Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain
Abstract
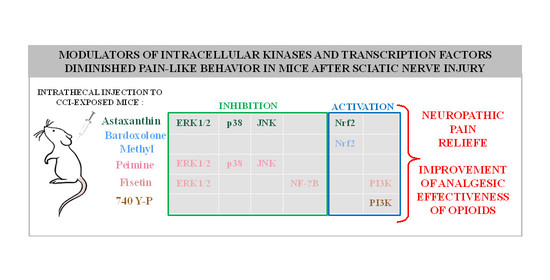
1. Introduction
2. Results
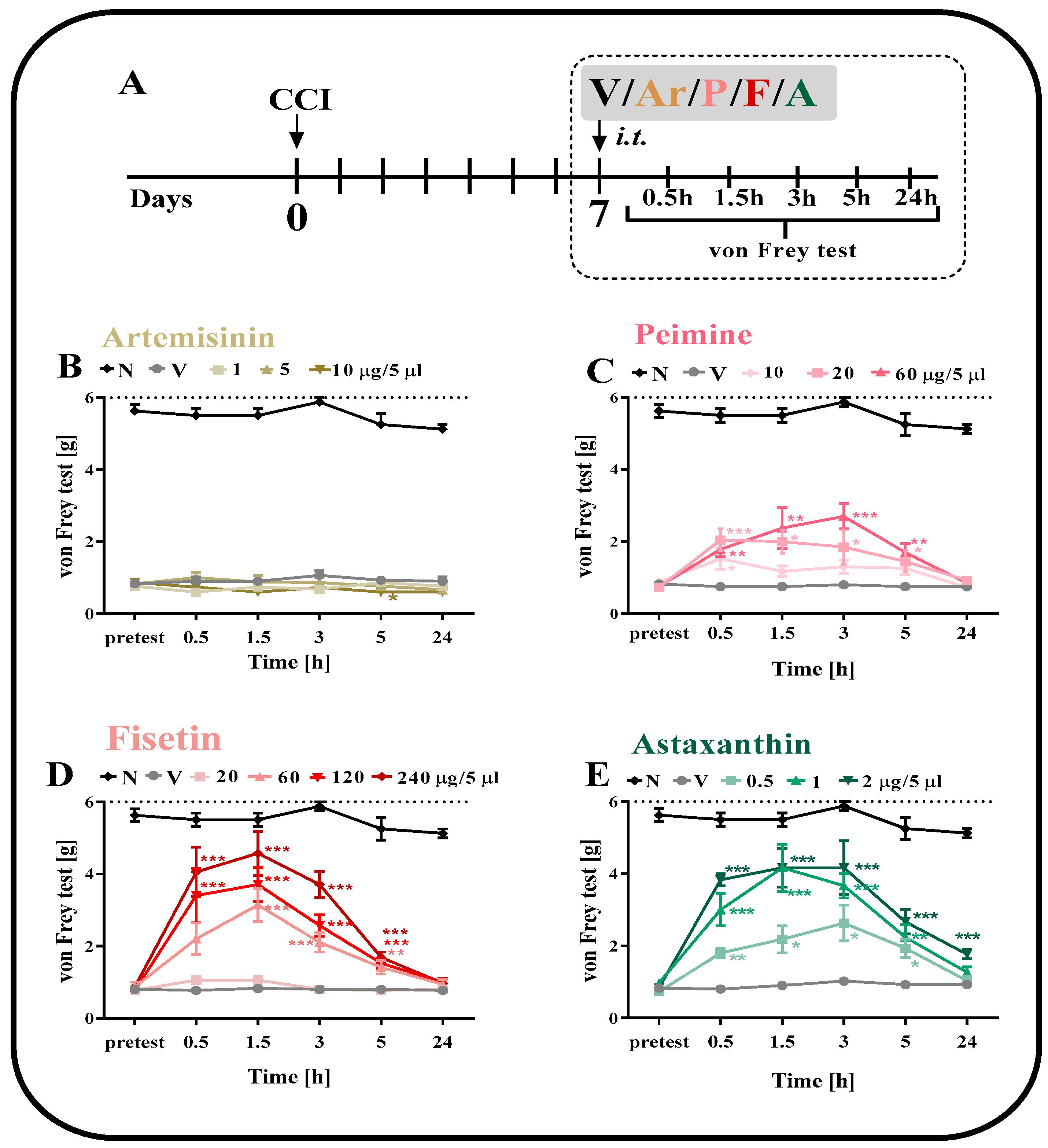
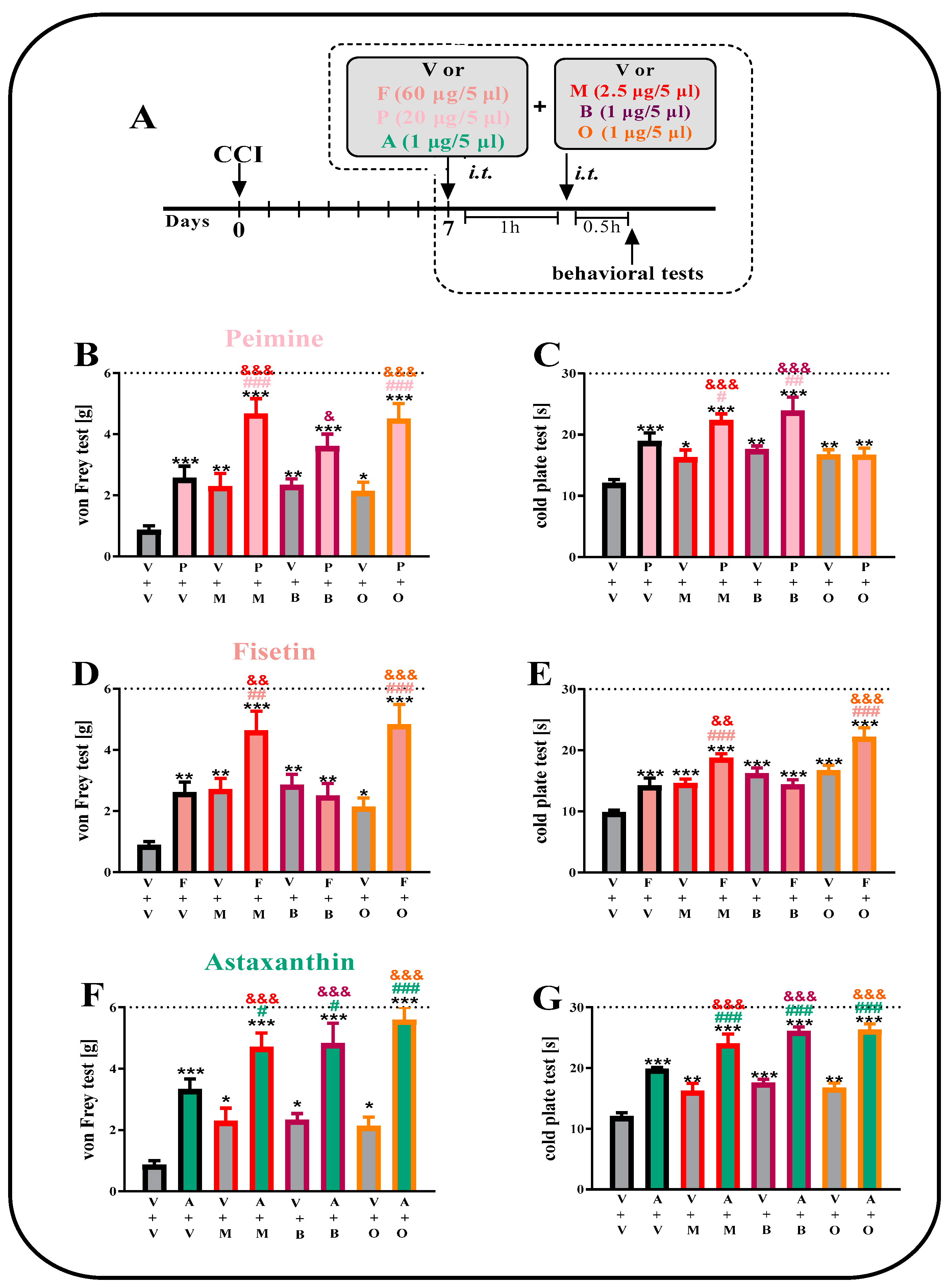
2.1. The effect of a Single i.t. Administration of Artemisinin, Peimine, Fisetin and Astaxanthin on Tactile Hypersensitivity on the 7th Day after CCI
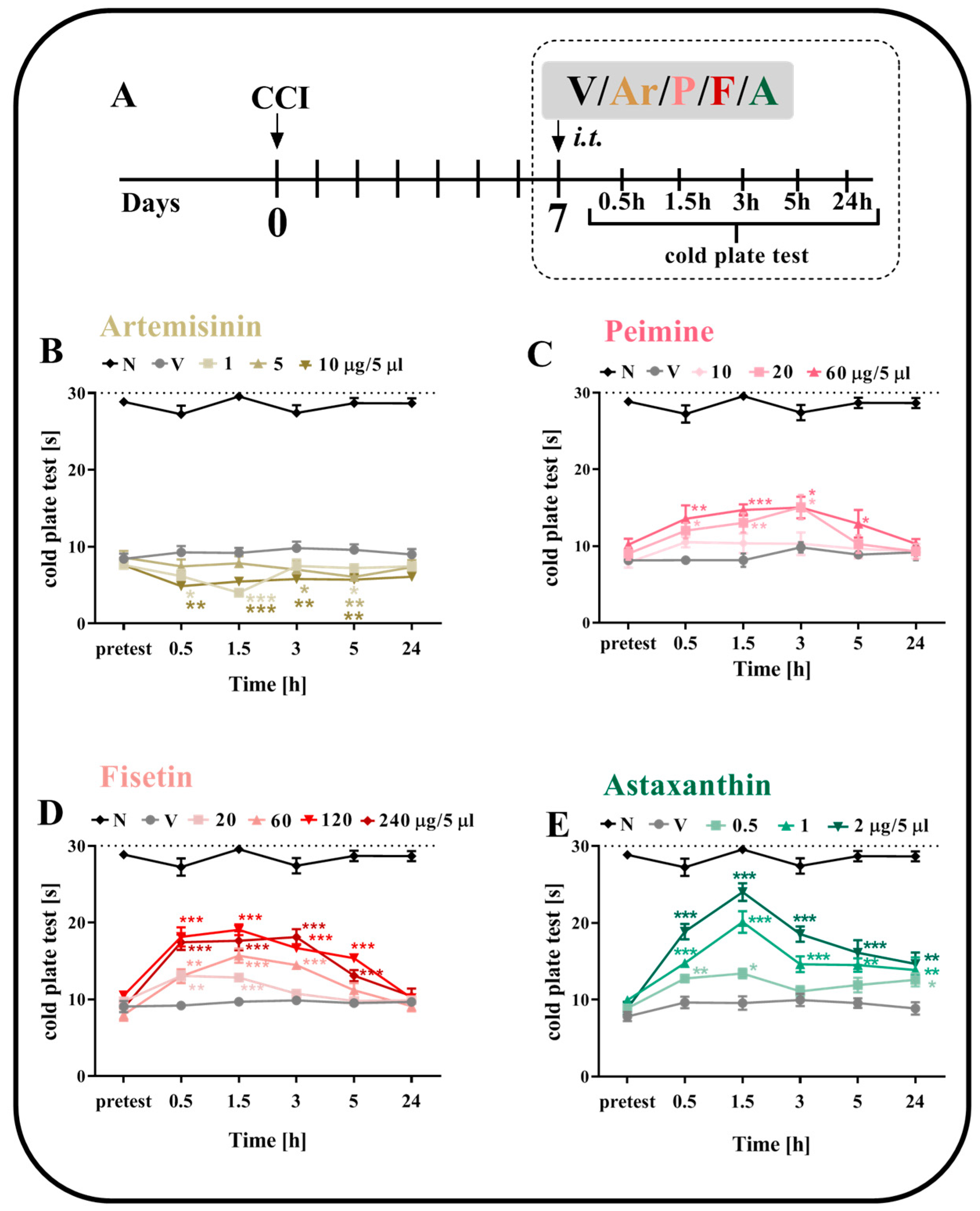
2.2. The Effect of a Single i.t. Administration of Artemisinin, Peimine, Fisetin and Astaxanthin on Thermal Hypersensitivity on the 7th Day after CCI
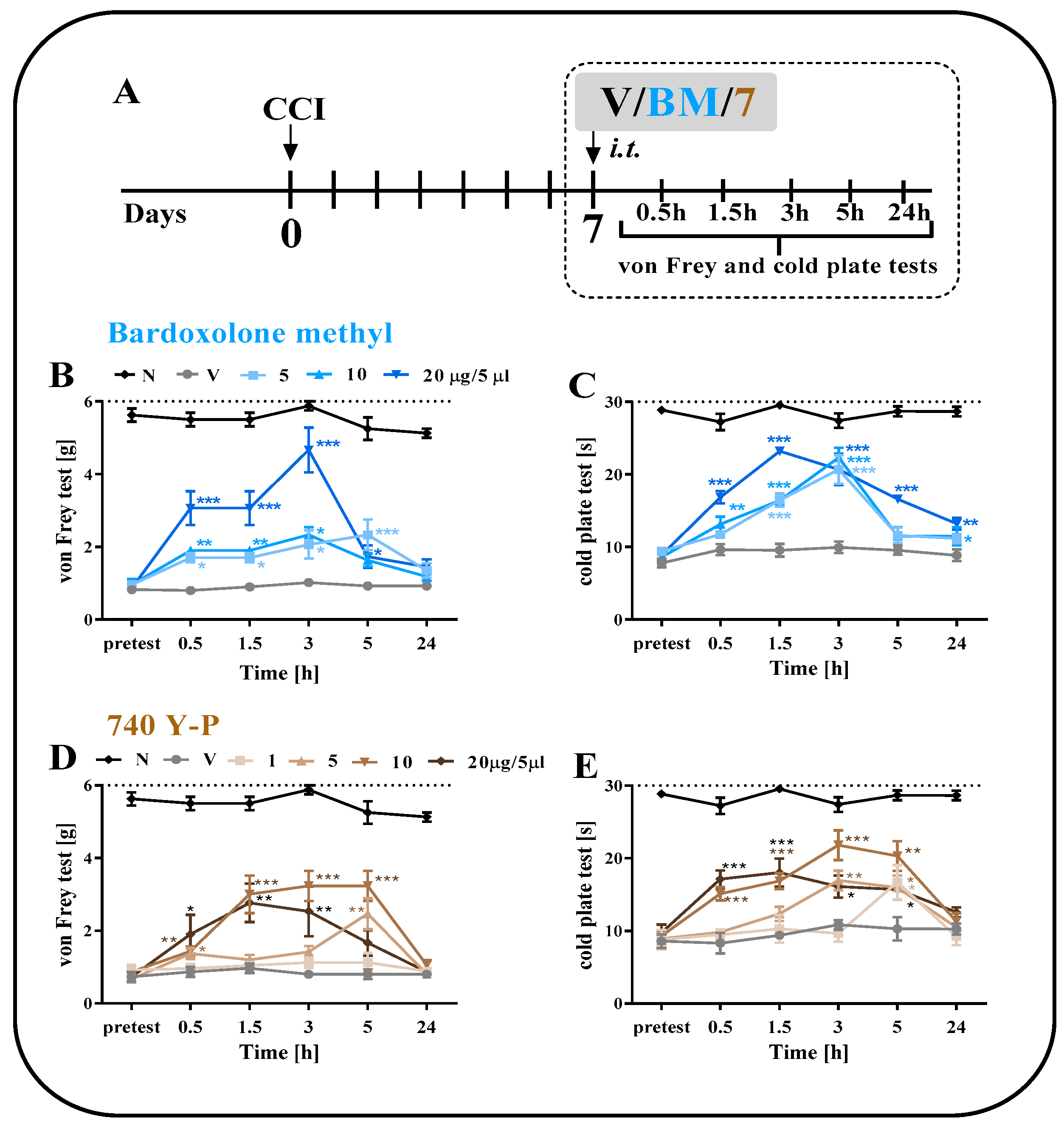
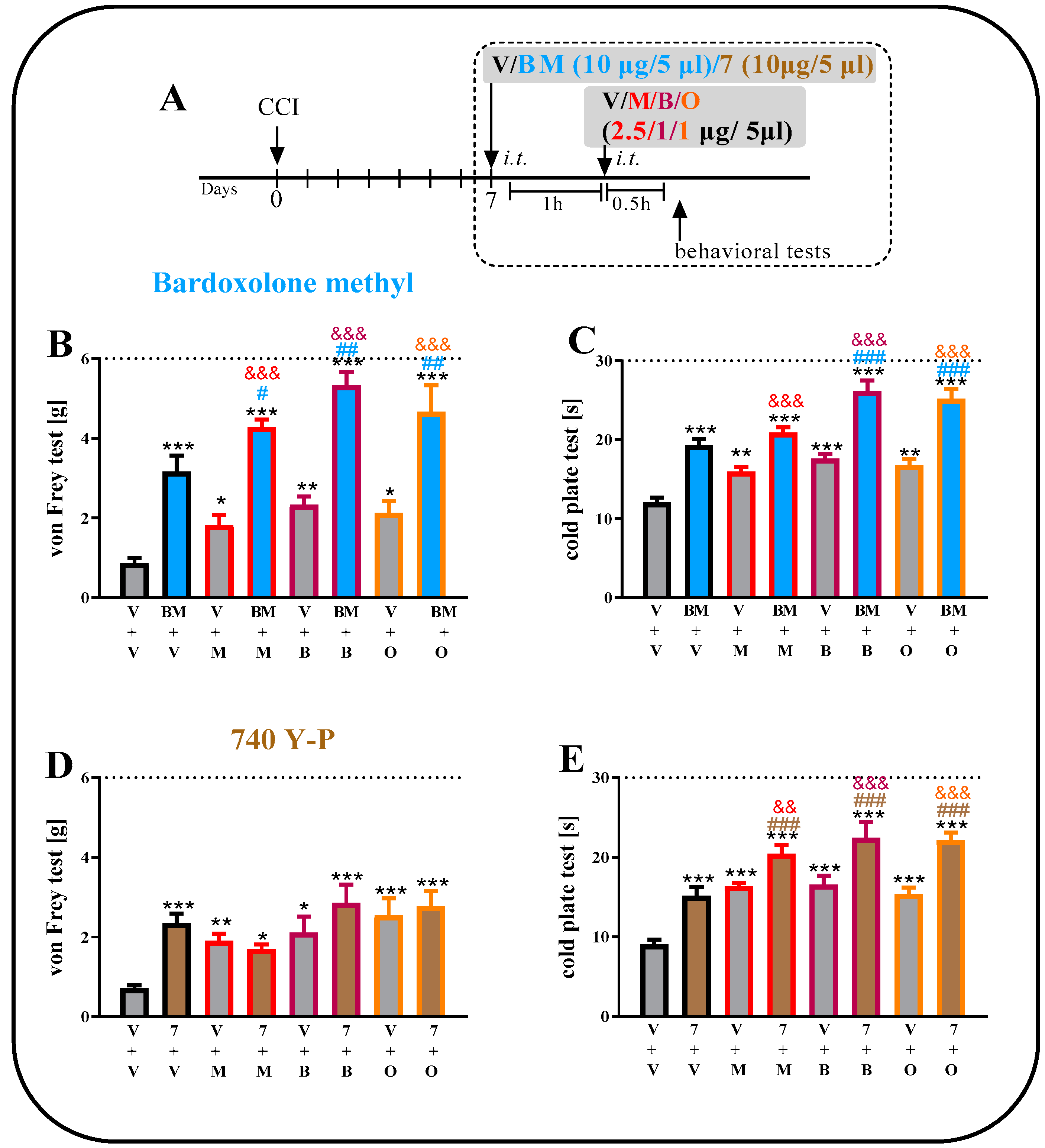
2.3. The Effect of a Single i.t. Administration of Bardoxolone Methyl or 740 Y-P on Pain-Related Behaviors on Day 7 after CCI
2.4. Comparison of the Analgesic Effects of a Single i.t. Administration of Artemisinin, Fisetin, Peimine, Astaxanthin, Bardoxolone Methyl or 740 Y-P on Day 7 after CCI
2.5. The Effect of a Single i.t. Administration of Peimine, Fisetin and Astaxanthin on Opioid Effectiveness on the 7th Day after CCI
2.6. The Effect of a Single i.t. Administration of Bardoxolone Methyl or 740 Y-P on Opioid Effectiveness on the 7th Day after CCI
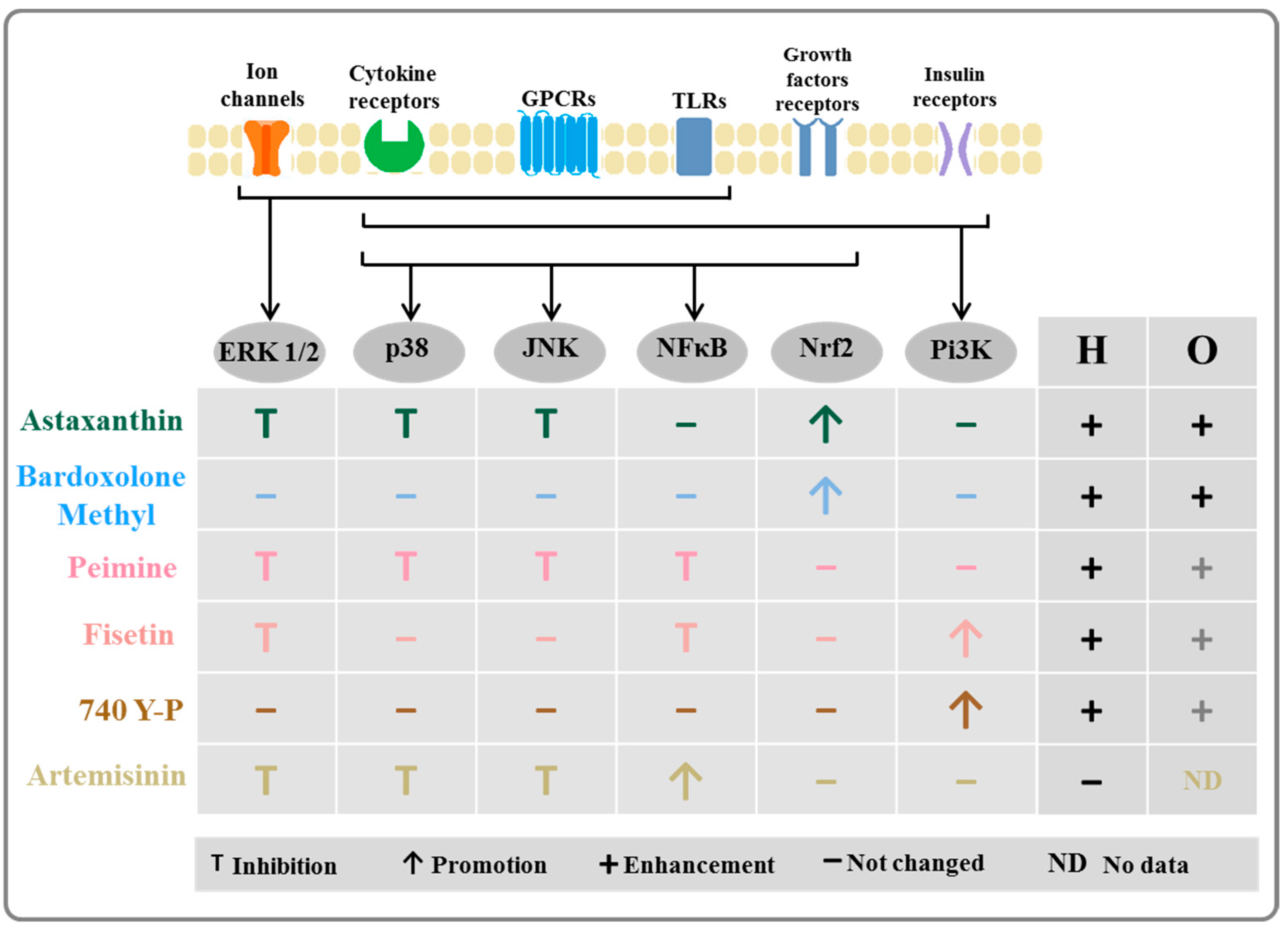
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Neuropathic Pain Model
4.3. Behavioral Tests
4.3.1. Von Frey Test
4.3.2. Cold Plate Test
4.4. Pharmacological Study
4.4.1. Drugs and Routes of Administration
4.4.2. Dose-Dependence Study
4.4.3. Administration with Opioids
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dworkin, R.H.; O’Connor, A.B.; Audette, J.; Baron, R.; Gourlay, G.K.; Haanpää, M.L.; Kent, J.L.; Krane, E.J.; Lebel, A.A.; Levy, R.M.; et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clin. Proc. 2010, 85, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Prim. 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsuda, M. Microglia and neuropathic pain. Glia 2009, 57, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef]
- Ji, R.-R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef]
- Ji, R.R.; Gereau IV, R.W.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.X.; Zhuang, Z.Y.; Woolf, C.J.; Ji, R.R. p38 Mitogen-Activated Protein Kinase Is Activated after a Spinal Nerve Ligation in Spinal Cord Microglia and Dorsal Root Ganglion Neurons and Contributes to the Generation of Neuropathic Pain. J. Neurosci. 2003, 23, 4017. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Noguchi, K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004, 74, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Quirion, R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin. Ther. Targets 2005, 9, 699–713. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Makuch, W.; Rojewska, E.; Pilat, D.; Mika, J. Inhibition of intracellular signaling pathways NF-κB and MEK1/2 attenuates neuropathic pain development and enhances morphine analgesia. Pharmacol. Rep. 2014, 66, 845–851. [Google Scholar] [CrossRef]
- Qu, Y.J.; Jia, L.; Zhang, X.; Wei, H.; Yue, S.W. MAPK Pathways Are Involved in Neuropathic Pain in Rats with Chronic Compression of the Dorsal Root Ganglion. Evid.-Based Complement. Altern. Med. 2016, 2016, 6153215. [Google Scholar] [CrossRef]
