Abstract
This review briefly summarizes the data on the mechanisms of development of the adaptability of Acinetobacters to various living conditions in the environment and in the clinic. A comparative analysis of the genomes of free-living and clinical strains of A. lwoffii, as well as the genomes of A. lwoffii and A. baumannii, has been carried out. It has been shown that plasmids, both large and small, play a key role in the formation of the adaptability of Acinetobacter to their living conditions. In particular, it has been demonstrated that the plasmids of various strains of Acinetobacter differ from each other in their structure and gene composition depending on the lifestyle of their host bacteria. Plasmids of modern strains are enriched with antibiotic-resistant genes, while the content of genes involved in resistance to heavy metals and arsenic is comparable to plasmids from modern and ancient strains. It is concluded that Acinetobacter plasmids may ensure the survival of host bacteria under conditions of various types of environmental and clinical stresses. A brief overview of the main mechanisms of horizontal gene transfer on plasmids inherent in Acinetobacter strains is also given.
1. Introduction
Bacteria of the genus Acinetobacter are gram-negative coccobacilli belonging to the family Moraxellaceae, class γ-proteobacteria. Acinetobacters are aerobic chemoorganotrophic saprophytes. Strains of Acinetobacter are among the most ubiquitous bacteria and live in a wide variety of ecological niches. They are widespread in various soils, fresh and sea water, on surface covers and in the intestines of insects, animals and humans, and on plants [1,2]. Some strains, predominantly A. baumannii, are causative agents of nosocomial infections such as septicemia, pneumonia, meningitis, urinary tract infections, skin infections, gastroenteritis and wound infections, as well as infectious diseases in animals [3,4]. A. baumannii strains are also particularly dangerous because many of them are resistant to all antibacterial drugs used in clinic [5,6]. The number of studies of clinical strains of Acinetobacter increases from year to year. One of the most important questions facing researchers is the question about the mechanisms underlying the amazing plasticity of representatives of the genus Acinetobacter and their ability to adapt to a wide range of living conditions [7,8,9].
The first attempt to compare the structure of the genomes of different Acinetobacter strains was made in 2008 [10]. Three strains were chosen for comparison, two of which belonged to the A. baumannii species and one to the A. baylyi species. The strain AYE of A. baumannii was a human isolate; the strain SDF was isolated from lice, and the strain ADP1 (BD413) of A. baylyi was a mutant of the naturally transformable strain BD4 isolated from soil [2,11]. The findings show that the genome structure of the three compared strains is partly determined by life in different ecological niches. In particular, multidrug resistance was found only in the clinical AYE strain [10]. To the surprise of the researchers, the clinical strain had the greatest metabolic potential. However, most of the differences found cannot be unambiguously attributed to adaptation. Perhaps multiple resistance to antibiotics is the only reliable example of adaptation to the conditions of existence in the clinic. Obviously, it is impossible to find a clue to the plasticity of Acinetobacter by comparing the genomes of only three strains from two species.
In further studies, chromosomes and plasmids of various Acinetobacter species were sequenced and characterized. However, only very limited information about the comparative genomics of strains characterized by different lifestyles could be obtained from these works [9,12].
We had at our disposal a collection of ancient strains of Acinetobacter isolated from the permafrost of the Kolyma Lowland, which guarantees that these strains had never experienced anthropogenic influence. Five strains of A. lwoffii isolated from permafrost samples aged from 15 thousand to 1.8 million years were selected from this collection for the comparative analysis of genomes. Representatives of this species are known to exist both in the environment and on human skin and mucous membrane [13]. In addition, strains of this species have been increasingly reported as hospital pathogens associated with bacteremia [14]. We sequenced the genomes of these strains and performed a comparative study of plasmids and chromosomes of modern and ancient strains.
Thanks to these studies, we were able to get an idea of how rapid adaptation to habitat conditions is ensured in representatives of the genus Acinetobacter. In this minireview, we summarize our findings and recently published results of the other authors and try to answer the question about the role of Acinetobacter plasmids in shaping the adaptability of host bacteria in different habitats.
