: F5,23 = 199.9, p < 0.001) (Figure S1A–C). The relative CLas titer was highest in the digestive system (5 day-old males: F3,15 = 35.53, p < 0.001; 10 day-old males: F3,15 = 89.94, p < 0.001; 15 day-old males: F3,15 = 404.4, p < 0.001) (Figure S1D,E).
2.2. Distribution and Relative Titers of CLas in CLas-Positive Adult Females
As shown in Figure 4A, CLas had widespread distribution in the CLas-positive adult female brain and salivary glands. There was no CLas signal in the CLas-negative adult female brain or salivary glands. In addition, fluorescence intensity (F2,11 = 0.74; p > 0.05) and the relative titer (F2,11 = 0.07; p > 0.05) of CLas in the female brain showed no significant difference during different developmental stages (Figure 4B,D), but those in the female salivary glands displayed a significant decrease at 10 DAE and 15 DAE compared with 5 DAE (F2,11 = 12.03; p < 0.01; F2,11 = 22.50; p < 0.001) (Figure 4C,E).
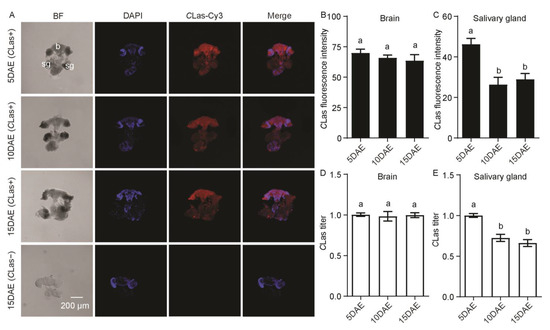
Figure 4.
Analysis of distribution and relative titers of CLas in CLas-positive adult female brain and salivary glands by FISH and RT-PCR. (A) Representative confocal images of CLas distribution in the brain and salivary glands of CLas-positive females at 5 DAE, 10 DAE, and 15 DAE and CLas-negative adult females at 15 DAE. DAE: days after eclosion. b: brain, sg: salivary gland. CLas−: CLas-negative, CLas+: CLas-positive. Scale bar is 200 μm. (B) CLas fluorescence intensity in female brain. (C) CLas fluorescence intensity in female salivary glands. (D) Relative CLas titer in female brain. (E) Relative CLas titer in female salivary glands. The different letters at the top of the columns in (B–E) figures indicate statistically significant differences (in all cases p < 0.05).
CLas was also found to localize in the gut and Malphigian tubules of CLas-positive females (Figure 5A). In addition, CLas was not detected in the gut or Malphigian tubules of CLas-negative females at 15 DAE (Figure 5A). There was no obvious difference in CLas fluorescence intensity in the female gut (F2,11 = 0.02; p > 0.05) (Figure 5B) but was significantly increased in the Malphigian tubules during development (F2,11 = 37.67; p < 0.001) (Figure 5C). In addition, the relative CLas titer in the female digestive system increased markedly with development (F2,11 = 31.67; p < 0.001) (Figure 5D).
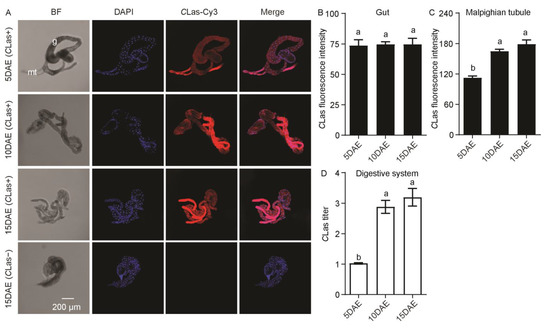
Figure 5.
Analysis of distribution and relative titer for CLas in CLas-positive adult female digestive system by FISH and RT-PCR. (A) Representative confocal images of CLas distribution in the digestive system of CLas-positive females at 5 DAE, 10 DAE, and 15 DAE and CLas-negative adult females at 15 DAE. DAE: days after eclosion. mt: Malpighian tubule, g: gut. CLas−: CLas-negative, CLas+: CLas-positive. Scale bar is 200 μm. (B) CLas fluorescence intensity in female gut. (C) CLas fluorescence intensity in female Malpighian tubules. (D) Relative CLas titer in female digestive system. The different letters at the top of the columns in (B–D) figures indicate statistically significant differences (in all cases p < 0.05).
