Abstract
Non-coding RNAs, particularly lncRNAs and miRNAs, have recently been shown to regulate different steps in viral infections and induction of immune responses against viruses. Expressions of several host and viral lncRNAs have been found to be altered during viral infection. These lncRNAs can exert antiviral function via inhibition of viral infection or stimulation of antiviral immune response. Some other lncRNAs can promote viral replication or suppress antiviral responses. The current review summarizes the interaction between ncRNAs and herpes simplex virus, cytomegalovirus, and Epstein–Barr infections. The data presented in this review helps identify viral-related regulators and proposes novel strategies for the prevention and treatment of viral infection.
1. Introduction
The human genome consists of a variety of non-protein-coding DNA. A proportion of these genomic regions are transcribed into RNA. These non-coding RNAs (ncRNAs) are believed to have diverse roles in cellular functions. In addition to those with characterized functions, further functional ncRNAs certainly need to be discovered and categorized [1]. Since the first evidence of biological functions of transfer and ribosomal RNAs in the 1950s, several other classes of ncRNAs have been identified. Two classes of these transcripts, namely, long ncRNAs (lncRNAs) and microRNAs (miRNAs), have gained special attention because of their regulatory roles on gene expression. While lncRNAs regulate gene expression at different levels, miRNAs mainly act at a post-transcriptional level. LncRNAs and miRNAs have another distinctive feature arising from their size. While the former group is longer than 200 nt, the latter are approximately 22 nt in length. LncRNAs interplay with other RNA species, particularly miRNAs, in a way that they sequester them and decrease their bioavailability [2]. Besides this, lncRNAs have biological functions through serving as scaffolds and enhancer RNAs [3].
The regulatory role of miRNAs on gene expression is mediated through miRNAs pairing with the miRNA recognition elements in the mRNAs. These elements are found particularly in the 3’ untranslated region (UTR) of transcripts; however, they are also present in 5′UTRs and coding regions. When the RNA-induced silencing complex is recruited to miRNA recognition elements, the target mRNA is destabilized, or its expression is repressed [4]. Based on an individual miRNA’s ability to target numerous miRNA recognition elements and suppression of hundreds of mRNAs, it is estimated that more than 60% of human protein-coding genes can be targeted by miRNAs [2].
NcRNAs, particularly lncRNAs, have recently been shown to regulate different steps in viral infections and induce immune responses against viruses [5]. Expressions of several host and viral lncRNAs have been found to be altered during viral infection [2,6]. These lncRNAs can exert antiviral function via inhibition of viral infection or stimulation of antiviral immune response. Some other lncRNAs can promote viral replication or suppress antiviral responses [2].
In the current review, we summarize the interaction between ncRNAs and herpes simplex virus (HSV), cytomegalovirus (CMV), and Epstein–Barr (EBV) infections.
2. ncRNAs and HSV Infection
Kaposi’s sarcoma-associated HSV-encoded miRNAs have been shown to affect the expression of host lncRNAs such as Maternally Expressed 3 (MEG3), antisense non-coding RNA in the INK4 locus (ANRIL), and Urothelial Cancer Associated 1 (UCA1) in favor of cancer development. More than 120 host lncRNAs have been identified as putative targets for viral miRNAs. Notably, in addition to the miRNA-dependent route, this type of HSV can affect the expression of host lncRNAs through direct interactions between lncRNAs and latency-related proteins. The impact of HSV on UCA1 expression has pro-proliferative and pro-migratory effects on endothelial cells [7].
HSV-1 has also been shown to increase the expression of Nuclear Enriched Abundant Transcript 1 (NEAT1) and the establishment of paraspeckles through influencing Signal Transducer And Activator Of Transcription 3 (STAT3). NEAT1 and other paraspeckle constituents, namely, P54nrb and PSPC1, interact with HSV-1 genomic DNA. Paraspeckle Component 1 (PSPC1) binds with STAT3 to facilitate its recruitment to paraspeckles and increase its interplay with viral gene promoters. This interaction increases the expression of viral genes and the replication of viruses. Suppression of NEAT1 or STAT3 has improved the healing of HSV-1-related skin lesions in animal models [8]. Another study has shown that HSV infection can result in the construction of higher numbers of paraspeckles via increasing expression of NEAT1 [9]. NEAT1 has also been shown to cooperate with HEXIM1 to construct a multi-subunit complex that participates in the regulation of DNA-associated innate responses of the immune system. This complex encompasses DNA-PK subunits as well as paraspeckle proteins. In fact, binding of HEXIM1 to NEAT1 has an essential role in the assembly of this complex. This complex has a vital participation in induction of innate immune responses against foreign DNA via induction of cGAS-STING-IRF3 pathway [10].
A high throughput RNA sequencing experiment has shown over-expression of lncRNAs in murine 661 W cells following HSV-1 infection. U90926 RNA has been identified as the most over-expressed lncRNA after infection with this virus. Being located in the nucleus, U90926 enhanced replication of HSV-1 DNA and increased proliferation of this virus. U90926-silenced cells have exhibited higher survival rates [11].
During the latent phase of HSV infection, one region of its genome which encodes the latency-associated transcript (LAT) is not silenced. This lncRNA has been first identified as an enhancer of HSV-1 reactivation. Yet, subsequent studies have shown this lncRNA’s role in the inhibition of cell apoptosis and enhancement of the formation of latency. Experiments in a rabbit model have revealed that reduction in LAT levels in neurons after the establishment of the latent phase decreases the capacity of HSV to reactivate. Thus, the HSV-1 LAT transcript is involved in the reactivation in an independent manner from its role in establishing latency [12].