2. Results
2.1. Comparison of the Chromosomes of A. lwoffii
To reveal specific genetic characteristics of ancient A. lwoffii strains preserved in permafrost and their differences from modern isolates, we conducted a comparative analysis of the genome structure of the five permafrost A. lwoffii strains mentioned above (Table 1) and modern environmental and clinical strains of this species (Table S1). It should be noted that analysis of the genomes of strains isolated from permafrost provides a unique opportunity to study microorganisms that existed before the onset of anthropogenic impact on the biosphere [15,16]. All strains were resistant to various salts of heavy metals and to some antibiotics (Table 1). To compare them with modern strains, we reiterated our previous analysis of A. lwoffii chromosomal sequences using all available genomes of A. lwoffii placed in the database by 1 July 2022. For the construction of a phylogenetic tree, we identified a set of 847 single-copy genes present in all genomes using the CD-HIT v4.6 clustering program with a 90% nucleotide global identity threshold. Individual alignment for each single copy gene nucleotide sequences was made using MAFFT v7.453; the alignments of 847 genes were concatenated and used as an input for the tree construction in PhyML v3.3 with default parameters. As a result of this analysis, it turned out that five of the strains retrieved from the database do not belong to the species A. lwoffii (Figure S1). We therefore removed them from the analysis and conducted it again without their genomes. The resulting tree is shown in Figure 1.

Table 1.
Analyzed permafrost strains of A. lwoffii.
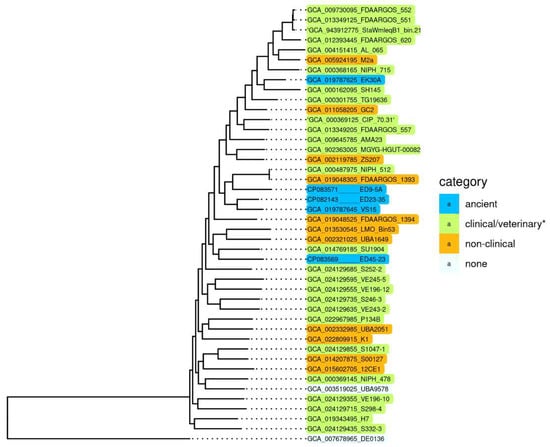
Figure 1.
Genome-based phylogeny of A. lwoffii. The maximum likelihood tree is based on concatenated nucleotide sequences of 847 single copy genes. * Strains isolated from samples of healthy and sick animals and humans. A. pseudolwoffii DE0136 was used to root the tree. The bootstrap values for all nodes are above 99%.
Surprisingly, we did not find any significant differences in the structure (sequences) of chromosomes that would distinguish permafrost strans from modern strains, including clinical ones [17]. It is clearly seen that there are no isolated groups of A. lwoffii strains adapted to any habitat. Moreover, phylogenetic analysis based on the whole genome sequences showed that permafrost (ancient) strains do not form a distinct cluster, and some of them are closely related to clinical isolates.
2.2. Comparison of the Plasmids of A. lwoffii
Since comparison of the complete genomes of A. lwoffii strains did not reveal significant differences between clinical and environmental, or modern and permafrost isolates, we decided to compare the plasmids of modern and ancient strains of A. lwoffii. In particular, we analyzed the content of heavy metal and antibiotic-resistant genes in them. For this analysis, the sequences of all A. lwoffii plasmids placed in the database by 1 July 2022 were used. We compiled a detailed list of all resistant genes found in the plasmids of modern and ancient A. lwoffii strains (Tables S2 and S3) and determined their average numbers per plasmid (Table 2). In this analysis, the presence of only complete operons (genes) of resistance was taken into account. The results show that modern plasmids are significantly enriched with antibiotic-resistant genes compared to ancient ones, while the content of genes for resistance to heavy metals and arsenic is comparable (Table 2). It should be emphasized that strains of A. lwoffii, unlike A. baumannii, are part of the normal microbiota of the skin and mucous membranes of healthy people [13]; therefore, it is likely that many strains isolated from human samples are not pathogenic. However, modern strains of A. lwoffii likely begin to accumulate the corresponding resistant genes when exposed to antibiotics, which is typical for clinical and veterinary specimens. It should be noted that the accumulation of new adaptive genes in A. lwoffii also occurs mainly on plasmids [17].