CLas was also detected in the female reproductive system, which includes the ovaries and spermathecae, but was not detected in the ovaries or spermathecae of CLas-negative females at 15 DAE (Figure 6A). Most of the ovaries at 5 DAE were small and all oocytes were at the pre-vitellogenic stage, but at 10 DAE they contained both pre-vitellogenic and vitellogenic oocytes, and the mature oocytes of elder females (15 DAE) were filled with CLas. CLas fluorescence intensity in the follicles increased significantly along with ovarian development (F2,11 = 63.13; p < 0.001), eventually filling with mature follicles, but the fluorescence intensity of CLas in the spermathecae decreased significantly (F2,11 = 29.91; p < 0.001) (Figure 6B,C). The relative CLas titer in the female reproductive system significantly increased with development (F2,11 = 65.19; p < 0.001) (Figure 6D).
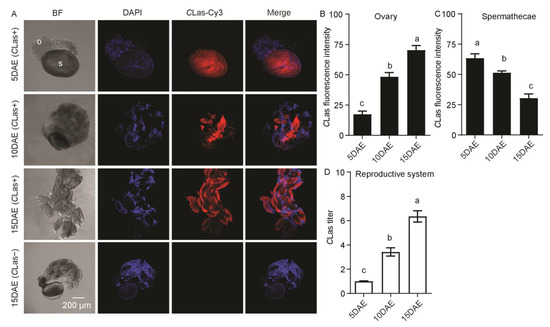
Figure 6.
Analysis of distribution and relative titer of CLas in CLas-positive adult female reproductive system by FISH and RT-PCR. (A) Representative confocal images of CLas distribution in reproductive system of CLas-positive adult females at 5 DAE, 10 DAE, and 15 DAE and CLas-negative adult females at 15 DAE. DAE: days after eclosion, o: ovary, s: spermathecae, CLas−: CLas-negative, CLas+: CLas-positive, Scale bar is 200 μm. (B) CLas fluorescence intensity in female ovaries. (C) CLas fluorescence intensity in female spermathecae. (D) Relative CLas titer in female reproductive system. The different letters at the top of the columns in (B–D) figures indicate statistically significant differences (in all cases p < 0.05).
In summary, CLas fluorescence intensity was highest in female Malpighian tubules, followed by the gut, brain, and spermathecae, and lowest in the ovaries of CLas-positive females at 5 DAE (F5,23 = 118.2, p < 0.001). In CLas-positive females at 15 DAE, CLas density was highest in the Malpighian tubules, followed by the gut, brain, and ovaries, and lowest in the spermathecae and salivary glands (F5,23 = 144.3, p < 0.001) (Figure S2A–C). The relative CLas titer was highest in the female digestive system, followed by the female reproductive system and brain, and lowest in the salivary glands (5 day-old males: F3,15 = 102.7, p < 0.001; 10 day-old males: F3,15 = 77.24, p < 0.001; 15 day-old males: F3,15 = 280.6, p < 0.001) (Figure S2D,E).
2.3. The Distribution and Dynamics of CLas in Embryos and Nymphs
In embryos observed at 18–24 h post-oviposition, the formed germ band extended and the proto-bacteriome appeared to be cellularized. At 42–48 h post-oviposition, the embryos were in the process of dorsal closure. The former cells were fused into a syncytium at 66–72 h post-oviposition, and then the embryos hatched at 90–96 h post-oviposition. CLas was observed throughout embryo development and the subsequent first–second-instar nymphs obtained from CLas-positive females (Figure 7A). No CLas signal was detected in the eggs or subsequent first–second-instar nymphs derived from CLas-negative females (Figure 7B). There was no significant difference in CLas fluorescence intensity during embryogenesis and first-instar nymphs, but it significantly increased in second-instar nymphs (F2,11 = 12.33; p < 0.001) (Figure 7C). Moreover, the relative CLas titer showed no significant difference during embryogenesis but significantly increased in the subsequent first–second-instar nymphs (F2,11 = 24.55; p < 0.001) (Figure 7D).