Kaposi’s sarcoma-associated HSV encodes a viral oncogene, namely, viral interferon regulatory factor 1 (vIRF1). This oncogene enhances migration potential and aggressiveness of endothelial cells through decreasing expression of miR-218-5p to release its targets HMGB2 and CMPK1 from its inhibitory effects. Functionally, vIRF1 suppresses the function of p53 to enhance the expression of DNA Methyltransferase 1 (DNMT1) and methylation of pre-miR-218-1 promoter (Figure 1). This process leads to the enhancement of the expression of OIP5 Antisense RNA 1 (OIP5-AS1), a lncRNA that sponges miR-218-5p. Cumulatively, the cellular lnc-OIP5-AS1/miR-218-5p axis is hijacked by vIRF1 to facilitate the invasiveness of HSV-associated tumors [13].
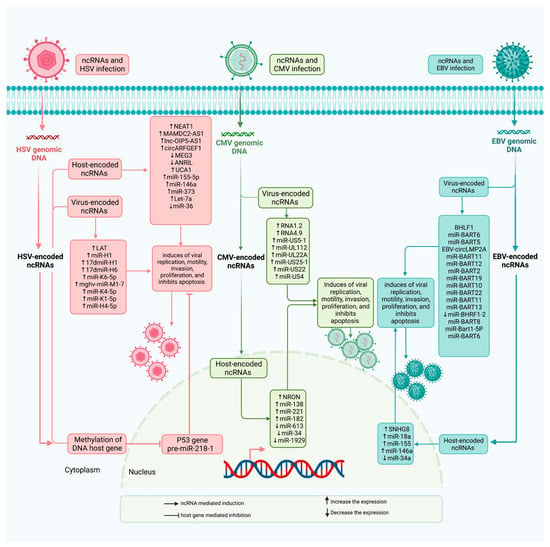
Figure 1.
lncRNAs influence immune defense responses through directly interacting with host DNA genome, effecters, and transcriptional factors, and through releasing different types on non-coding RNAs, such as lncRNAs and miRNAs. Light pink represents HSV infection, light blue represents CMV infection, and pale turquoise represents EBV infection.
miR-H1 as an HSV-1-encoded miRNA has been shown to target Ubiquitin Protein Ligase E3 Component N-Recognin 1 (Ubr1) to increase amassing of neurodegeneration-associated protein [14]. Another study has demonstrated the role of miR-H1/H6 in the facilitation of effective reactivation from latency [15]. Moreover, HSV-1 has been shown to encode miR-H2-3p, a miRNA that interferes with cytosolic DNA-induced antiviral innate immune responses through decreasing expression of DEAD-Box Helicase 41 (DDX41) [16]. Table 1 and Table 2 show the role of host and viral-encoded ncRNAs in HSV infection.

Table 1.
Host-encoded ncRNAs and HSV infection (HEXIM1-DNA-PK-paraspeckle components-ribonucleoprotein complex (HDP-RNP), infected cell protein 0 (ICP0), thymidine kinase (TK), virion protein 16 (VP16), High-mobility-group protein 2 (HMGB2), SRSF2 (Serine and arginine Rich Splicing Factor 2), Interleukin-1 receptor-associated kinase (IRAK), RBPJ (Recombination Signal Binding Protein For Immunoglobulin Kappa J Region), interferon-induced transmembrane protein 1 (IFITM1)). (↑ upregulation; ↓ down regulation).

Table 2.
Virus-encoded ncRNAs and HSV infection (DEAD-Box Helicase 41 (DDX41), EWSR1 (EWS RNA Binding Protein 1, Cytosolic arginine sensor for mTORC1 subunit 1 (CASTOR1), Suppressor Of Cytokine Signaling 2 (SOCS2)). (↑ upregulation; ↓ down regulation).
3. ncRNAs and CMV Infection
RNA1.2 has been recognized as one of four principal lncRNAs which are expressed by human CMV. This lncRNA has an essential function in manipulating the cellular NF-κB-dependent pathways of cytokine and chemokine production in the course of CMV infection. Thus, this lncRNA can affect host immune responses [29]. Another study has shown that human CMV encodes lncRNA4.9, which is localized to the viral replication compartment in the nucleus. Depletion of this lncRNA decreases CMVV DNA replication and its growth. Notably, CRISPR-Cas9-mediated targeting the RNA4.9 promoter results in the reduction of viral ssDBP levels implying the relation between ssDBP levels and oriLyt activity [30].
An in vitro study has shown the ability of human CMV in infecting primary human mammary epithelial cells. This infection leads to inactivation of Rb and p53 proteins, enhancement of telomerase activity, and activation of c-Myc and Ras as well as Akt and STAT3 signaling pathways. CMV-transformed cells exhibited a CMV signature associated with the lncRNA4.9 gene. The sequence of this lncRNA has also been detected in xenograft tumors originated from CMV-transformed cells. Most notably, similar lncRNA4.9 genomic sequences have been found in tumor samples of breast cancer patients [31].
Persistent CMV infection in elderlies has been associated with down-regulation of Non-Coding Repressor Of NFAT (NRON) lncRNA, while up-regulation of its immunity-associated target gene NFAT, in both CD28nullCD8+ T cells and CMVpp65CD8+ T cells (Figure 1). Thus, NRON has been suggested to contribute to CMV-induced CD28nullCD8+ T cell aging through affecting IL-4-associated NFAT signals [32].