Table 2.
The number of plasmid-encoded heavy metal and antibiotic-resistant genes (operons) in permafrost and modern A. lwoffii strains.
Many plasmids of modern A. lwoffii strains contain remnants of different heavy metals and arsenic resistance operons. For example, two variants of the ars-operon with an incomplete set of genes were found: (i) an operon contained a single arsH gene in which the N-terminus was deleted; and (ii) an operon that contained three genes, including arsH, in which the N-terminus was deleted; the repressor gene, and arsC. In both cases, the defective operon contained an IS element next to the arsH gene: IS1007 in the operon of the first type and IS66 in the second. Most likely, the formation of defective ars-operons is the result of the insertion of the IS element into the N terminus of the arsH gene. At the same time, only complete ars-operons were found in ancient bacterial strains [16].
Interestingly, the genomes of ancient permafrost strains of A. lwoffii and modern environmental strains (Tables S2 and S3) are more similar in terms of the number of plasmids and the content of heavy metal and antibiotic resistance genes in them. For instance, the plasmidome generated from an arsenic-resistant strain, A. lwoffii ZS207, contained nine plasmids in the size between 4.3 and 38.4 kb as well as one 186.6 kb megaplasmid. The mega-plasmid carries arsenic and heavy metals-resistant regions similar to those found in permafrost A. lwoffii strains [18]. Numerous plasmids (15) were also found in the strain A. lwoffii M2a isolated from a honey sample [19]. Although some of them were unassembled, it was possible to show that they contain genes of mercury and heavy metal salts resistance; antibiotic-resistant genes were not found in these plasmids [19]. The same pattern was observed in unassembled plasmids of the A. lwoffii strain GT2 [20,21]. These results show that both ancient and modern strains of A. lwoffii usually carry many plasmids, and these plasmids, especially large ones, usually contain different sets of genes for resistance to heavy metals and arsenic. At the same time, plasmids from environmental strains of A. lwoffii rarely bear antibiotic-resistant genes, in contrast to the strains isolated in the clinic (which prevail in the sequenced genomes of A. lwoffii, Table 2).
2.3. Comparative Structure of A. lwoffii and A. baumannii Genomes
It was interesting to compare the genomes of A. lwoffii and A. baumannii strains, since there are much more pathogenic strains among A. baumannii, and most of the sequences in the database belong to clinical isolates. A comparative analysis of the genomes of A. lwoffii and A. baumannii strains revealed a number of differences between them: (i) chromosome sizes in A. baumannii are larger than in A. lwoffii by about 20%; (ii) in contrast, the number of plasmids and their total size are greater in A. lwoffii than in A. baumannii; (iii) environmental strains of A. lwoffii outnumber A. baumannii strains in the number and diversity of heavy metal-resistant genes, and these genes are predominantly located on plasmids in A. lwoffii and in the chromosomes in A. baumannii (Table 3) [16,17].

Table 3.
Comparative structure of A. lwoffii and A. baumannii genomes [17].
In addition, it is known that chromosomes of A. baumannii can contain resistance and pathogenicity islands, the acquisition of which immediately gives many advantages to the strain living in the clinic [22,23,24]. These structures have not yet been found in strains of A. lwoffii [22]. Thus, different Acinetobacter species may have slightly different adaptation strategies associated with different contributions of plasmids and chromosomes to them. Analysis of available data shows that the genes of resistance to heavy metals are markers of adaptation to the environment and the genes of resistance to antibiotics are characteristics for clinical strains of Acinetobacter. Furthermore, it was demonstrated that in some species of Acinetobacter, such as A. lwoffii, plasmids are the main players in adaptation, while in other species, such as A. baumannii, their role is apparently less important, but still significant [7,16,25,26,27,28,29].