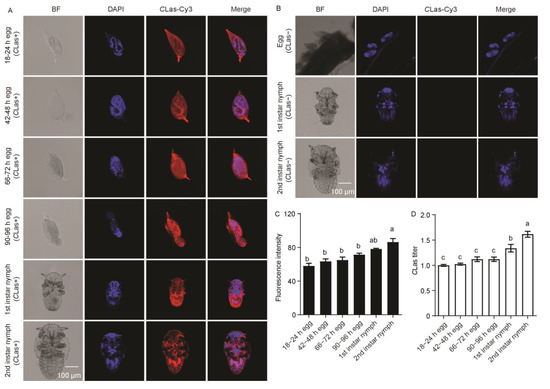
Figure 7.
Analysis of distribution and relative titer of CLas in the embryos and subsequent 1st–2nd-instar nymphs derived from CLas-positive females. (A) Representative confocal images of CLas distribution during embryogenesis and subsequent 1st–2nd-instar nymphs derived from CLas-positive females. Scale bar is 100 μm. (B) Representative confocal images of CLas distribution in the eggs and subsequent 1st–2nd-instar nymphs derived from CLas-negative females. Scale bar is 100 μm. (C) CLas fluorescence intensity during embryogenesis and subsequent 1st–2nd-instar nymphs. (D) Relative CLas titer during embryogenesis and subsequent 1st–2nd-instar nymphs. The different letters at the top of the columns in (C,D) figures indicate statistically significant differences (in all cases p < 0.05).
3. Discussion
Confocal imaging using FISH with a CLas probe enables us to visualize CLas’s location within vector insects. In the present study, the near-systemic invasion of CLas within all female and male dissected tissues, including the brain, salivary glands, gut, Malpighian tubules, and reproductive system, suggested a wide distribution of CLas in CLas-positive psyllids, which is in accordance with previous studies [4,7,8,9,10,19,20,21]. It was obvious that the CLas titer in certain tissues of D. citri was very low. Whether via relative quantification using normalization to a reference gene or absolute quantification using a standard curve, errors are inevitable in detection. FISH technology is visual and sensitive, and even weak probe signals can be captured. Therefore, by combining the results of FISH and qRT-PCR, the relative differences in CLas titers in D. citri can be quantified in various tissues at different ages/stages of the vector.
Along with adult development, the trend of CLas fluorescence intensity by FISH was consistent with that of the CLas titer by qRT-PCR in the same tissue. The CLas titer was highest in the digestive system in our study, which is consistent with previous reports [7,8,10]. However, CLas fluorescence intensity by FISH was highest in the Malpighian tubes in all examined tissues. These differing results may, on the one hand, be due to the difference in detection techniques. The FISH technique could be used to quantify and visualize the difference in CLas in any tissue of D. citri. For example, it could separately quantify CLas fluorescence intensity in the gut and Malpighian tubules, in female ovaries and the spermathecae, or in the male testis and sperm sac. However, qRT-PCR could only be used to quantify the corresponding digestive and reproductive systems because these tissues are closely connected in D. citri.
CLas fluorescence intensity and titers were significantly increased in both the digestive system and the female reproductive system with development, showed a marked decreased in both the salivary glands and the male brain, and showed no significant change in the female brain or male reproductive system. Ammar et al. [22] described the detailed ultrastructure of various tissues, and the epithelial cells of Malpighian tubules were vacuolated and lined with extensive microvilli in D. citri. Malpighian tubules in insects have discrete compartments and specific cell types that are highly specialized in excretory function [23]. In addition to CLas, the Wolbachia titer was highest in the Malpighian tubules [10]. The cause of these densely aggregated microbes in the Malpighian tubules is not yet known. It is possible that nitrogen metabolites in Malpighian tubules are essential for the development and replication of microbes. The effect of a large number of CLas gathered in the Malpighian tubes on the interactions between D. citri and CLas requires further study. The CLas fluorescence intensity and titer were lowest in the salivary glands of both males and females, which is consistent with a previous report [7]. Furthermore, the fluorescence intensity and titer of CLas were lower and gradually decreased in the sperm sac as it developed, which provided a possible explanation for the low sexual-transmission efficiency (3.6%) of CLas from CLas-positive males to CLas-negative females during mating [24].
