Abstract
Histone deacetylases tuin (HDT) is a plant-specific protein subfamily of histone deacetylation enzymes (HDAC) which has a variety of functions in plant development, hormone signaling and stress response. Although the HDT family’s genes have been studied in many plant species, they have not been characterized in Brassicaceae. In this study, 14, 8 and 10 HDT genes were identified in Brassica napus, Brassica rapa and Brassica oleracea, respectively. According to phylogenetic analysis, the HDTs were divided into four groups: HDT1(HD2A), HDT2(HD2B), HDT3(HD2C) and HDT4(HD2D). There was an expansion of HDT2 orthologous genes in Brassicaceae. Most of the HDT genes were intron-rich and conserved in gene structure, and they coded for proteins with a nucleoplasmin-like (NPL) domain. Expression analysis showed that B. napus, B. rapa, and B. oleracea HDT genes were expressed in different organs at different developmental stages, while different HDT subgroups were specifically expressed in specific organs and tissues. Interestingly, most of the Bna/Br/BoHDT2 members were expressed in flowers, buds and siliques, suggesting they have an important role in the development of reproductive organs in Brassicaceae. Expression of BnaHDT was induced by various hormones, such as ABA and ethylene treatment, and some subgroups of genes were responsive to heat treatment. The expression of most HDT members was strongly induced by cold stress and freezing stress after non-cold acclimation, while it was slightly induced after cold acclimation. In this study, the HDT gene family of Brassicaceae was analyzed for the first time, which helps in understanding the function of BnaHDT in regulating plant responses to abiotic stresses, especially freezing stresses.
1. Introduction
Posttranslational modifications of histones in plant genomes, such as acetylation, methylation, phosphorylation and ubiquitination, establish a rapid and reversible pattern of gene expression. The steady-state acetylation level of histones is achieved through the action of histone acetyl transferases (HATs) and histone deacetylation enzymes (HDACs) by adding an acetyl group to the N-terminal of histones, which is a marker of transcriptional activation in eukaryotes. Acetylation involves the acetylation of two amino acids, lysine and arginine. Compared with arginine, lysine acetylation is more conservative and more thoroughly studied [1]. Histone acetylation has been reported in different lysine residues of histones, such as H3 (K4, K9, K14, K18, K23 and K27), H4 (K5, K8, K12 and K16) and H2B (K5, K12, K15 and K20) [2]. Acetylation of lysine residues at the tail of histones reduces the amount of positive charge carried by histones and reduces their affinity to negatively charged DNA strands, which results in the unwinding of local DNA and histone octamers, thus promoting the binding of various protein factors involved in transcriptional regulation with DNA-specific sequences and then playing a transcriptional regulation role [3,4].
The histone deacetylases (HDAC) are a supergene family that is widespread in eukaryotes, including yeast, mammals and plants. HDACs are divided into three families: the RPD3/HDA1 family, SIR2 family and HDT family. The HDT (HD2) family is plant-specific histone deacetylase [5], which is completely different from the RPD3/HDA1 family in sequence [6]. It has also been shown that HDA evolution is associated with increased structural ductility/disorder, and two Brassicaceae-specific HDAs have been identified, as well as key mutations that affect the catalytic activity of individual HDAs [7]. RPD3 and SIR2 share sequence homology with yeast HDACs, whereas the HDT family shares no sequence homology with yeast HDACs [8,9].
More and more data have shown that HDACs play a key role in plant growth and development, including bud, flower [2,10,11], seed [3,12] and root development [13], and that they respond to biotic and abiotic stresses, such as drought [14], salt stress [15], low temperature [16], and ABA [5,17,18]. In Arabidopsis, there are four AtHDT members, AtHDT1 (AtHD2A), AtHDT2 (AtHD2B) [12], AtHDT3 (AtHD2C) [19] and AtHDT4 (AtHD2D) [20,21]. AtHDT1, AtHDT2 and AtHDT3 can mediate transcriptional inhibition in Arabidopsis [14,22]. AtHDT1 is highly expressed in Arabidopsis flowers and young siliques, while AtHDT2 is widely expressed in stems, leaves, flowers and young siliques [14]. Overexpression of AtHDT1 in Arabidopsis results in leaf abnormalities, delayed flowering and seed abortion [19]. Arabidopsis HDT1 and HDT2 affect leaf morphological development by regulating the expression of miR165/166 in Arabidopsis [23]. AtHDT3 is inhibited by abscisic acid, and its overexpression confers ABA sensitivity phenotypes, reduces transpiration and enhances tolerance to salt and drought stress [18].
HDTs have been studied in crops such as rice, soybean, tomato and potato [24,25]. Overexpression of HDT701 induced early flowering of hybrid rice under long-day conditions compared with the parents. HDT702 knockdown resulted in abnormal plant height and narrow leaf and stem segments [26]. ScHDT1 in potato (Solanum chacoense) is a homolog of Arabidopsis HDT1, which has been shown to increase the accumulation of transcripts in ovules after fertilization [27]. SlHDT3 was a positive regulator of fruit ripening by affecting ethylene synthesis and carotenoid accumulation [15,25].
HvHDAC2-1 in barley was induced by jasmonic acid (JA), ABA and salicylic acid (SA) [28]. Studies show that two HDTs act as key negative regulators of elicitor-induced cell death in tobacco [29]. Overexpression of HDT701 in rice decreases ABA, salt and osmotic stress resistance during seed germination [24]. In Arabidopsis, High Expression of Osmotically Responsive Gene (HOS15) interacts with HDT3 in the promoter region of the Cold-responsive (COR) gene, thereby mediating HDT3 degradation, leading to upregulation of the COR gene as part of the cold stress response [30].