2.4. Acquisition of New Genes in Small Plasmids of Acinetobacter
Comparative analysis of one of the most abundant groups of small plasmids, the representatives of which are very often found in both clinical and environmental strains of different species of Acinetobacter, allowed us to see how the formation of new variants occurs. We demonstrated that all members of this group have similar genes of plasmid maintenance (the backbone region), but they strongly differ in the structure of their accessory regions (Figure 2) [30]. Variants of the plasmid pRAY* with the aadB gene for resistance to kanamycin and gentamicin are most common among modern strains [31]. Three out of the five permafrost strains of A. lwoffii contain a related plasmid, pALWED1.8, about 4.1 kb in size with the aad27 gene for streptomycin and spectinomycin resistance. An almost identical plasmid was also found in modern Acinetobacter strains of different species [30]. All other related plasmids are arranged in a similar way (Figure 2). Another very common variant is a plasmid with detergent-resistant gene encoding alkyl/arylsulfatase [30]. However, there are many other rare variants that contain ORFs encoding hypothetical proteins with obscure functions or that do not contain any coding sequences. These sequences most often resemble regions of Acinetobacter chromosomes [30]. It can be proposed that small pieces of DNA are constantly being inserted into these plasmids, followed by their selection and wide distribution of most adaptive variants. However, the mechanism of active DNA acquisition by these plasmids is unclear.
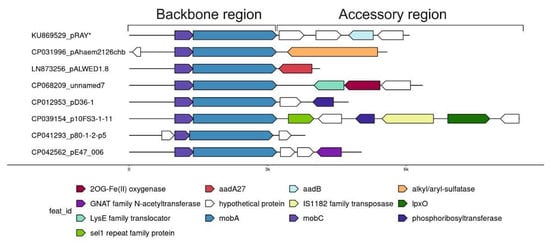
Figure 2.
Plasmid pRAY* and related small plasmids.
2.5. Acquisition of New Genes by Acinetobacter Mega-Plasmids
Very rapid DNA rearrangements and acquisition of new adaptive genes were also revealed in comparative analysis of the sequences of mega-plasmids. Two groups of researchers identified a new group of giant conjugative mega-plasmids about 300 kb in size and demonstrated their wide distribution in the environmental and clinical Acinetobacter strains and their participation in the horizontal transfer of antibiotic resistance genes [32,33,34]. All members of this very large group contain a common backbone region (almost 100 kb in size), which includes genes for replication, plasmid maintenance and conjugative transfer. At the same time, analysis of their accessory regions demonstrates high abundance of various resistance genes. In total, 221 different antibiotic-resistant genes were revealed in 21 mega-plasmids from clinical strains of Acinetobacter analyzed by Ghaly et al. [32]. In the study of Mindlin et al. [34], the difference in the structure of clinical and natural variants of mega-plasmids was demonstrated by the example of pALWED1.1 from an environmental permafrost strain of A. lwoffii and pAHTJR1 from a clinical A. haemolyticus strain. The permafrost plasmid contains only one antibiotic resistance gene, tet (H), with impaired function, and a large cluster of determinants of resistance to heavy metals include mercury, chromium, cobalt, zinc, cadmium and nickel. These genes are absent in the clinical version of the plasmid, as well as some other genes not associated with antibiotic resistance. At the same time, the clinical plasmid contains three regions with genes for resistance to different antibiotics.
For this review, we analyzed the content of determinants of resistance to heavy metals, arsenic and antibiotics in 36 mega-plasmids from Acinetobacter strains of various species and different origins (Figure 3). Antibiotic-resistant genes were identified with AMRFinderPlus v3.10 using default parameters. Metal-resistant genes were identified using a diamond v2.0.13.151 homology search against the BacMet2 database; alignment coverage of at least 80% of the reference gene and e-value of <1e-5 were used as cutoff parameters. For czc and cop operons, we additionally used manual selection of reference genes for the diamond search.