Previous studies reported that CLas was detected in the ovaries at a low frequency by qRCR [7,8,10]. In addition, Hosseinzadeh et al. (2019) reported that CLas was occasionally found in the follicle cells of females [25]. In the current study, CLas was detected in all female reproductive systems by FISH. At the early stage of ovarian development, there was no CLas dissected in the follicles by FISH, and CLas fluorescence intensity in the follicles increased significantly along with ovarian development, eventually filling with mature follicles. The corresponding CLas titer in the female reproductive system was significantly increased by RT-PCR. The differing results between these studies may have arisen from different developmental stages of D. citri. Previously published studies used psyllids at a certain stage, whereas we used insect vectors at three different developmental stages.
Transovarial transmission of microbes from female vectors to progeny is an important strategy for microbes to speed up transmission and is recognized as crucial to becoming an epidemic [13,14]. Transovarial transmission controls microbe dispersal in space and time and thus plays an important role in microbe outbreak, which has received constant attention [24,26,27]. However, most studies have focused on circulative–propagative or circulative–non-propagative plant viruses [15,28,29,30], and only a few have investigated circulative–propagative plant bacteria [24,26]. CLas moves through the psyllid body in a propagative–circulative manner [7,8]. Until now, only two cases have reported that CLas can be vertically transmitted at a low rate [17,18], which is contrary to our results.
Kelley and Pelz-Stelinski (2019) reported that nymphs exposed to CLas were more likely to pick it up via horizontal transmission than vertical transmission [18]. In order to reduce this possibility, partially CLas-resistant citrus plants—orange Jasmine (Murraya panifulata (L.) Jack) [31]—were used for egg laying by infected females to study transovarial transmission. CLas was observed in all laid eggs and subsequent first–second-instar nymphs, indicating that a high percentage of embryos and nymphs resulting from the infected D. citri mothers were infected with CLas. Although in M. panifulata CLas decreased gradually to an undetectable level, it is also possible that the older nymphs may have acquired the pathogen from the oviposition site. In addition, the epidermis of the nymph thickens gradually along with the development of the nymph, which makes it difficult for the nymphs to sufficiently decolorize, so the CLas probe could not penetrate the cuticle in the third–fifth-instar nymphs. Hence, the distribution and relative titers of CLas in subsequent third–fifth-instar nymphs were not shown in the present work. It is well documented that even with highly infected colonies of D. citri, when tested individually they transmit this bacterium to only up to 20–46% of test plants in different regions around the world [32]. As for transovarial transmission of CLas in D. citri, much lower percentages have been reported [17]. In future studies, actual transovarial transmission of CLas from infected D. citri mothers to their offspring needs to be verified by feeding first-generation nymphs, individually or in small groups, on young, susceptible, healthy citrus plants. These tests could show whether the high percentage of transovarially infected nymphs would lead to efficient inoculation of CLas into these test plants and the potential significance of transovarial transmission in the epidemiology of CLas/HLB.
CLas, one of the world’s most serious citrus pathogens, is considered to have spread from China to the rest of the world around the late 1800s [33]. D. citri invasion and the movement of CLas-infected materials are recognized as two important factors that are attributed to the rapid spread of CLas around the world. In the current study, D. citri efficiently transovarially transmitted CLas to their offspring. CLas-positive eggs—which are so small as to be almost invisible—laid by CLas-infected psyllids on plants can be transported over long distances with plant material by human activity. Thus, transovarial transmission may also make a significant contribution to the global spread of CLas in addition to the outbreak of HLB in fields.
4. Materials and Methods
4.1. Host Plants and Insect Colonies
The CLas-negative (healthy) D. citri colony was obtained from a laboratory culture continuously reared on healthy Citrus limon (L.) Osbeck plants. CLas-positive D. citri were obtained from a subculture of CLas-negative D. citri reared on CLas-positive C. limon. The two colonies were kept in the different incubators at 26 ± 1 °C, 65 ± 5% relative humidity (RH) with a 14L: 10D photoperiod. Both healthy and CLas-positive cultures were periodically tested to confirm the absence or presence of CLas by qPCR as previously reported [34]. The mean infection rate of CLas in the D. citri reared on CLas-positive plants was 90–100%. In order to explore the dynamics of transovarially transmitted CLas in D. citri, healthy orange Jasmine (M. panifulata (L.) Jack) plants (relatively resistant to CLas) were used for egg laying by infected females to study the dynamics of CLas in different stages of embryos and subsequent nymphs of D. citri.