B. napus (2n = 38, AACC) is a heterotetraploid hybrid species formed by the natural hybridization of Brassica rapa (2n = 20, AA) and Brassica oleracea (2n = 18, CC) through natural chromosome doubling [31]. In this study, we investigate the significant role of HDT genes in Brassicaceae plants. Therefore, we identified 32 HDT genes of B. napus, B. rapa and B. oleracea and compared their gene structures, chromosomal locations, evolutionary relationships and expression patterns in different tissues and under different abiotic/biotic stresses and plant hormonal treatments. Thus, the comprehensive analysis in this study provides a fundamental understanding of HDACs in development and stress responses in Brassicaceae plants.
2. Results
2.1. Identification and Characterization of HDT Genes in B. napus, B. rapa and B. oleracea
To identify HDT proteins in B. napus, B. rapa and B. oleracea, we performed a BLASTp search against the annotated proteins of B. napus, B.rapa and B. oleracea in Ensembl Plants (http://plants.ensembl.org/index.html, accessed on 13 August 2021) using Arabidopsis AtHDT protein (AtHDT1, AtHDT2, AtHDT3 and AtHDT4) sequences as queries. Sets of 14 (1 BnaHDT1, 10 BnaHDT2, 1 BnaHDT3 and 2 BnaHDT4), 8 (6 brHDT2, 1 brHDT3 and 1 brHDT4) and 10 (1 BoHDT1, 7 BoHDT2, 1 BoHDT3 and 1 BoHDT4) HDT proteins were identified in B. napus, B. rapa and B. oleracea, respectively (Figure S1).
All 32 HDTs in B. napus, B. rapa and B. oleracea contained conserved NPL domains. Among them, 27 HDT proteins were predicted to localize in the nucleus by using ProtComp Version 9.0; these protein are acidic proteins with PI values of less than 5, while 5 HDT2 proteins (Bra020207, Bo2g024200, Bo2g024210, Bo2g024220 and BnaA02g05820D) were weakly acidic or alkaline proteins, having no firm predicted subcellular localization, and their PI values were all greater than 6 (Table 1 and Figure S1).

Table 1.
List of HDT genes identified in Arabidopsis, B. rape, B. oleracea and B. napus.
All the HDT genes are divided into typical HDT and short abnormal HDT categories. Typical HDT gene members are HDT1, HDT2, HDT3 and HDT4, which have 215–356 amino acids and 5–11 exons (Table 1). It was discovered using isoelectric dot analysis that HDT is a typical acidic protein with a typical acidic region. Additionally, there are six short abnormal genes (Bra020206, Bo2g024150, Bra020204, BnaA02g05810D, BnaA02g05790D and BnaCnng34610D), which typically contain 162–184 amino acids and have three exons and two introns. According to gene structural analysis, all are lacking the last few amino acids of the carboxy-terminus. There is also a long HDT gene (Bo2g024200). The first half of the long gene is the typical structure of HDT, and the second half results from a fusion with another sequence (Figure S2).
2.2. Phylogenetic Analysis and Chromosomal Locations of HDT Genes in B. napus, B. rapa and B. oleracea
To explore the classification and evolutionary characteristics of the HDT proteins, an unrooted phylogenetic tree based on the 36 protein sequences of B. napus (14), B. rapa (8), B. oleracea (10) and Arabidopsis (4) HDT genes was constructed in MEGA X (Figure 1). According to the phylogenetic analysis, the HDT genes were divided into four groups: HDT1 (homologous to AT3G44750.1/AtHDT1), HDT2 (homologous to AT5G22650.1/AtHDT2), HDT3 (homologous to AT5G03740.1/AtHDT3) and HDT4 (homologous to AT2G27840.1/AtHDT4). HDT2 has six sets of homologs, while the other three have only one each. These results indicated that there was an expansion of HDT2 homologous genes in B. napus, B.rapa and B. oleracea.
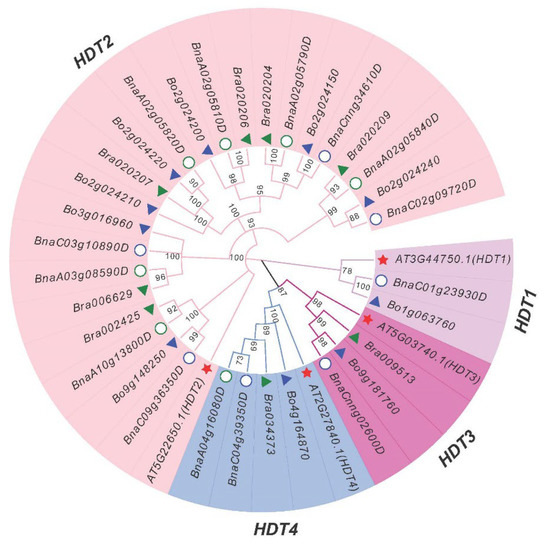
Figure 1.
Phylogenetic analysis of 36 HDT proteins from B. napus (14), B. rapa, (8), B. oleracea (10) and Arabidopsis (4).
A neighbor-joining phylogenetic tree was generated by MEGA X with full-length HDT sequences (1000 bootstrap replicates). The resulting four groups classified into four resulting subfamilies (HDT1, HDT2, HDT3 and HDT4, highlighted in purple, pink, red and blue, respectively) are labeled. According to the homologous gene sets among the Ar (B. rapa,), Co (B. oleracea), An and Cn subgenomes of B. napus, six Ar-Co-An-Cn pairs were identified among the 32 Bna/Br/BoHDT genes (Figure 1). The A genome of B. napus lacks the homologous genes corresponding to the B. rapa HDT1 gene Bra009513, and the C genome lacks the homologous genes corresponding to the B. oleracea HDT2 genes (Bo2g024210, Bo2g024220 and Bo2g024200), while the homologous genes in the HDT3 and HDT4 subgroups were relatively conserved (Figure 2 and Figure S3).
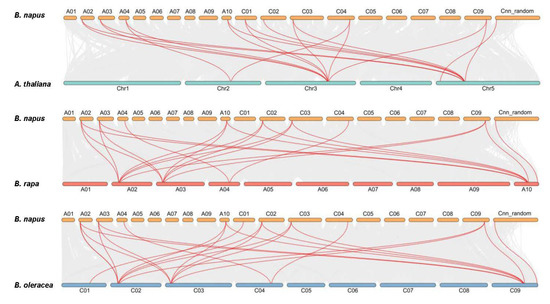
Figure 2.
