Quantifying the Detrimental Effects of Multiple Freeze/Thaw Cycles on Primary Human Lymphocyte Survival and Function
Abstract
1. Introduction
2. Results
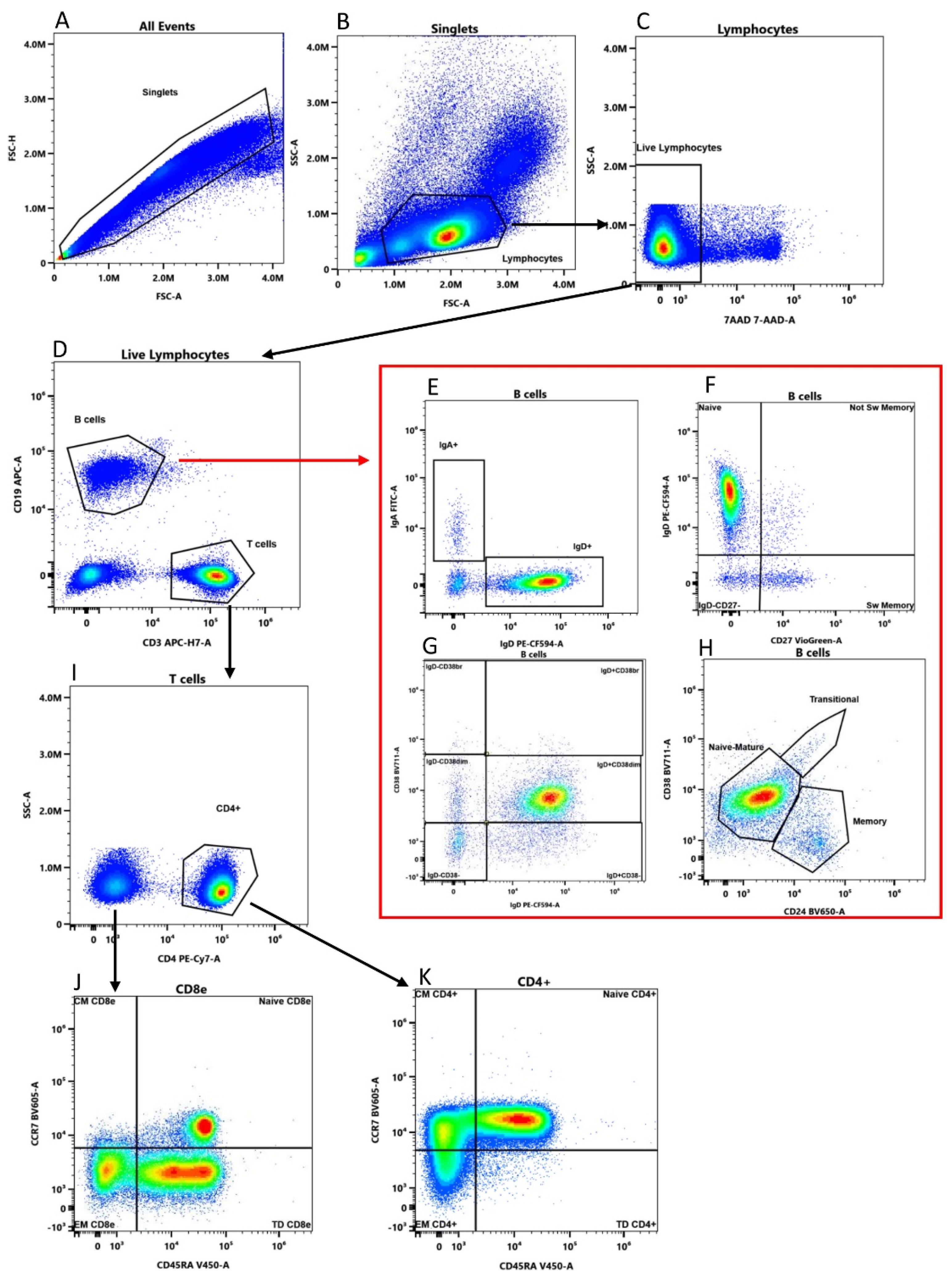
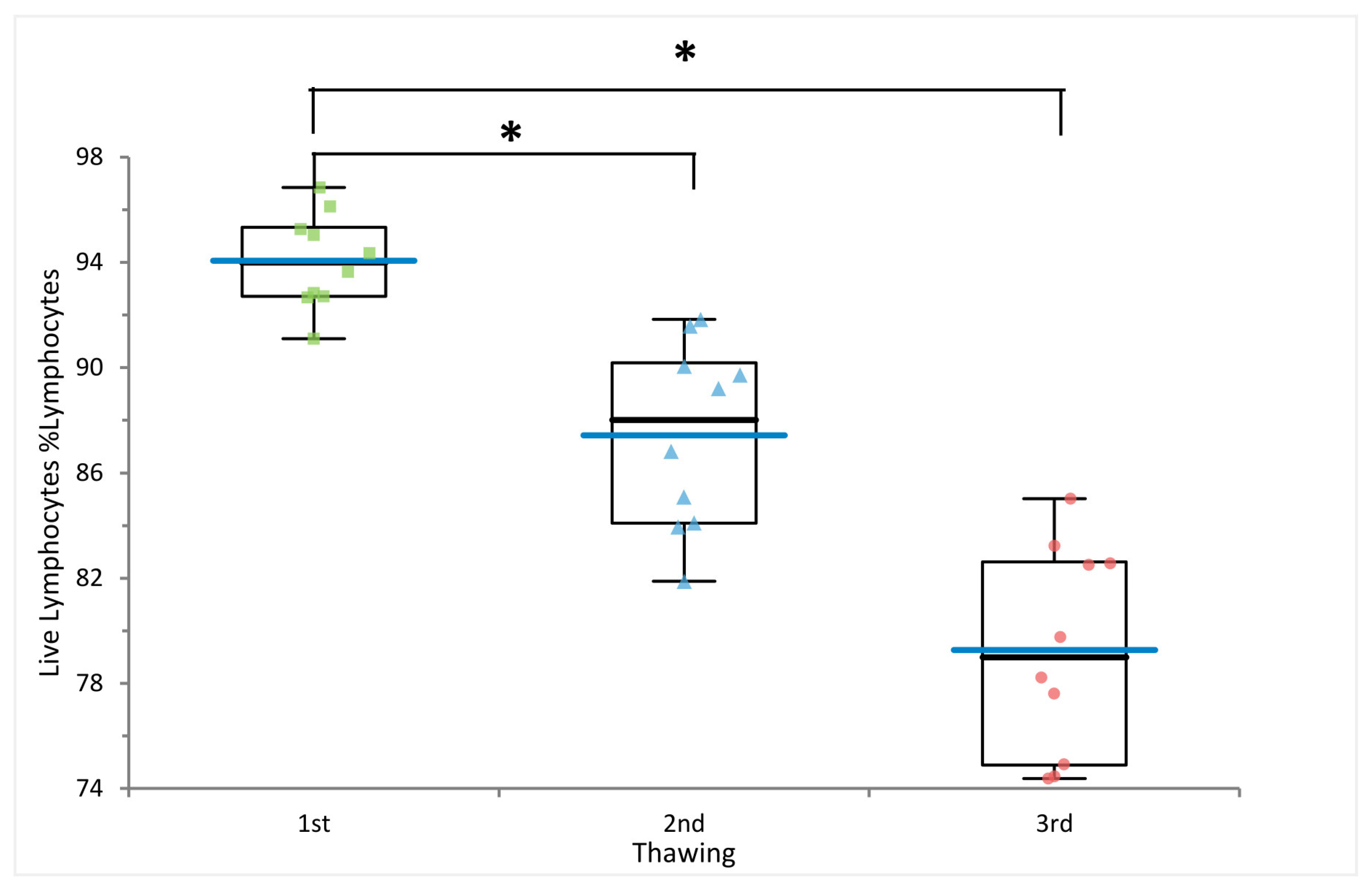
2.1. Multiple Freezing Cycles Affect Lymphocyte Viability but Not Cell Frequencies
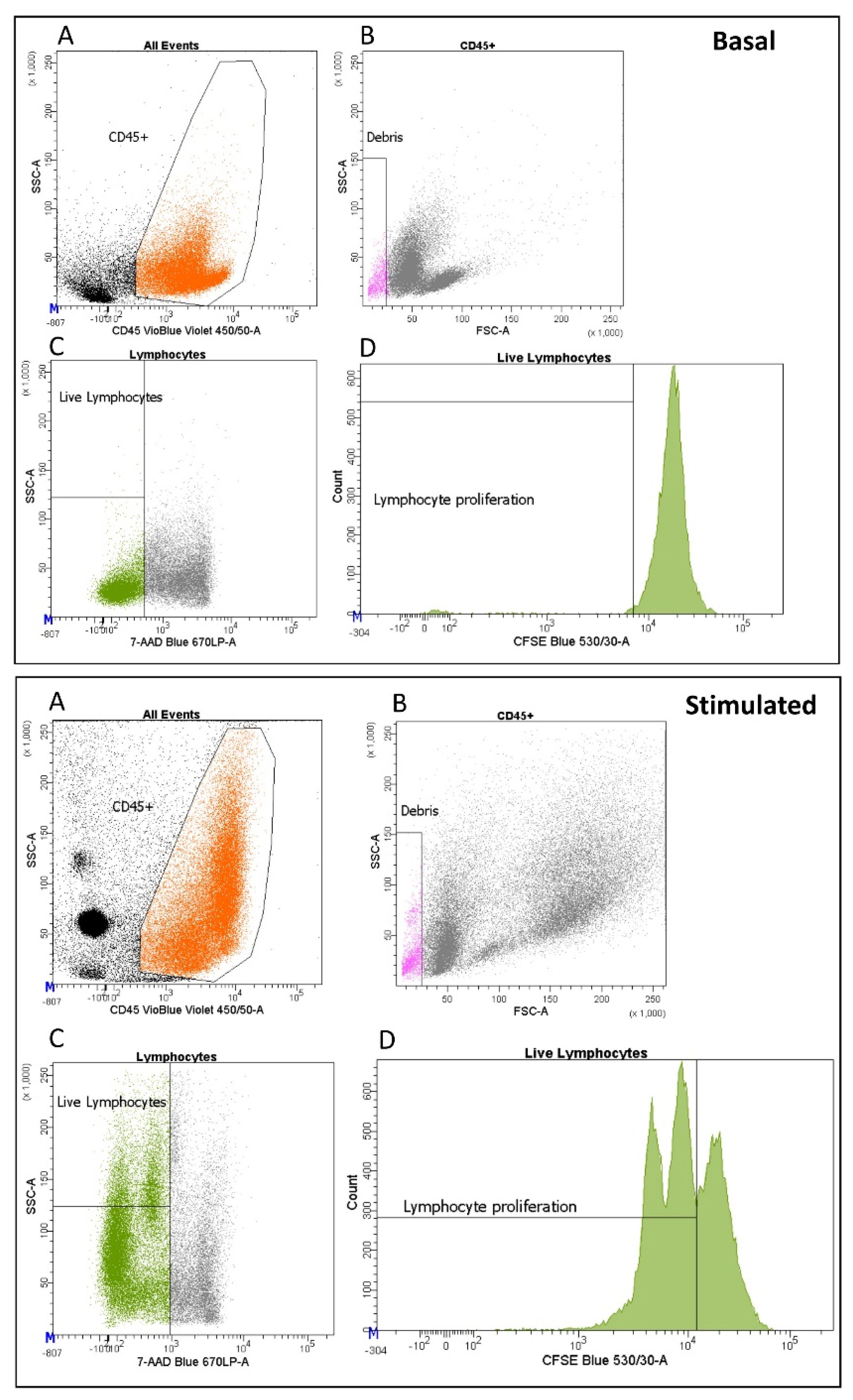
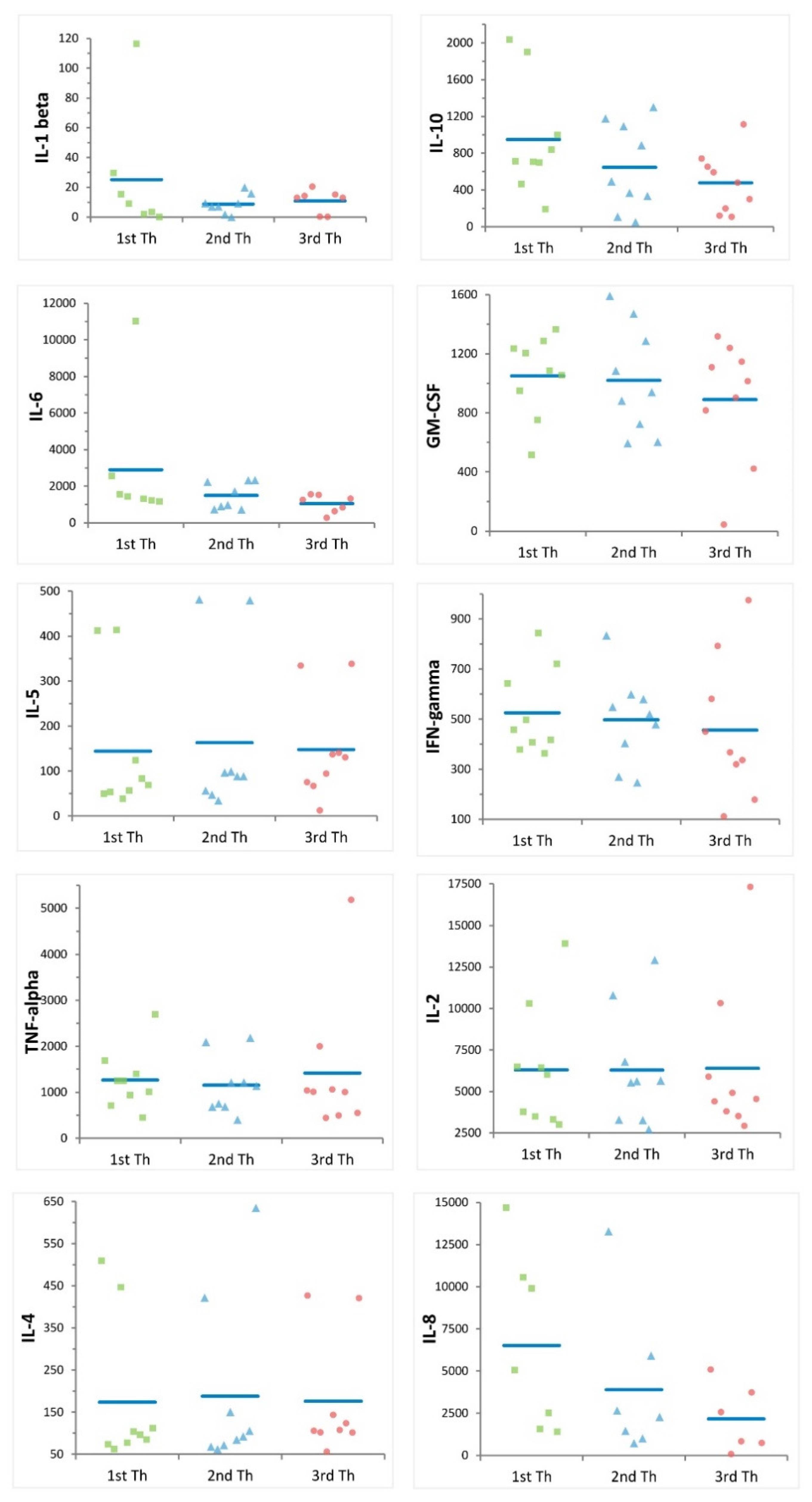
2.2. Multiple Freezing Cycles Affect Proliferation Activity but Not Cytokine Production
3. Discussion
4. Materials and Methods
4.1. The SardiNIA Dataset
4.2. Peripheral Blood Mononuclear Cell Isolation and Cryopreservation
4.3. Thawing, Cell Population Assessment and PBMC Refreezing
4.4. Cytek Aurora Settings
4.5. Proliferation Assay and Cytokine Production
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1266. [Google Scholar] [CrossRef] [PubMed]
- Patin, E.; Hasan, M.; Bergstedt, J.; Rouilly, V.; Libri, V.; Urrutia, A.; Alanio, C.; Scepanovic, P.; Hammer, C.; Jönsson, F.; et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol. 2018, 19, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M.; Quaye, L.; Mangino, M.; Beddall, M.H.; Mahnke, Y.; Chattopadhyay, P.; Tosi, I.; Napolitano, L.; Barberio, M.T.; Menni, C.; et