Experiments in animal models have shown that miR-1929-3p partakes in CMV-induced hypertensive vascular remodeling via inflammasome activation through Ednra/NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) axis [33].
Another study has shown that human CMV encodes several ncRNAs such as miR-US5-1 and miR-UL112-3p that protect CD34+ hematopoietic progenitors from apoptosis through inactivation of Forkhead box class O 3a (FOXO3a) [34]. miR-UL112-3p has an established role in the enhancement of the progression of glioblastoma [35]. Table 3 and Table 4 show the role of host and viral-encoded ncRNAs in CMV infection.

Table 3.
Host-encoded ncRNAs and CMV infection (Endothelin-1 (ET-1), Sirtuin 1 (SIRT1), NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3), FOXO3a (Forkhead box class O 3a)). (↑ upregulation; ↓ down regulation).

Table 4.
Virus-encoded ncRNAs and CMV infection (Tumor protein p63-regulated gene 1-like protein (TPRG1L), Monocyte Chemoattractant Protein-1 (MCP-1), CXCL1 (C-X-C Motif Chemokine Ligand 1, BCL2L11 (BCL2 Like 11), TUSC3 (Tumor Suppressor Candidate 3), GAB1 (GRB2 Associated Binding Protein 1)). (↑ upregulation; ↓ down regulation).
4. ncRNAs and EBV Infection
Nasopharyngeal carcinoma is one of the EBV-associated cancers. EBV has been shown to express very few viral proteins in nasopharyngeal carcinoma cells, probably in order to evade induction of immune responses. Yet, it expresses high amounts of EBV BamHI-A region rightward transcript (BART) miRNAs and lncRNAs (Figure 1). These ncRNAs are implicated in the pathogenesis of EBV-related disorders. The expression of BARTs has been shown to be regulated by the NF-κB pathway. In fact, EBV LMP1 as an effective activator of the NF-κB pathway can increase the expression of BARTs via this pathway. Meanwhile, BART miRNAs can decrease the expression of LMP1. NF-κB pathway and expression of BARTs construct an autoregulatory circuit to preserve EBV latency in nasopharyngeal carcinoma cells [46].
BHLF1 gene of EBV has been shown to encode several lncRNAs in linear and circular forms that participate in viral replication. However, an open reading frame has been detected in this gene in a proportion of EBV isolates. BHLF1 transcripts have also been detected during the latent phase. In fact, this lncRNA participates in important features of EBV latency, such as its capacity to induce the constant proliferation of B lymphocytes and their malignant transformation [47]. On the other hand, the EBV-encoded miRNA miR-BART6-3p has been shown to act as a tumor suppressor. This miRNA could inhibit metastasis and invasion processes and suppress the proliferation of EBV-related neoplasms via decreasing the expression of LOC553103. LOC553103 has been found to directly bind with the 3’UTR of STMN1 and increase its stability. Cumulatively, miR-BART6-3p/LOC553103/STMN1 molecular route can modulate levels of cell cycle-related proteins, which subsequently suppress the EBV-related proliferation of tumor cells [48].
Over-expression of EBV-miR-BART5-3p has been shown to promote the growth of nasopharyngeal and gastric cancer cells. This miRNA can directly target 3’UTR of TP53 and subsequently decrease Cyclin Dependent Kinase Inhibitor 1A (CDKN1A), BCL2 Associated X, Apoptosis Regulator (BAX), and Fas Cell Surface Death Receptor expression levels. Thus, this miRNA accelerates cell cycle progression and inhibits cell apoptosis. Most notably, BART5-3p participates in chemo/radioresistance. Furthermore, it increases p53 protein degradation [49].
Huang et al. have sequenced more than 11,000 lncRNAs and 144,000 protein-coding transcripts from four EBV-associated and EBV-negative gastric cancer samples in addition to their adjacent unaffected tissues. They have shown specific expression of SNHG8 in EBV-associated gastric cancer. This lncRNA has been demonstrated to influence the activity of numerous gastric cancer-specific signaling pathways and genes being affected by EBV [50]. Table 5 and Table 6 show the role of host and viral-encoded ncRNAs in EBV infection.

Table 5.
Host-encoded ncRNAs and EBV infection (TRIM28 (Tripartite Motif Containing 28), EIF4A2 (Eukaryotic Translation Initiation Factor 4A2), NAP1L1 (Nucleosome Assembly Protein 1 Like 1), PLD3 (Phospholipase D Family Member 3), RPL18A (Ribosomal Protein L18a), SMAD2 (SMAD Family Member 2), CXCR4 (C-X-C Motif Chemokine Receptor 4), Programmed death-ligand 1 (PD-L1)). (↑ upregulation; ↓ down regulation).

Table 6.
Virus-encoded ncRNAs and EBV infection (AT-rich interactive domain-containing protein 1A (ARID1A), latent membrane protein 1 (LMP1), Tubulin polymerization promoting protein 1 (Tppp1), DKK1 (Dickkopf WNT Signaling Pathway Inhibitor 1), NKIRAS2 (NFKB Inhibitor Interacting Ras Like 2)). (↑ upregulation; ↓ down regulation).