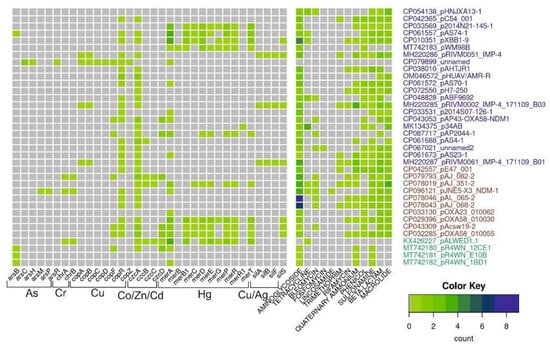
Figure 3.
Diversity and abundance of heavy metals and antibiotic-resistant genes in mega-plasmids. The number of resistant genes (color scale bar) is shown for each plasmid (rows). Plasmid names are colored according to their origin: from Homo sapiens, pets, poultry, in blue; from hospital and farm environments, including hospital sewage in red; from the environment in green.
This analysis shows that mega-plasmids isolated from strains related to the clinic or veterinary, including A. baumannii strains, are very rich in genes resistant to various antibiotics compared to plasmids from strains isolated from the environment. However, it should be noted that of the 36 plasmids whose complete sequences are available in the database, only 4 were isolated from environmental samples, 1 from a permafrost strain and 3 from strains isolated from marine king prawns. At the same time, many clinical plasmids contain certain operons of resistance to heavy metals and/or their relics. Interestingly, mercury-resistant operons are most often found in mega-plasmids. This may be due to the fact that mercury preparations had been used in medicine for a long time. At the same time, no complete operon of resistance to arsenic was found in mega-plasmids. Overall, analysis of mega-plasmids from predominantly clinical strains of Acinetobacter of various species reveals similar trends to the analysis of plasmids from A. lwoffii.
In large plasmids, in contrast to small ones, several mechanisms of acquisition of resistant genes are evident, including insertions of composite transposons and various integrons [34]. The latter are very common and are found in at least half of modern mega-plasmids. Moreover, some mega-plasmids contain 2–3 integrons [34]. Integrons are quite rare in unspoiled ecosystems but are abundant in the clinic, where they encode resistance to antibiotics [35,36,37]. The transfer of integrons mainly occurs through their insertion into both simple and compound transposons [38]. Similarly, compound transposons are constantly found in the clinic, while they are practically absent in environmental strains [39,40,41].
2.6. Mobile Genetic Elements and Mechanisms of Rearrangement of Acinetobacter Plasmids
Quite a long time ago, it was found that, unlike in other bacteria, mercury-resistant genes in Acinetobacters most often are part of defective transposons, with transposition genes almost completely lost [42]. In other bacteria, the loss of mobility of a mercury transposon usually leads to its elimination from cells. On the contrary, such defective transposons are widely distributed on different plasmids in Acinetobacter strains of various species [42]. Detailed analysis of the structure of defective mer transposons demonstrated that their distribution mainly depends on various systems of homologous and site-specific recombination. IS elements often act as homologous sites for recombination events. On the basis of DNA sequence analysis, possible mechanisms of translocation of defective mercury-resistant transposons via recombination events implicating nearby res (resolution) sites and IS elements were proposed (Figure 4) [42]. In this work, evidence was also obtained for an unusually active rearrangement of Acinetobacter plasmids, since at least 36 recombination events over 70 kb of sequenced plasmid DNA were documented [42]. In addition, a site-specific recombination system CinH-RS2, encoding a 189 aa CinH recombinase and a 119 bp recombination site RS2, is often found in Acinetobacter plasmids, including pKLH2, pKLH204 and pKLH205, [43,44,45]. It was shown that this recombination system recognizes not only its own res sites, but also the res sites of the Tn3 family transposons, as well as res sites of some plasmid cointegrates [43].