al. The Genetic Architecture of the Human Immune System: A Bioresource for Autoimmunity and Disease Pathogenesis. Cell 2015, 161, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Orrù, V.; Steri, M.; Sole, G.; Sidore, C.; Virdis, F.; Dei, M.; Lai, S.; Zoledziewska, M.; Busonero, F.; Mulas, A.; et al. Genetic variants regulating immune cell levels in health and disease. Cell 2013, 155, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Steri, M.; Orrù, V.; Idda, M.L.; Pitzalis, M.; Pala, M.; Zara, I.; Sidore, C.; Faà, V.; Floris, M.; Deiana, M.; et al. Overexpression of the Cytokine BAFF and Autoimmunity Risk. N. Engl. J. Med. 2017, 376, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Jenks, S.A.; Cashman, K.S.; Zumaquero, E.; Marigorta, U.M.; Patel, A.V.; Wang, X.; Tomar, D.; Woodruff, M.C.; Simon, Z.; Bugrovsky, R.; et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 2018, 49, 725–739.e6, Erratum in: Immunity 2020, 52, 203. [Google Scholar] [CrossRef] [PubMed]
- Orrù, V.; Steri, M.; Cucca, F.; Fiorillo, E. Application of Genetic Studies to Flow Cytometry Data and Its Impact on Therapeutic Intervention for Autoimmune Disease. Front. Immunol. 2021, 12, 714461. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Orrù, V.; Lai, S.; Lobina, M.; Steri, M.; Cucca, F.; Fiorillo, E. Comparison of Whole Blood Cryopreservation Methods for Extensive Flow Cytometry Immunophenotyping. Cells 2022, 11, 1527. [Google Scholar] [CrossRef] [PubMed]
- Braudeau, C.; Guen, N.S.; Chevreuil, J.; Rimbert, M.; Martin, J.C.; Josien, R. An easy and reliable whole blood freezing method for flow cytometry immuno-phenotyping and functional analyses. Cytom. Part B Clin. Cytom. 2021, 100, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, C.P.; Kohli, V.; Balion, C. A comprehensive assessment of immunophenotyping performed in cryopreserved peripheral whole blood. Cytom. Part B Clin. Cytom. 2017, 94, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Toh, Z.Q.; Reitsma, A.; Do, L.; Nathanielsz, J.; Licciardi, P.V. Effect of peripheral blood mononuclear cell cryopreservation on innate and adaptive immune responses. J. Immunol. Methods 2019, 465, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ticha, O.; Moos, L.; Bekeredjian-Ding, I. Effects of long-term cryopreservation of PBMC on recovery of B cell subpopulations. J. Immunol. Methods 2021, 495, 113081. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, C.; Jia, G.; Liu, Y.; Wang, N.; Yang, F.; Su, R.; Shang, Y.; Han, Y. Comprehensive evaluation of the effects of long-term cryopreservation on peripheral blood mononuclear cells using flow cytometry. BMC Immunol. 2022, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Nazarpour, R.; Zabihi, E.; Alijanpour, E.; Abedian, Z.; Mehdizadeh, H.; Rahimi, F. Optimization of Human Peripheral Blood Mononuclear Cells (PBMCs) Cryopreservation. Int. J. Mol. Cell. Med. 2012, 1, 88–93. [Google Scholar] [PubMed]
- Hønge, B.L.; Petersen, M.S.; Olesen, R.; Møller, B.K.; Erikstrup, C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLoS ONE 2017, 12, e0187440. [Google Scholar] [CrossRef] [PubMed]
- Bohnhorst, J.; Bjørgan, M.B.; Thoen, J.E.; Natvig, J.B.; Thompson, K.M. Bm1–Bm5 Classification of Peripheral Blood B Cells Reveals Circulating Germinal Center Founder Cells in Healthy Individuals and Disturbance in the B Cell Subpopulations in Patients with Primary Sjögren’s Syndrome. J. Immunol. 2001, 167, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Kondo, T.; Matsuki, F.; Takata, H.; Takiguchi, M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int. Immunol. 