4.2. Tissue Dissection and Sample Collection
Newly emerged adults (within 24 h) reared on CLas-positive plants were collected and relocated onto new CLas-positive plants, which were marked as 1 day after eclosion (1 DAE). Three different developmental stages (5 DAE, 10 DAE, and 15 DAE) of adult females and males were prepared. In the laboratory, adults were first anesthetized with CO2, the brain was connected to the salivary glands and the gut to the Malpighian tubules, and the reproductive system was then excised in 1 × phosphate-buffered saline (PBS) (Sangon Biotech, Shanghai, China) under a dissecting microscope (Motic SMZ-171). Following dissection, each sample was divided into two parts for conjoint analysis, one for FISH to detect CLas fluorescence intensity and the other for RT-PCR for relative CLas titers. All experiments were performed with four biological replications. Each replication included 40–45 insects for qRT-PCR. Four FISH tests were conducted and 60 samples per tissue were imaged to confirm the repeatability. In these experiments, the D. citri reared on CLas-positive plants had a high titer with a cycle threshold (CT) of less than 25 in qPCR monitoring and a 100% infection rate by FISH. The adult females and males of D. citri reared on CLas-negative C. limon plants at 15 DAE were dissected for negative control.
4.3. Preparation of Embryos and Subsequent Nymphs
In order to explore the dynamics of CLas in different stages of embryos and subsequent nymphs of D. citri, the female adults reared on the CLas-positive plants were allowed to lay eggs on healthy orange Jasmine (M. panifulata (L.) Jack) plants. After spawning for 12 h, the females were removed and tested to confirm the presence of CLas by qPCR. Results show that the infection rate of CLas in females was 100% and that all the females used were CLas-positive. According to the morphological characteristics [35,36], embryos of 24 h, 48 h, 72 h, and 96 h post-oviposition and subsequent first (1st)- and second (2nd—instar nymphs were collected. Four biological repetitions were conducted and imaged to confirm repeatability. As for FISH, a total of 200 embryos of each stage and 60 nymphs of each instar were tested. As for qRT-PCR, samples of different stages of embryos and nymphs were gently scraped off the plants with a soft brush, and each repetition included 40–50 nymphs.
4.4. Fluorescence In-Situ Hybridization (FISH)
FISH protocol was performed as previously described [8,10,37] with minor modifications. The samples of embryos, nymphs, and dissected adult tissues were fixed in Carnoy’s fixative (glacial acetic acid: ethanol: chloroform, 1:3:6, v:v:v) overnight at room temperature. After washing with 50% ethanol three times (10 min each time), the fixed samples were treated with 6% H2O2 in 80% ethanol until sufficiently decolorized. Then, the bleached samples were rinsed with PBST (1 × PBS: TritonX-100, 99.7:0.3, v:v) three times (20 min each time) and pre-incubated three times (10 min per incubation) with the hybridization buffer (20 mM Tris-HCl, pH 8.0, 0.9 M NaCl, 0.01% (wt/wol) sodium dodecyl sulfate, 30% (v:v) formamide) without the probe.
CLas probe (5′-Cy3-CATTATCTTCTCCGGCG -3′) [10] was labeled in 5′-terminal with fluorescence labeling of Cy3. The samples were hybridized within a hybridization buffer containing 10 pmol mL−1 of CLas probe at 25 °C overnight. After washing three times with PBST (20 min each time), nuclei were stained with DAPI (40, 60-diamidino-2-phenylindole; 0.1 mg mL−1) for 15 min and rinsed with PBST for 10 min. The stained samples were transferred to glass slides with spacers and mounted in mounting medium using a cover slip. The slides were viewed under a Leica TCS-SP8 (Leica Microsystems, Exton, PA, USA) confocal microscope using two excitation lasers, exciting near UV-diode 405 nm and 550 nm lasers to detect DAPI and the Cy3-signal, respectively. The acquisition setting and scanning setting were kept fixed throughout all experiments. Additionally, sequential scanning limited the potential signal overlap of the fluorescent probe. Image processing was completed using Leica LAS-AF software (v2.6.0). For the specificity of the probe signal, healthy insects (CLas-negative) at 15 DAE and were confirmed as negative controls.