- Gao, Y.J.; Cheng, J.K.; Zeng, Q.; Xu, Z.Z.; Decosterd, I.; Xu, X.; Ji, R.R. Selective inhibition of JNK with a peptide inhibitor attenuates pain hypersensitivity and tumor growth in a mouse skin cancer pain model. Exp. Neurol. 2009, 219, 146–155. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Kolosowska, N.; Piotrowska, A.; Zychowska, M.; Makuch, W.; Przewlocka, B.; Mika, J. PD98059 influences immune factors and enhances opioid analgesia in model of neuropathy. PLoS ONE 2015, 10, e0138583. [Google Scholar] [CrossRef]
- Chen, S.-P.; Zhou, Y.-Q.; Liu, D.-Q.; Zhang, W.; Manyande, A.; Guan, X.-H.; Tian, Y.; Ye, D.-W.; Omar, D.M. PI3K/Akt Pathway: A Potential Therapeutic Target for Chronic Pain. Curr. Pharm. Des. 2017, 23, 1860–1868. [Google Scholar] [CrossRef]
- Liu, W.; Lv, Y.; Ren, F. PI3K/Akt Pathway is Required for Spinal Central Sensitization in Neuropathic Pain. Cell. Mol. Neurobiol. 2018, 38, 747–755. [Google Scholar] [CrossRef]
- Beg, A.A.; Baltimore, D. An Essential Role for NF-κB in Preventing TNF-α-Induced Cell Death. Science 1996, 274, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Mayo, M.W.; Baldwin, A.S. TNF- and Cancer Therapy-Induced Apoptosis: Potentiation by Inhibition of NF-κB. Science 1996, 274, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Berghe, W.V.; Plaisance, S.; Boone, E.; De Bosscher, K.; Schmitz, M.L.; Fiers, W.; Haegeman, G. p38 and Extracellular Signal-regulated Kinase Mitogen-activated Protein Kinase Pathways Are Required for Nuclear Factor-κB p65 Transactivation Mediated by Tumor Necrosis Factor. J. Biol. Chem. 1998, 273, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.D.; Guo, Q.L.; Wang, E.; Ye, Z.; He, Z.H.; Zou, W.Y.; Cheng, Z.G.; Wang, Y.J. Intrathecal infusion of pyrrolidine dithiocarbamate for the prevention and reversal of neuropathic pain in rats using a sciatic chronic constriction injury model. Reg. Anesth. Pain Med. 2010, 35, 231–237. [Google Scholar] [CrossRef]
- Lee, M.K.; Han, S.R.; Park, M.K.; Kim, M.J.; Bae, Y.C.; Kim, S.K.; Park, J.S.; Ahn, D.K. Behavioral evidence for the differential regulation of p-p38 MAPK and p-NF-κB in rats with trigeminal neuropathic pain. Mol. Pain 2011, 7, 57. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Mei, W.; Tian, X.B.; Tian, Y.K.; Liu, D.Q.; Ye, D.W. The therapeutic potential of Nrf2 inducers in chronic pain: Evidence from preclinical studies. Pharmacol. Ther. 2021, 225, 107846. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, H.B.; Wu, X.P.; Guo, Y.L.; Cheng, W.D.; Qian, F. Fisetin alleviates sepsis-induced multiple organ dysfunction in mice via inhibiting p38 MAPK/MK2 signaling. Acta Pharmacol. Sin. 2020, 41, 1348–1356. [Google Scholar] [CrossRef]
- Watanabe, R.; Kurose, T.; Morishige, Y.; Fujimori, K. Protective Effects of Fisetin Against 6-OHDA-Induced Apoptosis by Activation of PI3K-Akt Signaling in Human Neuroblastoma SH-SY5Y Cells. Neurochem. Res. 2018, 43, 488–499. [Google Scholar] [CrossRef]
- Sundarraj, K.; Raghunath, A.; Panneerselvam, L.; Perumal, E. Fisetin Inhibits Autophagy in HepG2 Cells via PI3K/Akt/mTOR and AMPK Pathway. Nutr. Cancer 2021, 73, 2502–2514. [Google Scholar] [CrossRef]
- Yi, P.F.; Wu, Y.C.; Dong, H.B.; Guo, Y.; Wei, Q.; Zhang, C.; Song, Z.; Qin, Q.Q.; Lv, S.; Wu, S.C.; et al. Peimine impairs pro-inflammatory cytokine secretion through the inhibition of the activation of NF-κB and MAPK in LPS-induced RAW264.7 macrophages. Immunopharmacol. Immunotoxicol. 2013, 35, 567–572. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Chu, C.; Liu, S. Astaxanthin protects retinal ganglion cells from acute glaucoma via the Nrf2/HO-1 pathway. J. Chem. Neuroanat. 2020, 110, 101876. [Google Scholar] [CrossRef]