5. Impact of Drugs on the Expression of ncRNAs in Infected Patients
EBV-miR-BART22 has been shown to promote stemness properties, metastatic abilities of cancer cells, and increase their resistance to cisplatin. This viral-encoded miRNA directly targets the MAP2K4 and up-regulates MYH9 levels through PI3K/AKT/c-Jun signaling. Notably, cinobufotalin has been found to suppress miR-BART22-associated cisplatin resistance through enhancing expression of MAP2K4 to inhibit MYH9/GSK3β/β-catenin cascade and EMT process in nasopharyngeal carcinoma [71]. Another study has shown that RASG12V and irinotecan up-regulate BART3-3p expression to hamper gastric cancer cells’ senescence and lower NK cells and macrophages infiltration. In fact, BART3-3p reduces TP53, TP21, and inflammatory cytokines such as IL-1A, IL-1B, IL-6, and IL-8 [72]. Table 7 shows the impact of drugs on the expression of ncRNAs in infected patients.

Table 7.
Impact of drugs on the expression of ncRNAs in infected patients. (↑ upregulation; ↓ down regulation).
6. Diagnostic Value of Non-Coding RNAs in EBV-Infected Individuals
Expression levels of EBV-encoded miRNAs such as miR-BART2-5p, miR-BART7-3p, miR-BART9-3p, and miR-BART13-3p have been shown to distinguish patients with nasopharyngeal cancer from healthy controls with diagnostic values ranging from 0.87 to 0.97 (Table 8).

Table 8.
Diagnostic Value of ncRNAs in EBV-infected individuals.
7. Discussion
The interactions between ncRNAs and viral genes result in different pathophysiological consequences, thus affecting the clinical course of infection. In the current review, we have focused on the role of lncRNAs and miRNAs in HSV, CMV, and EBV infections and the induction of antiviral response. Most of the conducted studies have summarized the expression pattern of host transcripts. However, virus-derived ncRNAs have also been studied.
A possible mechanism for the oncogenic function of HSV is hijacking some cellular elements such as lncRNAs and miRNAs. Interference with this process possibly has therapeutic effects. Moreover, interference with viral-encoded ncRNAs might affect pathogenic processes in the course of viral infections. For instance, LAT-targeting ribozymes have been suggested as a possible strategy for treating recurrent HSV-related diseases such as herpes stromal keratitis [12].
A number of viral-encoded lncRNAs and miRNAs regulate the establishment of permanent latency, which might be a preceding phase of tumor development. Since this phase might be continued for a long time, it provides a window for therapeutic interventions in order to reverse the carcinogenic process. These viral transcripts have been shown to affect the expression of both protein-coding genes and ncRNAs encoded by the host cells. Thus, the interactions between cellular and viral ncRNAs are entirely complicated, necessitating the conduction of high throughput sequencing experiments and integrative bioinformatics analyses.
A number of cellular signaling pathways, such as NF-κB signaling, can affect viral latency. Additional investigations about the mechanisms by which NF-κB signaling can regulate the latency of viral particles might reveal novel therapeutic options for the treatment of viral-associated cancers.
Since most viral-encoded miRNAs and lncRNAs do not have similar sequences in the human genome, they can be considered appropriate diagnostic biomarkers, particularly for viral-associated malignancies. This type of application has been studies for EBV-encoded miRNAs such as miR-BART2-5p, miR-BART7-3p, miR-BART9-3p, and miR-BART13-3p, showing promising results in EBV-associated nasopharyngeal carcinoma. Further research in other types of viral-associated cancers is needed to expand the diagnostic application of these ncRNAs.
The data presented in this review helps identify viral-related regulators and proposes novel strategies for the prevention and treatment of viral infection.
Author Contributions
S.G.-F. wrote the draft and revised it. M.T. designed and supervised the study. B.M.H., G.S. and H.H.J. collected the data and desigend the figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genetics 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Cho, K.B.; Li, Y.; Tao, G.; Xie, Z.; Guo, B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 5758. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Fullwood, M.J. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P. The opening of pandora’s box: An emerging role of long noncoding RNA in viral infections. Front. Immunol. 2019, 9, 3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josset, L.; Tchitchek, N.; Gralinski, L.E.; Ferris, M.T.; Eisfeld, A.J.; Green, R.R.; Thomas, M.J.; Tisoncik-Go, J.; Schroth, G.P.; Kawaoka, Y.; et al. Annotation of long non-coding RNAs expressed in collaborative cross founder mice in response to respiratory virus infection reveals a new class of interferon-stimulated transcripts. RNA Biol. 2014, 11, 875–890. [Google Scholar] [CrossRef] [Green Version]