Syntenic relationship of HDAC genes in B. napus and three ancestral plant species. Grey lines in the background show the collinear blocks within rapeseed and other plant genomes, while the red lines highlight the syntenic HDAC gene pairs.
The An and Cn subgenomes of B. napus were collinear with the corresponding diploid Ar and Co genomes, most of the An-Ar and Cn-Co homologous pairs showed similar chromosomal locations [32]. As shown in Figure 2, the 14 BnaHDT genes were unevenly distributed on the 10 chromosomes of B. napus (chrA02-A04, A10, C01-C04, chrC09 and chrCnn_random. There was one tandem gene, BnaA02g05790D/BnaA02g05810D/BnaA02g05820D/BnaA02g05840D, on chromosome A02 in B. napus, which was homologous to the tandem gene Bra020204/Bra020206/Bra020207/Bra020209 on chromosome A02 in B. rapa, while the tandem pair (Bo2g024150/Bo2g024200/Bo2g024210/Bo2g024220/Bo2g024240) corresponding to B. olearcea on chromosome C02 in B. napus was lost (Figure 2 and Figure S3). Therefore, it was inferred that the tandem genome was mutated in the homologous evolution process.
2.3. Motif Analysis (MEME) of HDT
To further analyze the domains and motifs of the HDT proteins, 10 motifs were predicted by MEME (Figure S4) (http://meme-suite.org/tools/meme, accessed on 24 May 2022). As shown in Figure 3B, all HDT proteins contain Motif 3, and Motif 2-1-5-3 constitutes the NPL domain (Figure S1). All of the HDT1 proteins contain Motif 2-5-3-8-6-7-4-9. HDT2 proteins contain Motif 2-1-3-7, except Bo2g024210, which only contains Motif 2-1-3. HDT3 proteins contain Motif 2-1-5-3-6-6-7-4-9. HDT4 proteins contain Motif 2-5-3-6-7, while AT2G27840 only contains Motif 3-6-7. Two of the six short abnormal genes, Bra020206 and BnaA02g05810D, lack Motif 4-6-10, while Bo2g024150, Bra020204, BnaA02g05790D and BnaCnng34610D lack Motif 4-9-10. The long HDT gene (Bo2g024200) contains Motif 2-1-5-3-8-2-1-5-3-7 (Figure 3B).
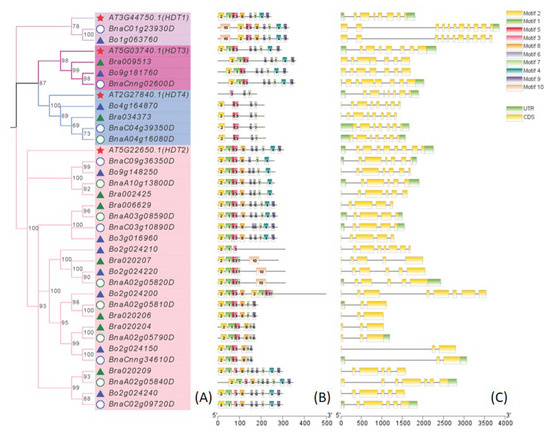
Figure 3.
Phylogenetic tree (A), gene motifs (B) and gene structure (C) of HDT of Arabidopsis, B. rapa, B. oleracea and B. napus. Neighbor-joining phylogenetic tree showing the relationship among 8 B. rapa (green triangle), 10 B. oleracea (blue triangle), 14 B. napus (circle) and 4 Arabidopsis HDT proteins (A). The resulting four groups are labeled (Group I–IV). Ten motifs in HDT proteins were identified by MEME tools (B). Yellow boxes, black lines and green boxes indicate exons, introns and untranslated regions, respectively (C).
2.4. Expression Profiling of HDT Genes in Different Tissues
Based on Arabidopsis eFP Browser data (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi, accessed on 24 May 2022) and RNA-Seq data (B. rapa: GSE43245, B. oleareaca: GSE42891 and B. napus: PRJNA394926) (Table S1) [33,34,35], the HDT gene was expressed in different vegetative and reproductive organs of the four species at different developmental stages (Figure 4). In general, the expression pattern of HDT differed between groups, but the expression pattern of the four species within the same subgroup was very similar. In Arabidopsis, AtHDTs were highly expressed in roots, carpels and flowers; BoHDTs and BrHDTs were highly expressed in leaf, bud and silique tissues in B. rapa and B. olearcea. In B. napus, BnaHDTs were highly expressed in bud, stamen and ovule tissues, as well as peel and silique tissues. The expressions of AtHDT2 and corresponding genes in B. rapa, B. olearcea (Bo2g024240 and Bra020209) and B. napus (BnaC02g09720D and BnaA02g05840D) were higher than those of other HDT genes in all tissues. In HDT2, the expression patterns of a pair of genes (Bo3g016960 and Bra006629) in B. olearcea and B. rapa were highly similar with corresponding pairs of gene (BnaC03g10890D and BnaA03g08590D) in B. napus, and the expression levels in stem, bud and silique tissues were higher than those in other tissues. Another pair of genes (Bo2g024150 and Bra020204) in B. olearcea and B. rapa had an expression pattern highly similar to that of a corresponding pair of genes (BnaCnng34610D and BnaA02g05790D) in B. napus. These genes are specifically expressed in bud, stamen and silique tissues. All the results suggested that Bna/Br/BoHDT2 members showed expression in flowers and siliques, which indicates their importance in propagative organ development in Brassicaceae plants.
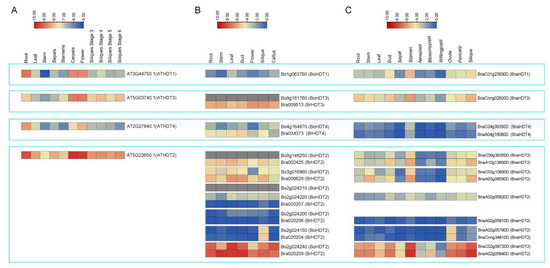
Figure 4.