- Heidari Khoei, H.; Fakhri, S.; Parvardeh, S.; Shams Mofarahe, Z.; Baninameh, Z.; Vardiani, M. Astaxanthin prevents the methotrexate-induced reproductive toxicity by targeting oxidative stress in male mice. Toxin Rev. 2019, 38, 248–254. [Google Scholar] [CrossRef]
- Eslami, M.; Esfandyari, S.; Aghahosseini, M.; Rashidi, Z.; Hosseinishental, S.; Brenjian, S.; Sobhani, A.; Amidi, F. Astaxanthin protects human granulosa cells against oxidative stress through activation of NRF2/ARE pathway and its downstream phase II enzymes. Cell J. 2021, 23, 319. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, X.; Wan, C.; Dong, D.; Wang, C.; Xi, Q.; Liu, Y.; Song, T. Astaxanthin alleviates inflammatory pain by regulating the p38 mitogen-activated protein kinase and nuclear factor-erythroid factor 2-related factor/heme oxygenase-1 pathways in mice. Food Funct. 2021, 12, 12381–12394. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, X.; Wu, H.; Liu, C. Artemisinin inhibits angiogenesis by regulating p38 MAPK/CREB/TSP-1 signaling pathway in osteosarcoma. J. Cell. Biochem. 2019, 120, 11462–11470. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, X.; Yang, C.; Jiang, Y.; Zhou, W.; Zheng, W. Artemisinin Attenuates Amyloid-Induced Brain Inflammation and Memory Impairments by Modulating TLR4/NF-κB Signaling. Int. J. Mol. Sci. 2022, 23, 6354. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.S.; Tauchi, M.; Katsuoka, F.; Flanders, K.C.; Liby, K.T.; Honda, T.; Gribble, G.W.; Johnson, D.A.; Johnson, J.A.; Burton, N.C.; et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol. Cancer Ther. 2007, 6, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, X.; Fan, J.; Chen, W.; Wang, J.; Zeng, Y.; Feng, X.; Yu, X.; Yang, X. Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)-induced acute kidney injury through Nrf2 pathway. Toxicology 2014, 318, 22–31. [Google Scholar] [CrossRef]
- Xu, B.; Li, J.; Xu, D.; Ran, Q. PLK4 inhibitor plus bortezomib exhibits a synergistic effect on treating multiple myeloma via inactivating PI3K/AKT signaling. Ir. J. Med. Sci. 2022, 192, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, S.R.; Chen, H.; Luo, Y.; Dong, Y.; Pan, H.L. Mitogen-activated protein kinase signaling mediates opioid-induced presynaptic NMDA receptor activation and analgesic tolerance. J. Neurochem. 2019, 148, 275–290. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, M.; Mao-Ying, Q.L.; Liu, H.; Wang, Z.F.; Zhang, M.T.; Wang, J.; Li, Q.; Liu, S.B.; Mi, W.L.; et al. Early single Aspirin-triggered Lipoxin blocked morphine anti-nociception tolerance through inhibiting NALP1 inflammasome: Involvement of PI3k/Akt signaling pathway. Brain Behav. Immun. 2015, 50, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; Dai, Y.; Wang, S.L.; Yamanaka, H.; Kobayashi, K.; Obata, K.; Chen, J.; Noguchi, K. Differential activation of p38 and extracellular signal-regulated kinase in spinal cord in a model of bee venom-induced inflammation and hyperalgesia. Mol. Pain 2008, 4, 17. [Google Scholar] [CrossRef]
- Crown, E.D.; Gwak, Y.S.; Ye, Z.; Johnson, K.M.; Hulsebosch, C.E. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 2008, 213, 257–267. [Google Scholar] [CrossRef]