- Sethuraman, S.; Gay, L.A.; Jain, V.; Haecker, I.; Renne, R. microRNA dependent and independent deregulation of long non-coding RNAs by an oncogenic herpesvirus. PLoS Pathog. 2017, 13, e1006508. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Fan, P.; Zhao, Y.; Zhang, S.; Lu, J.; Xie, W.; Jiang, Y.; Lei, F.; Xu, N.; Zhang, Y. NEAT1 modulates herpes simplex virus-1 replication by regulating viral gene transcription. Cell. Mol. Life Sci. 2017, 74, 1117–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, K.; Imamachi, N.; Akizuki, G.; Kumakura, M.; Kawaguchi, A.; Nagata, K.; Kato, A.; Sato, H.; Yoneda, M.; Kai, C.; et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol. Cell 2014, 53, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Morchikh, M.; Cribier, A.; Raffel, R.; Amraoui, S.; Cau, J.; Severac, D.; Dubois, E.; Schwartz, O.; Bennasser, Y.; Benkirane, M. HEXIM1 and NEAT1 long non-coding RNA form a multi-subunit complex that regulates DNA-mediated innate immune response. Mol. Cell 2017, 67, 387–399.e5. [Google Scholar] [CrossRef]
- Shirahama, S.; Onoguchi-Mizutani, R.; Kawata, K.; Taniue, K.; Miki, A.; Kato, A.; Kawaguchi, Y.; Tanaka, R.; Kaburaki, T.; Kawashima, H.; et al. Long noncoding RNA U90926 is crucial for herpes simplex virus type 1 proliferation in murine retinal photoreceptor cells. Sci. Rep. 2020, 10, 19406. [Google Scholar] [CrossRef]
- Watson, Z.L.; Washington, S.D.; Phelan, D.M.; Lewin, A.S.; Tuli, S.S.; Schultz, G.S.; Neumann, D.M.; Bloom, D.C. In vivo knockdown of the herpes simplex virus 1 latency-associated transcript reduces reactivation from latency. J. Virol. 2018, 92, e00812–e00818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, Q.; Feng, Q.; Wang, F.; Yan, Q.; Gao, S.J.; Lu, C. Oncogenic KSHV-encoded interferon regulatory factor up-regulates HMGB2 and CMPK1 expression to promote cell invasion by disrupting a complex lncRNA-OIP5-AS1/miR-218-5p network. PLoS Pathog. 2019, 15, e1007578. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, Q.; Wang, S.; Ren, Z.; Kitazato, K.; Yang, D.; Wang, Y. HSV-1-encoded microRNA miR-H1 targets Ubr1 to promote accumulation of neurodegeneration-associated protein. Virus Genes 2018, 54, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, E.R.; Nakayama, S.; Singh, P.; Vanni, E.A.; Arvin, A.M.; Neumann, D.M.; Bloom, D.C. Deletion of herpes simplex virus 1 microRNAs miR-H1 and miR-H6 impairs reactivation. J. Virol. 2020, 94, e00639-20. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, J.; Fan, S.; Liao, Y.; Feng, M.; Wang, L.; Zhang, Y.; Li, Q. Herpes Simplex Virus Type 1–Encoded miR-H2-3p Manipulates Cytosolic DNA–Stimulated Antiviral Innate Immune Response by Targeting DDX41. Viruses 2019, 11, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, L.; Wang, Y.; Luo, W.; Li, F.; Xiao, J.; Qin, S.; Wang, Z.; Song, X.; Jin, F.; et al. Single-cell RNA-sequencing analysis identifies host long noncoding RNA MAMDC2-AS1 as a co-factor for HSV-1 nuclear transport. Int. J. Biol. Sci. 2020, 16, 1586. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Jia, X.; Wang, F.; Sheng, L.; Song, P.; Cao, Y.; Shi, H.; Fan, W.; Ding, X.; Gao, S.J.; et al. CircRNA ARFGEF1 functions as a ceRNA to promote oncogenic KSHV-encoded viral interferon regulatory factor induction of cell invasion and angiogenesis by up-regulating glutaredoxin 3. PLoS Pathog. 2021, 17, e1009294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, K.; Wang, X.; Huang, W. MiR-155-5p modulates HSV-1 replication via the epigenetic regulation of SRSF2 gene expression. Epigenetics 2019, 14, 494–503. [Google Scholar] [CrossRef]
- Venuti, A.; Musarra-Pizzo, M.; Pennisi, R.; Tankov, S.; Medici, M.A.; Mastino, A.; Rebane, A.; Sciortino, M.T. HSV-1\EGFP stimulates miR-146a expression in a NF-κB-dependent manner in monocytic THP-1 cells. Sci. Rep. 2019, 9, 5157. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Zheng, G.; Di, C.; Zhang, J.; Wang, X.; Hong, Y.; Song, Y.; Chen, R.; Yang, Y.; Yan, Y.; et al. Latency-associated nuclear antigen inhibits lytic replication of Kaposi’s sarcoma-associated herpesvirus by regulating let-7a/RBPJ signaling. Virology 2019, 531, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Akula, S.M. miRNA-36 inhibits KSHV, EBV, HSV-2 infection of cells via stifling expression of interferon induced transmembrane protein 1 (IFITM1). Sci. Rep. 2017, 7, 17972. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, S.; Wang, J. MicroRNA-373 facilitates HSV-1 replication through suppression of type I IFN response by targeting IRF1. Biomed. Pharmacother. 