2008, 20, 1189–1199. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, V.; Fiorillo, E.; Cucca, F.; Orrù, V. Quantifying the Detrimental Effects of Multiple Freeze/Thaw Cycles on Primary Human Lymphocyte Survival and Function. Int. J. Mol. Sci. 2023, 24, 634. https://doi.org/10.3390/ijms24010634
Serra V, Fiorillo E, Cucca F, Orrù V. Quantifying the Detrimental Effects of Multiple Freeze/Thaw Cycles on Primary Human Lymphocyte Survival and Function. International Journal of Molecular Sciences. 2023; 24(1):634. https://doi.org/10.3390/ijms24010634
Chicago/Turabian StyleSerra, Valentina, Edoardo Fiorillo, Francesco Cucca, and Valeria Orrù. 2023. "Quantifying the Detrimental Effects of Multiple Freeze/Thaw Cycles on Primary Human Lymphocyte Survival and Function" International Journal of Molecular Sciences 24, no. 1: 634. https://doi.org/10.3390/ijms24010634
APA StyleSerra, V., Fiorillo, E., Cucca, F., & Orrù, V. (2023). Quantifying the Detrimental Effects of Multiple Freeze/Thaw Cycles on Primary Human Lymphocyte Survival and Function. International Journal of Molecular Sciences, 24(1), 634. https://doi.org/10.3390/ijms24010634