- Milligan, E.D.; O’Connor, K.A.; Armstrong, C.B.; Hansen, M.K.; Martin, D.; Tracey, K.J.; Maier, S.F.; Watkins, L.R. Systemic administration of CNI-1493, a p38 mitogen-activated protein kinase inhibitor, blocks intrathecal human immunodeficiency virus-1 gp120-induced enhanced pain states in rats. J. Pain 2001, 2, 326–333. [Google Scholar] [CrossRef]
- Cheng, K.I.; Wang, H.C.; Chuang, Y.T.; Chou, C.W.; Tu, H.P.; Yu, Y.C.; Chang, L.L.; Lai, C.S. Persistent mechanical allodynia positively correlates with an increase in activated microglia and increased P-p38 mitogen-activated protein kinase activation in streptozotocin-induced diabetic rats. Eur. J. Pain 2014, 18, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of p38 Mitogen-Activated Protein Kinase in Spinal Hyperactive Microglia Contributes to Pain Hypersensitivity Following Peripheral Nerve Injury. Glia 2004, 45, 89–95. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Jurga, A.M.; Makuch, W.; Przewlocka, B.; Mika, J. Involvement of pro- and antinociceptive factors in minocycline analgesia in rat neuropathic pain model. J. Neuroimmunol. 2014, 277, 57–66. [Google Scholar] [CrossRef]
- Wen, Y.R.; Suter, M.R.; Kawasaki, Y.; Huang, J.; Pertin, M.; Kohno, T.; Berde, C.B.; Decosterd, I.; Ji, R.R. Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology 2007, 107, 312–321. [Google Scholar] [CrossRef]
- Anand, P.; Shenoy, R.; Palmer, J.E.; Baines, A.J.; Lai, R.Y.K.; Robertson, J.; Bird, N.; Ostenfeld, T.; Chizh, B.A. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur. J. Pain 2011, 15, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, T.; Krishen, A.; Lai, R.Y.; Bullman, J.; Green, J.; Anand, P.; Scholz, J.; Kelly, M. A randomized, placebo-controlled trial of the analgesic efficacy and safety of the p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain from lumbosacral radiculopathy. Clin. J. Pain 2015, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Popiolek-Barczyk, K.; Pavone, F.; Mika, J. Comparison of the Expression Changes after Botulinum Toxin Type A and Minocycline Administration in Lipopolysaccharide-Stimulated Rat Microglial and Astroglial Cultures. Front. Cell. Infect. Microbiol. 2017, 7, 141. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Rojewska, E.; Piotrowska, A.; Barut, J.; Pawlik, K.; Ciapała, K.; Kreiner, G.; Mika, J. New insights into the analgesic properties of the XCL1/XCR1 and XCL1/ITGA9 axes modulation under neuropathic pain conditions—Evidence from animal studies. Front. Immunol. 2022, 13, 1058204. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Rojewska, E.; Makuch, W.; Przewlocka, B. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience 2010, 165, 1420–1428. [Google Scholar] [CrossRef]
- Mika, J.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur. J. Pharmacol. 2007, 560, 142–149. [Google Scholar] [CrossRef]
- Mika, J.; Wawrzczak-Bargiela, A.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naïve and neuropathic mice. Brain Behav. Immun. 2009, 23, 75–84. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Kreiner, G.; Nalepa, I.; Przewlocka, B.; Mika, J. Minocycline influences the anti-inflammatory interleukins and enhances the effectiveness of morphine under mice diabetic neuropathy. J. Neuroimmunol. 2013, 262, 35–45. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Sun, J.; Wang, X.M.; Tian, Y.K.; Wu, W.; Ye, D.W. Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol. Res. 2018, 134, 305–310. [Google Scholar] [CrossRef]