Expression of AtHDT (A), BraHDT and BoHDT (B), BnaHDT (C) in different tissues and organs. The expression levels (log10(FPKM) value) of HDT genes were indicated by differently colored rectangles.
2.5. Expression Profiling of HDT Genes under Abiotic Stress and Phytohormone Treatments
To reveal the roles of BnaHDTs in stress responsiveness in B. napus, their expression patterns upon various abiotic and phytohormone treatments were investigated (Table S2). As shown in Figure 5, the expression levels of most genes were increased after treatment with ABA for 6 h and ethylene treatment for 6 h. The HDT2 and HDT3 subgroups were more responsive to heat shock than the other two subgroups. Three genes in HDT2 (BnaA02g05840D, BnaC02g09720D and BnaA10g13800D) and one gene in HDT3 (BnaCnng02600D) were increased sharply after 12 h of heat shock. In addition, the expression of BnaA02g05840D in HDT2 was strongly induced after 12 h of low-temperature treatment. Overall, the HDT gene family is responsive to multiple hormonal and adversity stresses.
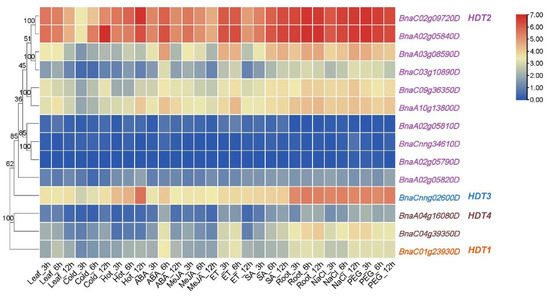
Figure 5.
Expression of BnaHDT genes under different abiotic stresses and plant hormone treatments. The expression levels (TPM values) of BnaHDT genes are indicated by differently colored rectangles. Leaf: untreated leaves; Cold: leaves treated with −4 °C. Hot: leaves treated with 40°C; ABA: leaves treated with 100 μM abscisic acid; MeJA: leaves treated with 100 μM methyl jasmonate; ETH: leaves treated with 10 μg/mL ethephon; SA: leaves treated with 1.0 mM salicylic acid. Root: untreated roots; NaCl: roots treated with 200 mM NaCl; PEG: roots treated with 20% polyethylene glycol 6000. The expression levels (log10(FPKM) value) of HDT genes are indicated by differently colored rectangles. Gene name color: Red represents BnaHDT1, purple represents BnaHDT2, blue represents BnaHDT3, and brown represents BnaHDT4.
2.6. Expression Profiling of HDT Genes under Low Temperature Stress
As mentioned previously, the two genes of HDT2 were highly expressed in all tissues and responded strongly to cold stress (Figure 5). In order to elucidate the potential function of BnaHDT in response to low-temperature stress, the transcriptional patterns of BnaHDT in B. napus were studied after different low-temperature stresses under conditions of cold acclimation/non-cold acclimation (Figure 6 and Table S3). Transcriptome data showed that compared with cold acclimation, the expression of HDT genes was more strongly induced by cold stress and freezing stress after non-cold acclimation. Compared to other genes, the expression of HDT2 gene pairs (BnaC02g09720D and BnaA02g05840D, BnaC09g36350D and BnaA10g13800D), the gene BnaC04g39350D of HDT4 and the gene BnaC01g23930D of HDT1 were particularly strongly expressed in response to cold stress and chilling stress. After cold acclimation of HX17 material, the expression of most genes induced by cold injury was stronger than that induced by freezing injury. After non-cold acclimation, the expression of most genes of HX17 material were all induced by cold and chilling injury. After cold acclimation of HX58 material, BnaC02g09720D, BnaA02g05840D and BnaC09g36350D of HDT2 and BnaC04g39350D of HDT4 were slightly induced by cold stress and freezing stress. After non-cold acclimation of HX58 material, the expression of BnaC02g09720D, BnaA02g05840D, BnaA03g08590D, BnaC09g36350D and BnaA10g13800D of HDT2 as well as BnaC04g39350D of HDT4 and BnaC01g23930D of HDT1 were strongly induced by cold stress and chilling stress. In conclusion, some members of the HDT gene family are induced by cold and freezing stresses.
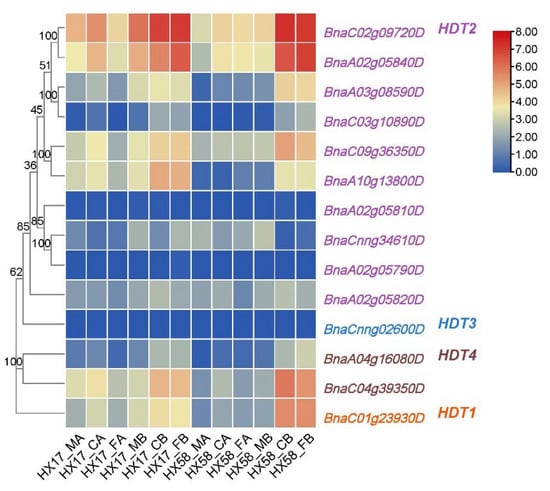
Figure 6.
Expression of BnaHDT genes under different low-temperature treatments.
Colored rectangles indicate FPKM or TPM values; gray rectangles indicate no data. HX17/58_MA represents untreated leaves of 6-week-old HX17 or HX58 (two early-maturing semi-winter B. napus varieties) seedlings. CA represents leaves of 6-week-old seedlings treated with cold acclimation (4 °C for two weeks) and then treated at 4 °C for 12 h. FA represents leaves of 6-week-old seedlings treated with cold acclimation (4 °C for two weeks) and then treated at −4 °C for 12 h. MB represents untreated leaves of 6-week-old seedlings. CB represents leaves of 6-week-old seedlings treated at 4 °C for 12 h. FB represents leaves of 6-week-old seedlings treated at −4 °C for 12 h. The expression levels (log10(FPKM) value) of HDT genes are indicated by differently colored rectangles. Gene name color: red represents BnaHDT1, purple represents BnaHDT2, blue represents BnaHDT3, and brown represents BnaHDT4.