2018, 97, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.A.; Foley, J.W.; Davison, A.J.; Sommer, M.; Liu, D.; Sung, P.; Moffat, J.; Zerboni, L.; Arvin, A.M. The latency-associated transcript locus of herpes simplex virus 1 is a virulence determinant in human skin. PLoS Pathog. 2020, 16, e1009166. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.; Manzano, M.; Chung, K.; Schipma, M.J.; Bartom, E.T.; Gottwein, E. The Oncogenic Kaposi’s Sarcoma-Associated Herpesvirus Encodes a Mimic of the Tumor-Suppressive miR-15/16 miRNA Family. Cell Rep. 2019, 29, 2961–2969.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Feldman, E.R.; Bullard, W.L.; Tibbetts, S.A. A gammaherpesvirus microRNA targets EWSR1 (Ewing sarcoma breakpoint region 1) in vivo to promote latent infection of germinal center B cells. Mbio 2019, 10, e00996-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Ju, E.; Gao, S.-J. Kaposi sarcoma–associated herpesvirus miRNAs suppress CASTOR1-mediated mTORC1 inhibition to promote tumorigenesis. J. Clin. Investig. 2019, 129, 3310–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Yang, J.; Liu, Y.; Fan, J.; Yang, H. HSV-2-encoded miRNA-H4 regulates cell cycle progression and Act-D-induced apoptosis in HeLa Cells by targeting CDKL2 and CDKN2A. Virol. Sin. 2019, 34, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Zhou, Z.; Yan, H.; Xiao, J. A herpes simplex virus type 2–encoded microRNA promotes tumor cell metastasis by targeting suppressor of cytokine signaling 2 in lung cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, B.; Kerr, K.; Gu, Q.; Nightingale, K.; Antrobus, R.; Suárez, N.M.; Stanton, R.J.; Wang, E.C.; Weekes, M.P.; Davison, A.J. Human cytomegalovirus long non-coding RNA1. 2 suppresses extracellular release of the pro-inflammatory cytokine IL-6 by blocking NF-κB activation. Front. Cell. Infect. Microbiol. 2020, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Tai-Schmiedel, J.; Karniely, S.; Lau, B.; Ezra, A.; Eliyahu, E.; Nachshon, A.; Kerr, K.; Suárez, N.; Schwartz, M.; Davison, A.J.; et al. Human cytomegalovirus long noncoding RNA4. 9 regulates viral DNA replication. PLoS Pathog. 2020, 16, e1008390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Tripathy, M.K.; Pasquereau, S.; Al Moussawi, F.; Abbas, W.; Coquard, L.; Khan, K.A.; Russo, L.; Algros, M.-P.; Valmary-Degano, S.; et al. The human cytomegalovirus strain DB activates oncogenic pathways in mammary epithelial cells. EBioMedicine 2018, 30, 167–183. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-H.; Yu, X.-H.; Qu, G.-J.; Qiao, F.-F.; Han, H. Reduced expression of the lncRNA NRON is a potential hallmark of the CMV-amplified CD8+ T cell accumulations commonly seen in older humans. Exp. Gerontol. 2019, 115, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xi, D.; Shi, Y.; Wang, L.; Zhong, H.; Huang, Z.; Liu, Y.; Tang, Y.; Lu, N.; Wang, Y.; et al. MicroRNA-1929-3p participates in murine cytomegalovirus-induced hypertensive vascular remodeling through Ednra/NLRP3 inflammasome activation. Int. J. Mol. Med. 2021, 47, 719–731. [Google Scholar] [CrossRef]
- Hancock, M.H.; Crawford, L.B.; Perez, W.; Struthers, H.M.; Mitchell, J.; Caposio, P. Human cytomegalovirus UL7, miR-US5-1, and miR-UL112-3p inactivation of FOXO3a protects CD34+ hematopoietic progenitor cells from apoptosis. Msphere 2021, 6, e00986-20. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, K.; Wang, B.; Cai, Q. HCMV-encoded miR-UL112-3p promotes glioblastoma progression via tumour suppressor candidate 3. Sci. Rep. 2017, 7, 44705. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, L.; Wang, R.; Tuo, H.; Guo, Y.; Yi, L.; Wang, D.; Wang, J. miR-138 promotes migration and tube formation of human cytomegalovirus-infected endothelial cells through the SIRT1/p-STAT3 pathway. Arch. Virol. 2017, 162, 2695–2704. [Google Scholar] [CrossRef]
- Shi, L.; Fan, B.; Chen, D.; Guo, C.; Xiang, H.; Nie, Y.; Zhong, D.; Shi, X. Human cytomegalovirus protein UL136 activates the IL-6/STAT3 signal through MiR-138 and MiR-34c in gastric cancer cells. Int. J. Clin. Oncol. 2020, 25, 1936–1944. [Google Scholar] [CrossRef]
- Yan, B.; Ma, H.; Jiang, S.; Shi, J.; Yang, Z.; Zhu, W.; Kong, C.; Chen, L.; Yan, H.; Ma, C. microRNA-221 restricts human cytomegalovirus replication via promoting type I IFN production by targeting SOCS1/NF-κB pathway. Cell Cycle 2019, 18, 3072–3084. [Google Scholar] [CrossRef]