- Ciruela, A.; Dixon, A.K.; Bramwell, S.; Gonzalez, M.I.; Pinnock, R.D.; Lee, K. Identification of MEK1 as a novel target for the treatment of neuropathic pain. Br. J. Pharmacol. 2003, 138, 751–756. [Google Scholar] [CrossRef]
- Han, M.; Huang, R.Y.; Du, Y.M.; Zhao, Z.Q.; Zhang, Y.Q. Early intervention of ERK activation in the spinal cord can block initiation of peripheral nerve injury-induced neuropathic pain in rats. Sheng Li Xue Bao 2011, 63, 106–114. [Google Scholar] [PubMed]
- Zhuang, Z.Y.; Gerner, P.; Woolf, C.J.; Ji, R.R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Quirion, R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain 2002, 99, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhu, C.; Li, Z.H.; Liu, X.Y.; Sun, S.K.; Zhang, T.; Luo, Z.J.; Zhang, H.; Li, W.Y. Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain. J. Neuroinflammation 2015, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.Y.; Wen, Y.R.; Zhang, D.R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef]
- He, N.; Qu, Y.J.; Li, D.Y.; Yue, S.W. RIP3 Inhibition ameliorates chronic constriction injury-induced neuropathic pain by suppressing JNK signaling. Aging 2021, 13, 24417–24431. [Google Scholar] [CrossRef]
- Ying, M.; Liu, H.; Zhang, T.; Jiang, C.; Gong, Y.; Wu, B.; Zou, L.; Yi, Z.; Rao, S.; Li, G.; et al. Effect of artemisinin on neuropathic pain mediated by P2X4 receptor in dorsal root ganglia. Neurochem. Int. 2017, 108, 27–33. [Google Scholar] [CrossRef]
- Dehkordi, F.M.; Kaboutari, J.; Zendehdel, M.; Javdani, M. The antinociceptive effect of artemisinin on the inflammatory pain and role of GABAergic and opioidergic systems. Korean J. Pain 2019, 32, 160–167. [Google Scholar] [CrossRef]
- Elisei, L.M.S.; Moraes, T.R.; Malta, I.H.; Charlie-Silva, I.; Sousa, I.M.O.; Veras, F.P.; Foglio, M.A.; Fraceto, L.F.; Galdino, G. Antinociception induced by artemisinin nanocapsule in a model of postoperative pain via spinal TLR4 inhibition. Inflammopharmacology 2020, 28, 1537–1551. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Zhang, L.; Sun, T.; Fu, Z. Intrathecal injection of ozone alleviates CCI-induced neuropathic pain via the GluR6-NF-κB/p65 signalling pathway in rats. Mol. Med. Rep. 2021, 23, 231. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Sun, Y.; Li, H.; Mi, W.; Jiang, Y. Analgesic effects of TLR4/NF-κB signaling pathway inhibition on chronic neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Biomed. Pharmacother. 2018, 107, 526–533. [Google Scholar] [CrossRef]
- Miao, J.; Zhou, X.; Ji, T.; Chen, G. NF-κB p65-dependent transcriptional regulation of histone deacetylase 2 contributes to the chronic constriction injury-induced neuropathic pain via the microRNA-183/TXNIP/NLRP3 axis. J. Neuroinflammation 2020, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Kolosowska, N.; Piotrowska, A.; Makuch, W.; Rojewska, E.; Jurga, A.M.; Pilat, D.; Mika, J. Parthenolide Relieves Pain and Promotes M2 Microglia/Macrophage Polarization in Rat Model of Neuropathy. Neural Plast. 2015, 2015, 676473. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, W.; Pan, L.; Zhang, A.; Chen, Q.; Xu, K.; Lu, H.; Chen, Y. Peimine, a main active ingredient of Fritillaria, exhibits anti-inflammatory and pain suppression properties at the cellular level. Fitoterapia 2016, 111, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alberola-Die, A.; Encinar, J.A.; Cobo, R.; Fernández-Ballester, G.; González-Ros, J.M.; Ivorra, I.; Morales, A. Peimine, an Anti-Inflammatory Compound from Chinese Herbal Extracts, Modulates Muscle-Type Nicotinic Receptors. Int. J. Mol. Sci. 2021, 22, 11287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Cui, W.G.; Ma, Q.; Zhou, W.H. Fisetin exerts antihyperalgesic effect in a mouse model of neuropathic pain: Engagement of spinal serotonergic system. Sci. Rep. 2015, 5, srep09043. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.L.; Liu, X.; Wang, C.; Zhou, D.S.; Ma, Q.; Zhou, W.H.; Hu, Z.Y. Antinociceptive effects of fisetin against diabetic neuropathic pain in mice: Engagement of antioxidant mechanisms and spinal GABAA receptors. Pharmacol. Res. 2015, 102, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFκB signaling pathways in SKH-1 hairless mice. Photochem. Photobiol. 2015, 91, 225–234. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Wang, L.; Liu, P.; Zhao, S.; Li, H.; Jiang, Y.; Guo, Y.; Wang, X. Resveratrol inhibits paclitaxel-induced neuropathic pain by the activation of pi3k/akt and sirti/pgcia pathway. J. Pain Res. 2019, 12, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Zhu, C. Biological and neurological activities of astaxanthin (Review). Mol. Med. Rep. 2022, 26, 300. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, X.; Song, T. Astaxanthin alleviates neuropathic pain by inhibiting the MAPKs and NF-κB pathways. Eur. J. Pharmacol. 2021, 912, 174575. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidve, P.; Prajapati, N.; Kalia, K.; Tiwari, V. Astaxanthin ameliorates behavioral and biochemical alterations in in-vitro and in-vivo model of neuropathic pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Koh, H.K.; Kim, D.S. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-κB-mediated signals in activated microglia. Int. Immunopharmacol. 2010, 10, 1560–1572. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, Q.; Liu, F.; Jing, C.; Ding, L.; Zhang, L.; Pang, C. Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci. Lett. 2018, 662, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, X.S.; Wang, H.D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.L.; Wang, X.L. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (nrf2-are) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.B.; Qiu, H.Y. Neuroprotective effect of tanshinone IIA against neuropathic pain in diabetic rats through the Nrf2/ARE and NF-κB signaling pathways. Kaohsiung J. Med. Sci. 2018, 34, 428–437. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.Y.; Zhang, L.Q.; Li, D.Y.; Wu, J.Y.; Gao, S.J.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. Nrf2 Activation Attenuates Chronic Constriction Injury-Induced Neuropathic Pain via Induction of PGC-1 α -Mediated Mitochondrial Biogenesis in the Spinal Cord. Oxid. Med. Cell. Longev. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Chen, N.; Sun, J.; Wang, X.M.; Cao, F.; Tian, Y.K.; Ye, D.W. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020, 41, 1041–1048. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Kumar, R.; Sherkhane, B.; Gundu, C.; Arruri, V.K.; Kumar, A. Bardoxolone Methyl Ameliorates Hyperglycemia Induced Mitochondrial Dysfunction by Activating the keap1-Nrf2-ARE Pathway in Experimental Diabetic Neuropathy. Mol. Neurobiol. 2020, 57, 3616–3631. [Google Scholar] [CrossRef]