2.7. Expression Profiling of HDT Genes under Biotic Stress Stress
In addition, we found that HDT also responds to biological stress. The transcriptional profiling of B. napus susceptible (Westar) and tolerant (ZY821) genotypes infected with S. sclerotiorum (GSE81545: https://www.ncbi.nlm.nih.gov/geo/qu-ery/acc.cgi?acc=GSE81545, accessed on 24 May 2022) (Table S4) showed that most of the BnaHDT genes were upregulated with S. sclerotiorum. In addition, the expression level was higher in tolerant plants than susceptible plants (Figure 7). The expression of BnaC02g09720D in susceptible plants (Westar) was higher than that in tolerant plants (ZY821). In summary, some members of the HDT family are induced by S. sclerotiorum.
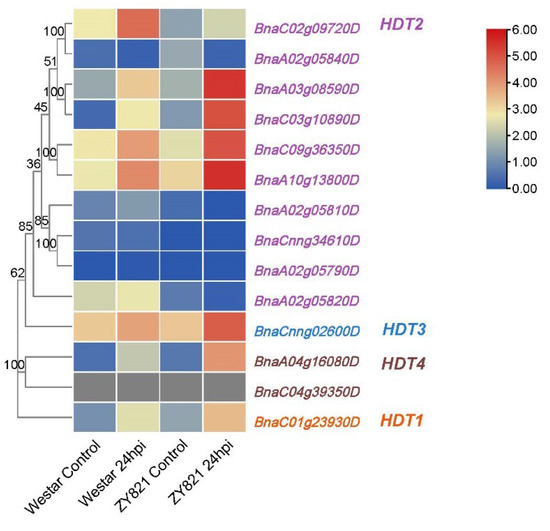
Figure 7.
Expression of BnaHDT genes under infection with S. sclerotiorum (B).
Colored rectangles indicate FPKM or TPM values; gray rectangles indicate no data. Gene name color: red represents BnaHDT1, purple represents BnaHDT2, blue represents BnaHDT3, and brown represents BnaHDT4.
Figure 7 is a heat map of BnaHDT expression levels (FPKM values) in susceptible (Westar) and tolerant (ZY821) genotypes of B. napus infected with S. sclerotiorum. Control: mock inoculated; 24 hpi; 24 h post-inoculation. The expression levels (log10(FPKM) value) of HDT genes are indicated by differently colored rectangles.
3. Discussion
The HDT family is a set of plant-specific histone deacetylases which plays a significance role in regulating plant development and resistance to biotic and abiotic stresses [19,22,28]. In Arabidopsis, HDT has four members, HD2A (HDT1), HD2B (HDT2) [14], HD2C (HDT3) [19] and HD2D (HDT4) [20,21]. In this study, we found 14, 10 and 8 HDT genes in B. napus, B. rapa and B. oleracea, respectively (Figure 1). In B. napus, the number of HDT genes in the An subgenome (7) and Cn subgenome (7) is almost the same as that in their diploid ancestors B oleracea (10) and B. rapa (8) (Table 1). This showed that most of the duplicated HDT genes were preserved after the whole genome duplication (WGD) event in B. napus. Homology analysis showed that three genes in B. oleracea (Bo2g024200, Bo2g024210 and Bo2g024220) and one gene in B. rapa (Bra009513) were lost during or after the WGD event in B. napus. HDT sequence alignment (Supplementary Document S1) revealed that most HDTs are conserved in Brassicaceae, indicating that these duplicated HDT genes can still retain the function of their ancestors in these species.
Among the HDT gene family, HDT2 is expanded in B. napus. The immediate consequences are threefold: The first is the weakening of the function of the HDT2 gene. The expression of Arabidopsis HDT2 gene occurs in almost all tissues, while some HDT2 genes are only specifically expressed in flowers, buds and ovule siliques in B. rapa, B. oleracea and B. napus, and some HDT2 genes are not expressed in all tissues. These loss-of-function members may be short HDT2 members (Figure 4). The second is tissue expression specificity. BnaA02g05790D and BnaCnng34610D are a pair of members with specifically high expression in ovules. A pair of genes (BnaC02g09720D and BnaA02g05840D) were expressed in all tissues. The final conseuence is the deletion of some HDT2 genes. There are seven HDT2 genes in B. rapa and seven HDT2 genes in B. oleracea. Theoretically there should be 14 HDT2 genes for B. napus, but in practice there are only 10, indicating that some HDT2 genes are lost. These variations have a certain impact on the growth and development of rapeseed and help it to better adapt to the environment. Compared with B. rapa and B. oleracea, BnaHDT2 is specifically expressed in flowers; presumably, HDT2 has attained a new function in B. napus.
HDT is regulated by various environmental stresses, has different tissue-specific expression patterns and has common and different functions in various developmental and physiological processes [14]. In Arabidopsis, HDT is mainly highly expressed in flowers, young siliques, stems, leaves and other tissues [14,19]. Like Arabidopsis, HDT genes of B. napus, B. rapa and B. oleracea are more highly expressed in reproductive organs such as flowers and young siliques. Yongfeng Hu et al. suggested that overexpression of HDT701 induced earlier flowering under long-daylight conditions in hybrid rice, as shown in Figure 4, and most BnaHDTs were highly expressed in stamens in B. napus [24], suggesting that BnaHDT may also have a regulatory effect on flowering. Marie Lagac’e found that ScHDT1 in potato (Solanum chacoense) increased the accumulation of HDT transcripts in ovules after fertilization [27], and we found that most HDT2 genes were specifically expressed in flowers, buds and ovules of B. napus, B. rapa and B. oleracea. It is speculated that HDT2 is related to reproductive growth. The expression of Arabidopsis HDT4 in different tissues is lower than that of other HDT members. Similar to Arabidopsis, the expression of HDT4 in various tissues of B. napus, B. rapa and B. oleracea was lower than that of other HDT members. The expression of Arabidopsis HDT2 in different tissues was higher compared to other HDT members. As with Arabidopsis, HDT2 (BnaA02g05840D, BnaC02g09720D) in B. napus, B. rapa and B. oleracea was also expressed more in various tissues than other HDT members. In summary, the function of HDT is conserved during gene evolution. Interestingly, we also found that most HDT genes are redundantly expressed in reproductive organs such as flowers and shoots, from which we can speculate that HDT genes have an important role in the reproductive functions of plants. This result indicates that HDT family genes have conserved and specific biological functions in various developmental and physiological processes of B. napus.