4.5. qRT-PCR
The CLas primers (:TCGAGCGCGTATGCAATACG; R: GCGTTATCCCGTAGAAAAAGGTAG) used in the study were the same as those previously reported [34]. For relative CLas-titer detection, total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) The first-strand cDNA was synthesized using the PrimeScript™ II 1st Strand cDNA Synthesis Kit (Takara, Beijing, China) according to the manufacturer’s protocol. Quantitative RT-PCR was performed using TB Green® Premix Ex Taq™ II (Takara, Beijing, China) on an ABI PRISM® 7500 Real-Time System (Applied Biosystems, Foster City, CA, USA). Beta-actin (Dcβ-ACT) was selected to normalize the CLas-expression level in D. citri.
4.6. Statistical Analysis
For the Cy3 channel of tissue and embryo images, the mean grayscale value of the fluorescence intensity of regions of interest (ROI) was analyzed [38]. All the data should be checked for normality and homogeneity of variance in advance. One-way ANOVA followed by the Tukey’s Honestly Significant Difference (HSD) test were used for multiple comparisons (different letters denoted by p < 0.05). All statistical analyses were performed using GraphPad Prism 9.0 software.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24108997/s1.
Author Contributions
Conceptualization, X.N. and Y.H.; methodology, X.N. and Y.H.; software, X.N. and D.W.; validation, X.N., J.L., Y.L., S.W., J.H. and S.T.; formal analysis, X.N., Y.L. and D.W.; investigation, X.N., J.L., Y.L., S.W., J.H. and S.T.; resources, X.N., Y.L., S.W., J.H. and S.T.; data curation, X.N. and D.W.; writing—original draft preparation, X.N. and Y.H.; writing—review and editing, X.N. and Y.C.; visualization, Y.H.; supervision, Y.H. and X.N.; project administration, X.N., Y.C. and Y.H.; funding acquisition, X.N. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China, grant number 32102193, and the Open Competition Program of Ten Major Directions of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province, grant number 2022SDZG07.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data is in this manuscript.
Acknowledgments
The authors would like to thank the editor and reviewers for the modifications to and suggestions for the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, N. The citrus Huanglongbing crisis and potential solutions. Mol. Plant Pathol. 2019, 12, 607–609. [Google Scholar] [CrossRef]
- Gottwald, T.; Poole, G.; McCollum, T.; Hall, D.; Hartung, J.; Bai, J.; Luo, W.; Posny, D.; Duan, Y.P.; Taylor, E.; et al. Canine olfactory detection of a vectored phytobacterial pathogen, Liberibacter asiaticus, and integration with disease control. Proc. Natl. Acad. Sci. USA 2020, 117, 3492–3501. [Google Scholar] [CrossRef]
- Capoor, S.P.; Rao, D.G.; Viswanath, S.M. Diaphorina citri Kuway. A vector of the greening disease of citrus in India. Indian J. Agric. Sci. 1967, 37, 572–576. [Google Scholar]
- Ammar, E.D.; Ramos, J.E.; Hall, D.G.; Dawson, W.O.; Shatters, R.G., Jr. Acquisition, replication and inoculation of Candidatus Liberibacter asiaticus following various acquisition periods on Huanglongbing-infected citrus by nymphs and adults of the Asian citrus psyllid. PLoS ONE 2016, 11, e0159594. [Google Scholar] [CrossRef]
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus Huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Inoue, H.; Ohnishi, J.; Ito, T.; Tomimura, K.; Ashihara, W. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann. Appl. Biol. 2010, 155, 29–36. [Google Scholar] [CrossRef]
- Ammar, E.D.; Shatters, R.G., Jr.; Lynch, C.; Hall, D.G. Detection and relative titer of Candidatus Liberibacter asiaticus in the salivary glands and alimentary canal of Diaphorina citri (Hemiptera: Psyllidae) vector of citrus huanglongbing disease. Ann. Entomol. Soc. Am. 2011, 104, 526–533. [Google Scholar] [CrossRef]