- Chien, J.Y.; Chou, Y.Y.; Ciou, J.W.; Liu, F.Y.; Huang, S.P. The effects of two Nrf2 activators, bardoxolone methyl and omaveloxolone, on retinal ganglion cell survival during ischemic optic neuropathy. Antioxidants 2021, 10, 1466. [Google Scholar] [CrossRef]
- Schembri, E. Are Opioids Effective in Relieving Neuropathic Pain? SN Compr. Clin. Med. 2019, 1, 30–46. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Mika, J. The importance of chemokines in neuropathic pain development and opioid analgesic potency. Pharmacol. Rep. 2018, 70, 821–830. [Google Scholar] [CrossRef]
- Wen, Y.R.; Tan, P.H.; Cheng, J.K.; Liu, Y.C.; Ji, R.R. Microglia: A promising target for treating neuropathic and postoperative pain, and morphine tolerance. J. Formos. Med. Assoc. 2011, 110, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Leánez, S.; Pol, O. The inhibition of the nitric oxide-cGMP-PKG-JNK signaling pathway avoids the development of tolerance to the local antiallodynic effects produced by morphine during neuropathic pain. Eur. J. Pharmacol. 2012, 685, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Okerman, T.; Jurgenson, T.; Moore, M.; Klein, A.H. Inhibition of the phosphoinositide 3-kinase-AKT-cyclic GMP-c-Jun N-terminal kinase signaling pathway attenuates the development of morphine tolerance in a mouse model of neuropathic pain. Mol. Pain 2021, 17, 17448069211003375. [Google Scholar] [CrossRef]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Pol, O. Treatment with 5-fluoro-2-oxindole Increases the Antinociceptive Effects of Morphine and Inhibits Neuropathic Pain. Cell. Mol. Neurobiol. 2021, 41, 995–1008. [Google Scholar] [CrossRef]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane inhibited the nociceptive responses, anxiety—And depressive-like behaviors associated with neuropathic pain and improved the anti-allodynic effects of morphine in mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Corbett, A.D.; Paterson, S.J.; Kosterlitz, H.W. Selectivity of Ligands for Opioid Receptors. In Opioids; Springer: Berlin/Heidelberg, Germany, 1993; pp. 645–679. [Google Scholar] [CrossRef]
- Nasseef, M.T.; Singh, J.P.; Ehrlich, A.T.; McNicholas, M.; Park, D.W.; Ma, W.; Kulkarni, P.; Kieffer, B.L.; Darcq, E. Oxycodone-Mediated Activation of the Mu Opioid Receptor Reduces Whole Brain Functional Connectivity in Mice. ACS Pharmacol. Transl. Sci. 2019, 2, 264–274. [Google Scholar] [CrossRef]
- Gudin, J.; Fudin, J. A Narrative Pharmacological Review of Buprenorphine: A Unique Opioid for the Treatment of Chronic Pain. Pain Ther. 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhou, S.; Kochukov, M.Y.; Westlund, K.N.; Carlton, S.M. Plasticity in intact A delta- and C-fibers contributes to cold hypersensitivity in neuropathic rats. Neuroscience 2007, 150, 182–193. [Google Scholar] [CrossRef]
- Baba, H.; Doubell, T.P.; Woolf, C.J. Peripheral Inflammation Facilitates Aβ Fiber-Mediated Synaptic Input to the Substantia Gelatinosa of the Adult Rat Spinal Cord. J. Neurosci. 1999, 19, 859. [Google Scholar] [CrossRef]