- He, X.; Teng, J.; Cui, C.; Li, D.; Wen, L. MicroRNA-182 inhibits HCMV replication through activation of type I IFN response by targeting FOXO3 in neural cells. Exp. Cell Res. 2018, 369, 197–207. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, P.; Qian, D.; Hu, M.; Zhang, L.; Shi, H.; Wang, B. MicroRNA-613 is downregulated in HCMV-positive glioblastoma and inhibits tumour progression by targeting arginase-2. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Hancock, M.H.; Mitchell, J.; Goodrum, F.D.; Nelson, J.A. Human cytomegalovirus miR-US5-2 downregulation of GAB1 regulates cellular proliferation and UL138 expression through modulation of epidermal growth factor receptor signaling pathways. Msphere 2020, 5, e00582-20. [Google Scholar] [CrossRef] [PubMed]
- Hancock, M.H.; Crawford, L.B.; Pham, A.H.; Mitchell, J.; Struthers, H.M.; Yurochko, A.D.; Caposio, P.; Nelson, J.A. Human cytomegalovirus miRNAs regulate TGF-β to mediate myelosuppression while maintaining viral latency in CD34+ hematopoietic progenitor cells. Cell Host Microbe 2020, 27, 104–114.e4. [Google Scholar] [CrossRef] [PubMed]
- Mikell, I.; Crawford, L.B.; Hancock, M.H.; Mitchell, J.; Buehler, J.; Goodrum, F.; Nelson, J.A. HCMV miR-US22 down-regulation of EGR-1 regulates CD34+ hematopoietic progenitor cell proliferation and viral reactivation. PLoS Pathog. 2019, 15, e1007854. [Google Scholar] [CrossRef] [Green Version]
- Skinner, C.M.; Ivanov, N.S.; Barr, S.A.; Chen, Y.; Skalsky, R.L. An Epstein-Barr virus microRNA blocks interleukin-1 (IL-1) signaling by targeting IL-1 receptor 1. J. Virol. 2017, 91, e00530-17. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Qi, Y.; Huang, Y.; Liu, Z.; Ma, Y.; Guo, X.; Jiang, S.; Sun, Z.; Ruan, Q. Human cytomegalovirus miR-US4-5p promotes apoptosis via downregulation of p21-activated kinase 2 in cultured cells. Mol. Med. Rep. 2017, 16, 4171–4178. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, R.J.; Tong, S.; Zhang, G.; Zong, J.; Chen, Y.; Jin, D.Y.; Chen, M.R.; Pan, J.; Chen, H. NF-κB signaling regulates expression of Epstein-Barr virus BART microRNAs and long noncoding RNAs in nasopharyngeal carcinoma. J. Virol. 2016, 90, 6475–6488. [Google Scholar] [CrossRef] [Green Version]
- Yetming, K.D.; Lupey-Green, L.N.; Biryukov, S.; Hughes, D.J.; Marendy, E.M.; Miranda, J.L.; Sample, J.T. The BHLF1 locus of Epstein-Barr virus contributes to viral latency and B-cell immortalization. J. Virol. 2020, 94, e01215–e01220. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, W.; Wu, Y.; Wei, F.; Gong, Z.; Bo, H.; Wang, Y.; Li, X.; Xiang, B.; Guo, C.; et al. Epstein-Barr virus-encoded miR-BART6-3p inhibits cancer cell proliferation through the LOC553103-STMN1 axis. FASEB J. 2020, 34, 8012–8027. [Google Scholar]
- Zheng, X.; Wang, J.; Wei, L.; Peng, Q.; Gao, Y.; Fu, Y.; Lu, Y.; Qin, Z.; Zhang, X.; Lu, J.; et al. Epstein-Barr virus microRNA miR-BART5-3p inhibits p53 expression. J. Virol. 2018, 92, e01022-18. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Ji, Y.; Hu, D.; Chen, B.; Zhang, H.; Li, C.; Chen, G.; Luo, X.; Zheng, X.W.; Lin, X. SNHG8 is identified as a key regulator of epstein-barr virus (EBV)-associated gastric cancer by an integrative analysis of lncRNA and mRNA expression. Oncotarget 2016, 7, 80990. [Google Scholar] [CrossRef]
- Mai, S.; Xiao, R.; Shi, L.; Zhou, X.; Yang, T.; Zhang, M.; Weng, N.; Zhao, X.; Wang, R.; Liu, J.; et al. MicroRNA-18a promotes cancer progression through SMG1 suppression and mTOR pathway activation in nasopharyngeal carcinoma. Cell Death Dis. 2019, 10, 819. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Zhang, Y.; Liu, W.; Xiao, H.; Qi, Y.; Li, J.; Luo, B. Latent membrane protein 2A inhibits expression level of Smad2 through regulating miR-155-5p in EBV-associated gastric cancer cell lines. J. Med. Virol. 2020, 92, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Liu, W.; Xiao, H.; Zhang, Q.; Wang, J.; Luo, B. LMP1–miR-146a–CXCR4 axis regulates cell proliferation, apoptosis and metastasis. Virus Res. 2019, 270, 197654. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Stroopinsky, D.; Alimperti, S.; Jiao, A.L.; Pyzer, A.R.; Cippitelli, C.; Pepe, G.; Severa, M.; Rosenblatt, J.; Etna, M.P.; et al. Epstein− Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia 2019, 33, 132–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-M.; Hur, D.Y.; Hong, S.-W.; Kim, J.H. EBV-encoded EBNA1 regulates cell viability by modulating miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem. Biophys. Res. Commun. 2017, 494, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.P.; Chen, J.N.; Dong, M.; Xiao, Z.D.; Feng, Z.Y.; Pan, Y.H.; Zhang, Y.; Du, Y.; Zhang, J.Y.; Bi, Y.H.; et al. Epstein–Barr virus-derived circular RNA LMP 2A induces stemness in EBV-associated gastric cancer. EMBO Rep. 2020, 21, e49689. [Google Scholar] [CrossRef] [PubMed]