- Ammar, E.D.; Shatters, R.G., Jr.; Hall, D.G. Localization of Candidatus Liberibacter asiaticus, associated with citrus Huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 2011, 159, 726–734. [Google Scholar] [CrossRef]
- Ammar, E.D.; Achor, D.; Levy, A. Immuno-Ultrastructural localization and putative multiplication sites of Huanglongbing bacterium in Asian citrus psyllid Diaphorina citri. Insects 2019, 10, 422. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Ramsey, J.; Mann, M.; Bennett, L.; Hunter, W.B.; Shams-Bakhsh, M.; Hall, D.G.; Heck, M. Color morphology of Diaphorina citri influences interactions with its bacterial endosymbionts and ‘Candidatus Liberibacter asiaticus’. PLoS ONE 2019, 14, e0216599. [Google Scholar] [CrossRef]
- Ghanim, M.; Achor, D.; Ghosh, S.; Kontsedalov, S.; Lebedev, G.; Levy, A. ‘Candidatus Liberibacter asiaticus’ accumulates inside endoplasmic reticulum associated vacuoles in the gut cells of Diaphorina citri. Sci. Rep. 2017, 7, 16945. [Google Scholar] [CrossRef]
- Moya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: Genomic insights into prokaryote-animal symbioses. Nat. Rev. Genet. 2008, 9, 218–229. [Google Scholar] [CrossRef]
- Lequime, S.; Paul, R.E.; Lambrechts, L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016, 12, e1005548. [Google Scholar] [CrossRef]
- Tesh, R.B.; Chaniotis, B.N.; Johnson, K.M. Vesicular stomatitis virus (Indiana serotype): Transovarial transmission by phlebotomine sandlies. Science 1972, 175, 1477–1479. [Google Scholar] [CrossRef]
- Wei, J.; He, Y.Z.; Guo, Q.; Guo, T.; Liu, Y.Q.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 6746–6751. [Google Scholar] [CrossRef]
- Mims, C.A. Vertical transmission of viruses. Microbiol. Rev. 1981, 45, 267–286. [Google Scholar] [CrossRef]
- Pelz-Stelinski, K.S.; Brlansky, R.H.; Ebert, T.A.; Rogers, M.E. Transmission parameters for Candidatus liberibacter asiaticus by Asian citrus psyllid (Hemiptera: Psyllidae). J. Econ. Entomol. 2010, 103, 1531–1541. [Google Scholar] [CrossRef]
- Kelley, A.J.; Pelz-Stelinski, K.S. Maternal contribution of Candidatus Liberibacter asiaticus to Asian citrus psyllid (Hemiptera: Liviidae) nymphs through oviposition site inoculation and transovarial transmission. J. Econ. Entomol. 2019, 112, 2565–2568. [Google Scholar] [CrossRef]
- Mann, M.; Fattah-Hosseini, S.; Ammar, E.D.; Stange, R.; Warrick, E.; Sturgeon, K.; Shatters, R.; Heck, M. Diaphorina citri nymphs are resistant to morphological changes Induced by "Candidatus Liberibacter asiaticus" in midgut epithelial cells. Infect. Immun. 2018, 86, e00889-17. [Google Scholar] [CrossRef]
- Kruse, A.; Fattah-Hosseini, S.; Saha, S.; Johnson, R.; Warwick, E.; Sturgeon, K.; Mueller, L.; MacCoss, M.J.; Shatters, R.G., Jr.; Heck, M.C. Combining ‘omics and microscopy to visualize interactions between the Asian citrus psyllid vector and the Huanglongbing pathogen Candidatus Liberibacter asiaticus in the insect gut. PLoS ONE 2017, 12, e0179531. [Google Scholar] [CrossRef]
- Ukuda-Hosokawa, R.; Sadoyama, Y.; Kishaba, M.; Kuriwada, T.; Anbutsu, H.; Fukatsu, T. Infection density dynamics of the citrus greening bacterium “Candidatus Liberibacter asiaticus” in field populations of the psyllid Diaphorina citri and its relevance to the efficiency of pathogen transmission to citrus plants. Appl. Environ. Microbiol. 2015, 81, 3728–3736. [Google Scholar] [CrossRef]