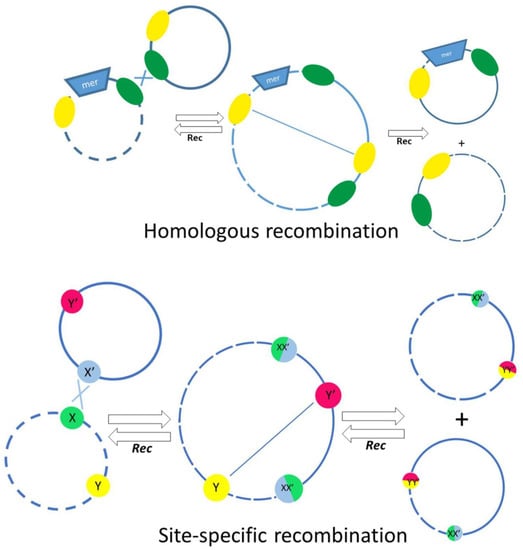
Figure 4.
The main mechanisms involved in the exchange of genetic material between plasmids, involving homologous recombination (top) or site-specific recombination depending on res sites and the action of resolvases (bottom). The res sites are indicated by colored circles in the bottom panel.
Recently, a novel class of mobile genetic elements, the transposition of which likely depends on the action of the dif/Xer recombination system, was discovered in Acinetobacter species [46].
In other bacteria, the recombination site, called the dif site is located only in the chromosome, and the tyrosine recombinase XerC/XerD participates in the resolution of chromosomal dimers after replication [47,48]. It turned out that in Acinetobacter, dif-like sites (pdif) are often found in plasmids [28,49,50,51]. Genes located between two plasmid pdif sites can be transferred between plasmids, since identical genes have been found in different plasmids [49,50,52]. Although this has not yet been experimentally proven, it is believed that this transfer involves the same XerC and XerD proteins that recognize the chromosomal dif site [46,50,51]. This gene transfer system is likely active in plasmids from both environmental and clinical strains, although the genes in these two cases are different. In plasmids from clinical strains, these genes predominantly encode resistance to antibiotics [49,50,53,54,55,56], whereas in environmental strains, various genes are found, including those that are associated with adaptation to their habitats [50,51].
3. Conclusions
Among the members of the genus Acinetobacter, A. baumannii is undoubtedly the most actively investigated and therefore the best studied. The important role of plasmids in the acquisition of unprecedented multidrug resistance by clinical strains of this species has been confirmed by a huge number of studies conducted around the world [8,12,56,57,58,59]. However, little was known about the significance of plasmids in other species of the genus, since nonclinical strains were rarely studied. Our studies of Acinetobacter strains isolated from permafrost have shown that in environmental strains, the contribution of plasmids to the adaptation to living conditions may be even higher than in clinical strains. Analysis of available data allows us to conclude that, to achieve rapid exchange of plasmid genes, Acinetobacters uses not only the mechanisms common in other bacteria but also an arsenal of their own. In particular, unlike other bacteria, horizontal transfer of mercury resistance operons on Acinetobacter plasmids is carried out not by the transposition of functionally active mercury transposons, but by various recombination events of aberrant, and therefore functionally inactive, mercury transposons [42]. Another example is the use of the dif/Xer site-specific recombination system, which in other bacteria functions only on chromosomes, to disseminate adaptive plasmid genes [46]. It seems that the adaptation strategy of Acinetobacter spp. is to constantly pass different genes through their plastidome. Hernández-González et al. [60] described horizontal gene transfer as “gene flow”. The term flow seems to most clearly reflect the essence of what happens in the plasmids of Acinetobacter. Considering that many Acinetobacter plasmids are mobilizable, and widespread mega-plasmids are able not only to move themselves but also to mobilize other plasmids [34], it is clear that the necessary genes easily become available to all Acinetobacter strains. Then, there is a selection of options to be adapted depending on the given living conditions.