It is well known that ABA is an indispensable hormone in plant stress response and plays an important role in abiotic stress. ABA inhibited the expression of AtHDT3 in Arabidopsis [18]. We found that most BnaHDTs were induced by ABA (Figure 5). In Arabidopsis, HDT3 is responsive to cold stress, whereas in our study, BnaHDT2 expression was found to be strongly induced by cold induction for reasons that remain to be explored subsequently. Furthermore, most members of HDT of B. napus, especially BnaC02g09720D and BnaA02g05840D, were slightly induced by low-temperature stress but were strongly induced by low temperature (4 °C) and freezing (−4 °C) under non-cold acclimation conditions. BnaHDT3 specifically responds to heat stress, is barely expressed under cold and freezing stress and is induced after infection with S. sclerotiorum in tolerant (ZY821) genotypes of B. napus, suggesting that this gene may have a function in responding to biotic stresses. In conclusion, HDT genes can play an important role in resisting many adversity stresses, like heat, cold, freezing, ABA and infection of S. sclerotiorum in B. napus. This suggests a functional differentiation of the HDT family.
4. Materials and Methods
4.1. Identification of the HDT Gene Family
TAIR (https://www.Arabidopsis.org/index.jsp, accessed on 13 August 2021) was used to obtain the nucleotide and protein sequences for Arabidopsis AtHDT. Using BLASTp (E-value < 1 × 10−5) in Ensembl genomes (http://ensemblgenomes.org/, accessed on 13 August 2021), four AtHDT proteins were used as query sequences to search for the HDT proteins of B. napus, B. rapa and B. oleracea. In ExPasy and Ensembl Plants (https://web.ex-pasy.org/compute_pi/, accessed on 13 August 2021), the molecular weights (MW), isoelectric points (IP) and subcellular localizations of HDT proteins were predicted [36].
4.2. Analysis of Gene Structure, Motif Composition
NCBI (https://www.ncbi.nlm.nih.g-ov/cdd) and Pfam (http://pfam.xfam.org, accessed on 16 August 2021) were used to characterize the HDT domain [37]. MEME (http://me-me.nbcr.net/meme/cgi-bin/meme.cgi, accessed on 27 August 2021) was used to analyze the conserved motifs. To examine the gene structures and motif composition, TBtools version 1.095 was used [38].
4.3. Phylogenetic Analysis and Chromosomal Locations
ClustalW was used to align the multiple sequences of all detected HDT proteins (from Arabidopsis, B. napus, B. rapa and B. oleracea), and MEGA X was used to build a phylogenetic tree using the neighbor-joining (NJ) phylogenetic technique with 1000 bootstrap replicates [39]. TBtools version 1.095 was used to examine the gene chromosomal localization [38].
4.4. Plant Materials and Treatments, Heat Map Analysis of the HDT Transcriptome Data
Rapeseed ZS11 (the semi-winter cultivar Zhongshuang 11) [33] seeds were germinated on filter paper, and the seedlings were then transplanted into pots with soil or vermiculite and nurtured in a growth chamber for six weeks. Based on RNA-Seq data, the expression patterns of ZS11’s roots, stems, leaves, buds, sepals, stamens, new pistils, blossoming pistils, wilting pistils, siliques, pericarps and ovules were examined [40]. B. rapa, and B. oleracea’s RNA-Seq data were evaluated to determine the pattern of expression in various tissues [41].
Hormone treatments were performed by sprinkling leaves with 100 μM abscisic acid (ABA), 100 μM methyl jasmonate (MeJA), 1 mM salicylic acid (SA) and 10 μg/mL ethephon (ETH) solutions during the 8:00 a.m.–8:00 p.m. period. To simulate salt and PEG stresses, seedlings were righted with NaCl (200 mM) or PEG-6000 (20%) solution. To simulate hot and cold stresses, seedlings were grown in a chamber at 40 °C or 4 °C. Leaf samples were collected at 3, 6 and 12 h time points during stress treatment. For chilling and freezing treatments with or without cold acclimation, two early-maturing semi-winter rapeseed varieties (HX17 and HX58) were used [42]. They were treated as described previously. Seedlings were cultured in incubators under 20 °C (14 h light: 6:00 a.m.–8:00 p.m.)/16 °C (10 h dark: 8:00 p.m.–6:00 a.m.) for 4 weeks and then treated at 4 °C (14 days)→4 °C (12 h) (CA) or −4 °C (12 h) (FA), 20 °C/16 °C (light/dark) for 6 weeks→4 °C (12 h) (CB), 20 °C (14 h light: 6:00 a.m.–8:00 p.m.)/16 °C(10 h dark: 8:00 p.m.–6:00 a.m.) for 6 weeks→−4 °C (12 h) (FB). For the acclimation condition, after the 14 days at 4 °C, 4 °C/−4 °C (12 h), they were treated at 4 °C or −4 °C from 8:00 p.m.–8:00 a.m. (10 h dark and 2 h light) [43]. Then, the third leaves from the top were collected at 8:00 a.m. after cold treatment and stored at −80 °C immediately until RNA extraction.
Expression data after inoculation with S. sclerotiorum were obtained from the GEO database (GEO: GSE81545) [44]. TBtools version 1.095 was employed to generate heat maps of the expression profile values of BnaHDT genes [38].