- Kase, K.; Saito, M.; Nakajima, S.; Takayanagi, D.; Saito, K.; Yamada, L.; Ashizawa, M.; Nakano, H.; Hanayama, H.; Onozawa, H.; et al. ARID1A deficiency in EBV-positive gastric cancer is partially regulated by EBV-encoded miRNAs, but not by DNA promotor hypermethylation. Carcinogenesis 2021, 42, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, D.; Wei, F.; Xiong, F.; Zhang, S.; Gong, Z.; Shi, L.; Li, X.; Xiang, B.; Ma, J.; et al. EBV-miR-BART12 accelerates migration and invasion in EBV-associated cancer cells by targeting tubulin polymerization-promoting protein 1. FASEB J. 2020, 34, 16205–16223. [Google Scholar] [CrossRef] [PubMed]
- Lung, R.W.M.; Tong, J.H.M.; Ip, L.M.; Lam, K.H.; Chan, A.W.H.; Chak, W.P.; Chung, L.; Yeung, W.W.; Hau, P.; Chau, S.; et al. EBV–encoded miRNAs can sensitize nasopharyngeal carcinoma to chemotherapeutic drugs by targeting BRCA1. J. Cell. Mol. Med. 2020, 24, 13523–13535. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, D.; Xie, Z.; He, H.; Duan, Z. The Oncogenic Role of miR-BART19-3p in Epstein-Barr Virus-Associated Diseases. BioMed Res. Int. 2020, 2020, 5217039. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Lee, S.K. EBV miR-BART10-3p promotes cell proliferation and migration by targeting DKK1. Int. J. Biol. Sci. 2019, 15, 657. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Gong, L.P.; Chen, J.N.; Zhang, X.F.; Zhang, Y.W.; Hui, D.Y.; Zhao, X.X.; Wu, X.Y.; Shao, C.K. EBV-miR-BART10-3p and EBV-miR-BART22 promote metastasis of EBV-associated gastric carcinoma by activating the canonical Wnt signaling pathway. Cell. Oncol. 2020, 43, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Q.; Liao, S.; Zhong, K.; Jin, Y.; Zeng, T. Epstein-Barr virus-encoded miR-BART11 promotes tumor-associated macrophage-induced epithelial-mesenchymal transition via targeting FOXP1 in gastric cancer. Virology 2020, 548, 6–16. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Liu, W.; Zhang, Q.; Xiao, H.; Song, H.; Luo, B. MiR-BART1-5p targets core 2β-1, 6-acetylglucosaminyltransferase GCNT3 to inhibit cell proliferation and migration in EBV-associated gastric cancer. Virology 2020, 541, 63–74. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Y.; Yang, C.; Wan, C.; Dai, X.; Sun, Y.; Meng, J.; Lu, Y.; Li, Y.; Zhang, Z.; et al. Downregulation of ABI2 expression by EBV-miR-BART13-3p induces epithelial-mesenchymal transition of nasopharyngeal carcinoma cells through upregulation of c-JUN/SLUG signaling. Aging 2020, 12, 340. [Google Scholar] [CrossRef]
- Cristino, A.S.; Nourse, J.; West, R.A.; Sabdia, M.B.; Law, S.C.; Gunawardana, J.; Vari, F.; Mujaj, S.; Thillaiyampalam, G.; Snell, C.; et al. EBV microRNA-BHRF1-2-5p targets the 3′ UTR of immune checkpoint ligands PD-L1 and PD-L2. Blood 2019, 134, 2261–2270. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, J.; Tang, Y.; Lin, Y.; Wang, L.; Li, Y.; Liu, C.; Wu, D.; Cai, L. EBV encoded miRNA BART8-3p promotes radioresistance in nasopharyngeal carcinoma by regulating ATM/ATR signaling pathway. Biosci. Rep. 2019, 39, BSR20190415. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Zong, J.; Lin, W.; Wang, M.; Xu, Y.; Zhou, R.; Lin, S.; Guo, Q.; Chen, H.; Ye, Y.; et al. EBV-miR-BART8-3p induces epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 283. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zhou, R.; Zong, J.F.; Lin, W.S.; Tong, S.; Guo, Q.J.; Lin, C.; Lin, S.J.; Chen, Y.X.; Chen, M.R.; et al. Epstein-Barr virus-coded miR-BART13 promotes nasopharyngeal carcinoma cell growth and metastasis via targeting of the NKIRAS2/NF-κB pathway. Cancer Lett. 2019, 447, 33–40. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, J.; Guo, X.; Wu, G.; Jiao, Y.; Faleti, O.D.; Liu, P.; Liu, T.; Long, Y.; Chong, T.; et al. EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018, 14, e1007484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Qin, Z.; Wang, J.; Zheng, X.; Lu, J.; Zhang, X.; Wei, L.; Peng, Q.; Zheng, Y.; Ou, C.; et al. Epstein-Barr virus miR-BART6-3p inhibits the RIG-I pathway. J. Innate Immun. 2017, 9, 574–586. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Q.; Liu, X.; Lin, X.; Tang, Z.; Liu, C.; Zhou, J.; Zhao, M.; Li, X.; Cheng, Z.; et al. Cinobufotalin powerfully reversed EBV-miR-BART22-induced cisplatin resistance via stimulating MAP2K4 to antagonize non-muscle myosin heavy chain IIA/glycogen synthase 3β/β-catenin signaling pathway. EBioMedicine 2019, 48, 386–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zheng, X.; Qin, Z.; Wei, L.; Lu, Y.; Peng, Q.; Gao, Y.; Zhang, X.; Zhang, X.; Li, Z.; et al. Epstein–Barr virus miR-BART3-3p promotes tumorigenesis by regulating the senescence pathway in gastric cancer. J. Biol. Chem. 2019, 294, 4854–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Guo, Q.; Lin, K.; Chen, H.; Chen, Y.; Xu, Y.; Lin, C.; Su, Y.; Chen, Y.; Chen, M.; et al. Circulating Epstein-Barr virus microRNAs BART7-3p and BART13-3p as novel biomarkers in nasopharyngeal carcinoma. Cancer Sci. 2020, 111, 1711. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).