- Ammar, E.D.; Hall, D.G.; Shatters, R.G., Jr. Ultrastructure of the salivary glands, alimentary canal and bacteria-like organisms in the Asian citrus psyllid, vector of citrus huanglongbing disease bacteria. J. Microsc. Ultrastruct. 2017, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Cicero, J.M.; Brown, J.K.; Roberts, P.D.; Stansly, P.A. The digestive system of Diaphorina citri and Bactericera cockerelli (hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2009, 102, 650–665. [Google Scholar] [CrossRef]
- Mann, R.S.; Pelz-Stelinski, K.; Hermann, S.L.; Tiwari, S.; Stelinski, L.L. Sexual transmission of a plant pathogenic bacterium, Candidatus Liberibacter asiaticus, between conspecific insect vectors during mating. PLoS ONE. 2011, 6, e29197. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Shams-Bakhsh, M.; Mann, M.; Fattah-Hosseini, S.; Bagheri, A.; Mehrabadi, M.; Heck, M. Distribution and variation of bacterial endosymbiont and “Candidatus Liberibacter asiaticus” titer in the Huanglongbing insect vector, Diaphorina citri Kuwayama. Microb. Ecol. 2019, 78, 206–222. [Google Scholar] [CrossRef]
- Nachappa, P.; Levy, J.; Pierson, E.; Tamborindeguy, C. Diversity of endosymbionts in the potato psyllid, Bactericera cockerelli (Triozidae), vector of zebra chip disease of potato. Curr. Microbiol. 2011, 62, 1510–1520. [Google Scholar] [CrossRef]
- Pedro, S.A.; Abelman, S.; Tonnang, H.E. Predicting rift valley fever inter-epidemic activities and outbreak patterns: Insights from a stochastic host-vector model. PLoS Negl. Trop. Dis. 2016, 10, e0005167. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, W.W.; Zhang, F.J.; Chen, X.Y.; Li, L.; Liu, Q.F.; Zhou, Y.J.; Wei, T.Y.; Fang, R.X.; Wang, X.F. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014, 10, e1003949. [Google Scholar] [CrossRef]
- Ammar, E.D.; Tsai, C.W.; Whitfield, A.E.; Redinbaugh, M.G.; Hogenhout, S.A. Cellular and molecular aspects of rhabdovirus interactions with insect and plant hosts. Annu. Rev. Entomol. 2009, 54, 447–468. [Google Scholar] [CrossRef]
- Falk, B.W.; Tsai, J.H. Biology and molecular biology of viruses in the genus tenuivirus. Annu. Rev. Phytopathol. 1998, 36, 139–163. [Google Scholar] [CrossRef]
- Alves, M.N.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L.; Ferro, J.A.; Peña, L. Early population dynamics of “Candidatus Liberibacter asiaticus” in susceptible and resistant genotypes after inoculation with infected Diaphorina citri feeding on young shoots. Front. Microbiol. 2021, 12, 683923. [Google Scholar] [CrossRef] [PubMed]
- Ammar, E.D.; Shatters, R.G.; Heck, M. Huanglongbing pathogens: Acquisition, transmission and vector interactions. In Asian Citrus Psyllid: Biology, Ecology and Management of the Huanglongbing Vector; Qureshi, J.A., Stansly, P.A., Eds.; CAB International: Wallingford, CT, USA, 2020; pp. 113–139. [Google Scholar]
- Da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identiÞcation of Candidatus Liberibacter species associated with citrus Huanglongbing. J. Microbiol. Methods 2006, 66, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Ikeda, N.; Fujikami, M.; Nakabachi, A. Behavior of bacteriome symbionts during transovarial transmission and development of the Asian citrus psyllid. PLoS ONE 2017, 12, e0189779. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef]
- Kliot, A.; Kontsedalov, S.; Lebedev, G.; Brumin, M.; Cathrin, P.B.; Marubayashi, J.M. Fluorescence in situ hybridizations (FISH) for the localization of viruses and endosymbiotic bacteria in plant and insect tissues. J. Vis. Exp. 2014, 84, e51030. [Google Scholar]
- Van Bogaert, N.; De Jonghe, K.; Van Damme, E.J.; Maes, M.; Smagghe, G. Quantitation and localization of pospiviroids in aphids. J. Virol. Methods. 2015, 211, 51–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).