5. Conclusions
In this study, we identified 14, 8 and 10 HDT proteins in B. napus, B. rapa and B. oleracea, respectively, by exploring the important role of HDT genes in Brassicaceae plants. It was found that the HDT2 homologous gene of Brassicaceae plants was super-amplified, and all HDT proteins had conserved NPL (nucleoplastin-like) domains. In addition, we found that the Bna/Br/BoHDT gene was expressed differently in different tissues at different developmental stages, mainly in flowers and pods, and responded to low-temperature and freezing stresses. This study will lay a foundation for further understanding the biological function of HDT subfamily genes in B. napus and the study of low-temperature stress, especially freezing stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24010525/s1.
Author Contributions
Conceptualization, X.H, L.Q. and C.G.; methodology, P.X. and W.L.; software, R.R., Y.K., Y.L. and Y.J.; validation, X.H. and C.G.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Key Research and Development Program. Funder: Science and Technology Innovation Program of Hunan Province. Funding Number: 2020RC2057).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are available from the corresponding author on request (xiepan@hunau.edu.cn).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Aliev, G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin. Cancer Biol. 2022, 83, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Demidov, D.; Houben, A.; Schubert, I. Chromosomal histone modification patterns—From conservation to diversity. Trends Plant Sci. 2006, 11, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zentner, G.E.; Henikoff, S. Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 2013, 20, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, Y.Y.; Liu, X.; Yang, S.; Lu, Q.; Cui, Y.; Wu, K. HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J. Exp. Bot. 2012, 63, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.S.; Tian, L. HD2-type histone deacetylases: Unique regulators of plant development and stress responses. Plant Cell Rep. 2021, 40, 1603–1615. [Google Scholar] [CrossRef]
- Hollender, C.; Liu, Z. Histone Deacetylase Genes in Arabidopsis Development. J. Integr. Plant Biol. 2008, 50, 875–885. [Google Scholar] [CrossRef]
- Yruela, I.; Moreno-Yruela, C.; Olsen, C.A. Zn(2+)-Dependent Histone Deacetylases in Plants: Structure and Evolution. Trends Plant Sci. 2021, 26, 741–757. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Zhang, W.; Zhao, J.H.; Zhang, J.X.; Wu, K.Q.; Tian, L.N.; Duan, J. Histone acetyltransferases in rice (Oryza sativa L.): Phylogenetic analysis, subcellular localization and expression. BMC Plant Biol. 2012, 12, 145. [Google Scholar] [CrossRef]
- Bourque, S.; Jeandroz, S.; Grandperret, V.; Lehotai, N.; Aime, S.; Soltis, D.E.; Miles, N.W.; Melkonian, M.; Deyholos, M.K.; Leebens-Mack, J.H.; et al. The Evolution of HD2 Proteins in Green Plants. Trends Plant Sci. 2016, 21, 1008–1016. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Z.; Liu, Y.; An, Z.; Peng, M.; Shen, W.H.; Dong, A.; Yu, Y. MRG1/2 histone methylation readers and HD2C histone deacetylase associate in repression of the florigen gene FT to set a proper flowering time in response to day-length changes. New Phytol. 2020, 227, 1453–1466. [Google Scholar] [CrossRef]
- Farhi, J.; Tian, G.; Fang, H.; Maxwell, D.; Xing, T.; Tian, L. Histone deacetylase HD2D is involved in regulating plant development and flowering time in Arabidopsis. Plant Signal. Behav. 2017, 12, e1300742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yano, R.; Takebayashi, Y.; Nambara, E.; Kamiya, Y.; Seo, M. Combining association mapping and transcriptomics identify HD2B histone deacetylase as a genetic factor associated with seed dormancy in Arabidopsis thaliana. Plant J. 2013, 74, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Torres-Garcia, J.; Latrasse, D.; Benhamed, M.; Schilderink, S.; Zhou, W.; Kulikova, O.; Hirt, H.; Bisseling, T. Plant-Specific Histone Deacetylases HDT1/2 Regulate GIBBERELLIN 2-OXIDASE2 Expression to Control Arabidopsis Root Meristem Cell Number. Plant Cell 2017, 29, 2183–2196. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Q.; Tian, L.N.; Malik, K.; Brown, D.; Miki, B. Functional analysis of HD2 histone deacetylase homologues in Arabidopsis thaliana. Plant J. 2000, 22, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gao, Q.; Chen, G.; Guo, J.E.; Guo, X.; Tang, B.; Hu, Z. SlHDA5, a Tomato Histone Deacetylase Gene, Is Involved in Responding to Salt, Drought, and ABA. Plant Mol. Biol. Rep. 2017, 36, 36–44. [Google Scholar] [CrossRef]
- Zhu, J.H.; Yun, D.J.; Bressan, R.A.; Jeong, J.; Zhu, Y.; Sokolchik, I.; Miyazaki, S.; Zhu, J.K.; Hasegawa, P.M.; Bohnert, H.J. Involvement of Arabidopsis HOS15 In histone deacetylation and cold tolerance. Proc. Natl. Acad. Sci. USA 2008, 105, 4945–4950. [Google Scholar] [CrossRef]
- Han, Z.; Yu, H.; Zhao, Z.; Hunter, D.; Luo, X.; Duan, J.; Tian, L. AtHD2D Gene Plays a Role in Plant Growth, Development, and Response to Abiotic Stresses in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 310. [Google Scholar] [CrossRef]
- Sridha, S.; Wu, K. Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. Plant J. 2006, 46, 124–133. [Google Scholar] [CrossRef]
- Zhou, C.; Labbe, H.; Sridha, S.; Wang, L.; Tian, L.; Latoszek-Green, M.; Yang, Z.; Brown, D.; Miki, B.; Wu, K. Expression and function of HD2-type histone deacetylases in Arabidopsis development. Plant J. 2004, 38, 715–724. [Google Scholar] [CrossRef]