- Khan, M.I.; Momeny, M.; Ostadhadi, S.; Jahanabadi, S.; Ejtemaei-Mehr, S.; Sameem, B.; Zarrinrad, G.; Dehpour, A.R. Thalidomide attenuates development of morphine dependence in mice by inhibiting PI3K/Akt and nitric oxide signaling pathways. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 39–48. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Ciapała, K.; Mika, J. Kynurenic acid and zaprinast diminished CXCL17-evoked pain-related behaviour and enhanced morphine analgesia in a mouse neuropathic pain model. Pharmacol. Rep. 2019, 71, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Rojewska, E.; Mika, J. Comparison of the effects of chemokine receptors CXCR2 and CXCR3 pharmacological modulation in neuropathic pain model—In vivo and in vitro study. Int. J. Mol. Sci. 2021, 22, 11074. [Google Scholar] [CrossRef]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Administration Diminishes Hypersensitivity and Enhances the Analgesic Potency of Morphine and Buprenorphine in a Mouse Model of Neuropathic Pain. Front. Immunol. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Pawlik, K.; Ciapała, K.; Mika, J. Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model. Brain Sci. 2023, 13, 579. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Ciapała, K.; Rojewska, E.; Makuch, W.; Mika, J. Comparison of the beneficial effects of RS504393, maraviroc and cenicriviroc on neuropathic pain-related symptoms in rodents: Behavioral and biochemical analyses. Int. Immunopharmacol. 2020, 84, 106540. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapała, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but not CCL12, play a significant role in the development of pain-related behavior and opioid-induced analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef]
- Fairbanks, C.A. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv. Drug Deliv. Rev. 2003, 55, 1007–1041. [Google Scholar] [CrossRef] [PubMed]
| ED50 | Von Frey Test | Cold Plate Test |
|---|---|---|
| artemisinin | - | - |
| fisetin | 68.5 (27.3 ± 171.5) | 40.5 (14.7 ± 111.3) |
| peimine | 214.2 (19.8 ± 2321.5) | 60.5 (14.3 ± 256.6) |
| astaxanthin | 0.7 (0.2 ± 1.9) | 0.6 (0.4 ± 0.8) |
| bardoxolone methyl | 22.0 (4.9 ± 98.4) | 4.8 (2.5 ± 9.1) |
| 740 Y-P | 0.7 (20.4 ± 200.6) | 7.5 (2.0 ± 27.9) |
| %MPE | Von Frey Test | Cold Plate Test |
|---|---|---|
| vehicle | −0.22 ± 1.99 | 0.76 ± 4.12 |
| artemisinin (10 µg/5 µL) | 3.38 ± 8.32 | −10.34 ± 4.83 |
| fisetin (240 µg/5 µL) | 71.57 ± 12.07 *** | 41.31 ± 5.79 *** |
| peimine (60 µg/5 µL) | 30.91 ± 10.73 * | 22.76 ± 2.65 ** |
| astaxanthin (2 µg/5 µL) | 64.82 ± 10.14 *** | 71.05 ± 6.14 *** |
| bardoxolone methyl (20 µg/5 µL) | 42.96 ± 8.77 ** | 67.85 ± 2.10 *** |
| 740 Y-P (20 µg/5 µL) | 38.64 ± 9.93 ** | 38.30 ± 13.14 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciapała, K.; Rojewska, E.; Pawlik, K.; Ciechanowska, A.; Mika, J. Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 9000. https://doi.org/10.3390/ijms24109000
Ciapała K, Rojewska E, Pawlik K, Ciechanowska A, Mika J. Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain. International Journal of Molecular Sciences. 2023; 24(10):9000. https://doi.org/10.3390/ijms24109000
Chicago/Turabian StyleCiapała, Katarzyna, Ewelina Rojewska, Katarzyna Pawlik, Agata Ciechanowska, and Joanna Mika. 2023. "Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain" International Journal of Molecular Sciences 24, no. 10: 9000. https://doi.org/10.3390/ijms24109000
APA StyleCiapała, K., Rojewska, E., Pawlik, K., Ciechanowska, A., & Mika, J. (2023). Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain. International Journal of Molecular Sciences, 24(10), 9000. https://doi.org/10.3390/ijms24109000