Comparative analysis of the nucleotide sequences of more than 40 plasmids of permafrost strains of A. lwoffii and 75 plasmids of modern strains of A. lwofii made it possible to trace how the set of genes changes in accessory regions. It was also possible to show that accumulation of antibiotic resistance genes in modern strains occurs not only in modern clinical but also in modern environmental strains, indicating a strong anthropogenic impact on the biosphere associated with the production and widespread use of antibiotics over the past seventy years.
Thus, we come to the conclusion that the adaptability of Acinetobacter is provided not only by their high metabolic potential encoded in the chromosome, but to a large extent by their plasmids, although this contribution can obviously be different in different species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231810893/s1.
Author Contributions
Conceptualization, M.P. and O.M; validation, M.P, O.M. and S.M.; formal analysis, O.M., A.B. and A.M.; data curation, M.P., O.M. and S.M.; writing—original draft preparation, M.P.; writing—review and editing, O.M., A.M. and S.M.; visualization, A.B. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
O.M., S.M. and M.P were supported by the Ministry of Education and Science of Russian Federation within the framework of the Kurchatov Genome Center development Program (Agreement 075-15-2019-1664). A.M. and A.B. were supported by the Ministry of Science and Higher Education of the Russian Federation (№ 122040800186-8).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to A. Kulbachinskiy for helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baumann, P.; Doudoroff, M.; Stanier, R.Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J. Bacteriol. 1968, 95, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Juni, E. Genetics and physiology of Acinetobacter. Ann. Rev. Microbiol. 1978, 32, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.S.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Jee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Doughari, H.J.; Ndakidemi, P.A.; Human, I.S.; Benade, S. The Ecology, Biology and Pathogenesis of Acinetobacter spp.: An Overview. Microbes Environ. 2011, 26, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; de Breij, A.; Adams, M.D.; Cerqueira, G.M.; Mocali, S.; Galardini, M.; Nibbering, P.H.; Earl, A.M.; Ward, D.V.; Paterson, D.L.; et al. The success of acinetobacter species; genetic, metabolic and virulence attributes. PLoS ONE 2012, 7, e46984. [Google Scholar] [CrossRef]
- Fondi, M.; Bacci, G.; Brilli, M.; Papaleo, M.C.; Mengoni, A.; Vaneechoutte, M.; Dijkshoorn, L.; Fani, R. Exploring the evolutionary dynamics of plasmids: The Acinetobacter pan-plasmidome. BMC Evol. Biol. 2010, 10, 59. [Google Scholar] [CrossRef]
- Imperi, F.; Antunes, L.C.; Blom, J.; Villa, L.; Iacono, M.; Visca, P.; Carattoli, A. The genomics of Acinetobacter baumannii: Insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life 2011, 63, 1068–1074. [Google Scholar] [CrossRef]
- Jia, J.; Liu, M.; Feng, L.; Wang, Z. Comparative genomic analysis reveals the evolution and environmental adaptation of Acinetobacter johnsonii. Gene 2022, 808, 145985. [Google Scholar] [CrossRef]
- Vallenet, D.; Nordmann, P.; Barbe, V.; Poirel, L.; Mangenot, S.; Bataille, E.; Dossat, C.; Gas, S.; Kreimeyer, A.; Lenoble, P.; et al. Comparative analysis of Acinetobacters: Three genomes for three lifestyles. PLoS ONE 2008, 3, e1805. [Google Scholar] [CrossRef]