- Dangl, M.; Brosch, G.; Haas, H.; Loidl, P.; Lusser, A. Comparative analysis of HD2 type histone deacetylases in higher plants. Planta 2001, 213, 280–285. [Google Scholar] [CrossRef]
- Ritu, P.; Andreas, M.; Napoli, C.A.; Selinger, D.A.; Pikaard, C.S.; Richards, E.J.; Bender, J.; Mount, D.W.; Jorgensen, R.A. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversfication of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002, 30, 5036–5055. [Google Scholar]
- Wu, K.Q.; Tian, L.N.; Zhou, C.H.; Brown, D.; Miki, B. Repression of gene expression by Arabidopsis HD2 histone deacetylases. Plant J. 2003, 34, 8. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Ishikawa, T.; Watanabe, K.; Terakura, S.; Iwakawa, H.; Okada, K.; Machida, C.; Machida, Y. Histone deacetylases and ASYMMETRIC LEAVES2 are involved in the establishment of polarity in leaves of Arabidopsis. Plant Cell 2007, 19, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Zhang, W.; Wu, K.; Zheng, F.; Tian, L.; Liu, X.; Duan, J. Expression and functional analysis of the plant-specific histone deacetylase HDT701 in rice. Front. Plant Sci. 2014, 5, 764. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.E.; Hu, Z.; Li, F.; Zhang, L.; Yu, X.; Tang, B.; Chen, G. Silencing of histone deacetylase SlHDT3 delays fruit ripening and suppresses carotenoid accumulation in tomato. Plant Sci. 2017, 265, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qin, F.; Huang, L.; Sun, Q.; Li, C.; Zhao, Y.; Zhou, D.X. Rice histone deacetylase genes display specific expression patterns and developmental functions. Biochem. Biophys. Res. Commun. 2009, 388, 266–271. [Google Scholar] [CrossRef]
- Marie Lagac, S.C.C.; Geneviève, M.; Daniel, P.M. Fertilization induces strong accumulation of a histone deacetylase (HD2) and of other chromatin-remodeling proteins in restricted areas of the ovules. Plant Mol. Biol. 2003, 53, 759–769. [Google Scholar] [CrossRef]
- Demetriou, K.; Kapazoglou, A.; Tondelli, A.; Francia, E.; Stanca, M.A.; Bladenopoulos, K.; Tsaftaris, A.S. Epigenetic chromatin modifiers in barley: I. Cloning, mapping and expression analysis of the plant specific HD2 family of histone deacetylases from barley, during seed development and after hormonal treatment. Physiol. Plant 2009, 136, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Bourque, S.; Dutartre, A.; Hammoudi, V.; Blanc, S.; Dahan, J.; Jeandroz, S.; Pichereaux, C.; Rossignol, M.; Wendehenne, D. Type-2 histone deacetylases as new regulators of elicitor-induced cell death in plants. New Phytol. 2011, 192, 127–139. [Google Scholar] [CrossRef]
- Lim, C.J.; Park, J.; Shen, M.; Park, H.J.; Cheong, M.S.; Park, K.S.; Baek, D.; Bae, M.J.; Ali, A.; Jan, M.; et al. The Histone-Modifying Complex PWR/HOS15/HD2C Epigenetically Regulates Cold Tolerance. Plant Physiol. 2020, 184, 1097–1111. [Google Scholar] [CrossRef]
- Cheng, F.; Sun, R.; Hou, X.; Zheng, H.; Zhang, F.; Zhang, Y.; Liu, B.; Liang, J.; Zhuang, M.; Liu, Y.; et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016, 48, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Erratum: Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Jiang, L.; et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Wang, X.; Yu, J.Y.; Wu, J.; Li, W.S.; Huang, J.Y.; Dong, C.H.; Hua, W.; Liu, S. Comprehensive analysis of RNA-seq data reveals the complexity of the transcriptome in Brassica rapa. BMC Genom. 2013, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xie, S.; Xie, P.; Yao, M.; Liu, W.; Qin, L.; Liu, Z.; Zheng, M.; Liu, H.; Guan, M.; et al. Genome-wide identification of stress-associated proteins (SAP) with A20/AN1 zinc finger domains associated with abiotic stresses responses in Brassica napus. Environ. Exp. Bot. 2019, 165, 108–119. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, 412–419. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 9. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wang, W.; Liu, J.; Yao, M.; Guan, M.; Guan, C.; He, X. Phytochrome-interacting factor (PIF) in rapeseed (Brassica napus L.): Genome-wide identification, evolution and expression analyses during abiotic stress, light quality and vernalization. Int. J. Biol. Macromol. 2021, 180, 14–27. [Google Scholar] [CrossRef]
- Gao, P.; Quilichini, T.D.; Yang, H.; Li, Q.; Nilsen, K.T.; Qin, L.; Babic, V.; Liu, L.; Cram, D.; Pasha, A.; et al. Evolutionary divergence in embryo and seed coat development of U’s Triangle Brassica species illustrated by a spatiotemporal transcriptome atlas. New Phytol. 2022, 233, 30–51. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liao, L.; Xie, S.; Yao, M.; Xie, P.; Liu, W.; Kang, Y.; Huang, L.; Wang, M.; Qian, L.; et al. Comprehensive analyses of the annexin (ANN) gene family in Brassica rapa, Brassica oleracea and Brassica napus reveals their roles in stress response. Sci. Rep. 2020, 10, 4295. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ni, X.C.; Xie, P.; Liu, W.; Yao, M.; Kang, Y.; Qian, L.W.; Hua, W. Comparative Transcriptome Analyses Revealed Conserved and Novel Responses to Cold and Freezing Stress in Brassica napus L. G3 2019, 9, 2723–2737. [Google Scholar] [CrossRef] [PubMed]
- Girard, I.J.; Tong, C.; Becker, M.G.; Mao, X.; Huang, J.; De Kievit, T.; Fernando, W.G.D.; Liu, S.; Belmonte, M.F. RNA sequencing of Brassica napus reveals cellular redox control of Sclerotinia infection. J. Exp. Bot. 2017, 68, 5079–5091. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).