- Santala, S.; Santala, V. Acinetobacter baylyi ADP1-naturally competent for synthetic biology. Essays Biochem. 2021, 65, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Salto, I.P.; Torres Tejerizo, G.; Wibberg, D.; Pühler, A.; Schlüter, A.; Pistorio, M. Comparative genomic analysis of Acinetobacter spp. plasmids originating from clinical settings and environmental habitats. Sci. Rep. 2018, 8, 7783. [Google Scholar] [CrossRef]
- Figueiredo, S.; Poirel, L.; Seifert, H.; Mugnier, P.; Benhamou, D.; Nordmann, P. OXA-134, a naturally occurring carbapenem-hydrolyzing class D beta-lactamase from Acinetobacter lwoffii. Antimicrob. Agents Chemother. 2010, 54, 5372–5375. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.C.; Hsueh, P.R.; Yang, P.C.; Luh, K.T. Clinical and microbiological characteristics of bacteremia caused by Acinetobacter lwoffii. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Mindlin, S.Z.; Petrova, M.A.; Gorlenko, Z.M.; Soina, V.S.; Khachikian, N.A.; Karaevskaya, E.A. Multidrug-resistant bacteria in permafrost: Isolation, biodiversity, phenotypic and genotypic analysis. In New Permafrost and Glacier Research, 1st ed.; Krugger, M.I., Stern, H.P., Eds.; Nova Science: Hauppauge, NY, USA, 2009; pp. 89–105. [Google Scholar]
- Mindlin, S.; Petrenko, A.; Kurakov, A.; Beletsky, A.; Mardanov, A.; Petrova, M. Resistance of Permafrost and Modern Acinetobacter lwoffii Strains to Heavy Metals and Arsenic Revealed by Genome Analysis. Biomed. Res. Int. 2016, 16, 3970831. [Google Scholar] [CrossRef]
- Rakitin, A.L.; Ermakova, A.Y.; Beletsky, A.V.; Petrova, M.A.; Mardanov, A.V.; Ravin, N.V. Genome Analysis of Acinetobacter lwoffii Strains Isolated from Permafrost Soils Aged from 15 Thousand to 1.8 Million Years Revealed Their Close Relationships with Present-Day Environmental and Clinical Isolates. Biology 2021, 10, 871. [Google Scholar] [CrossRef]
- Walter, T.; Klim, J.; Jurkowski, M.; Gawor, J.; Köhling, I.; Słodownik, M.; Zielenkiewicz, U. Plasmidome of an environmental Acinetobacter lwoffii strain originating from a former gold and arsenic mine. Plasmid 2020, 110, 102505. [Google Scholar] [CrossRef]
- Veress, A.; Nagy, T.; Wilk, T.; Kömüves, J.; Olasz, F.; Kiss, J. Abundance of mobile genetic elements in an Acinetobacter lwoffii strain isolated from Transylvanian honey sample. Sci. Rep. 2020, 10, 2969. [Google Scholar] [CrossRef]
- Acer, O.; Güven, K.; Poli, A.; Donato, P.D.; Leone, L.; Buono, L.; Güven, R.G.; Nicolaus, B.; Finore, I. Acinetobacter mesopotamicus sp. nov., Petroleum-degrading Bacterium, Isolated from Petroleum-contaminated Soil in Diyarbakir, in the Southeast of Turkey. Curr. Microbiol. 2020, 77, 3192–3200. [Google Scholar] [CrossRef]
- Nemec, A. Strain “Acinetobacter mesopotamicus” GC2 Does Not Represent a Novel Species, but Belongs to the Species Acinetobacter lwoffii as Revealed by Whole-Genome Sequence-Based Analysis. Curr. Microbiol. 2021, 78, 369–370. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species. IUBMB Life 2011, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, M.; Hall, R.M. AbGR14, a novel antibiotic resistance island in multiply antibiotic-resistant Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 2017, 72, 2944–2947. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.L.N.; Morais-Rodrigues, F.; Hurtado, R.; Dos Santos, R.G.; Costa, D.C.; Barh, D.; Ghosh, P.; Alzahrani, K.J.; Soares, S.C.; Ramos, R.; et al. Pan-Resistome Insights into the Multidrug Resistance of Acinetobacter baumannii. Antibiotics 2021, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Fondi, M.; Rizzi, E.; Emilian, I.G.; Orlandini, V.; Berna, L.; Papaleo, M.C.; Perrin, E.; Maida, I.; Corti, G.; de Bellis, G.; et al. The genome sequence of the hydrocarbon-degrading Acinetobacter venetianus VE-C3. Res. Microbiol. 2013, 164, 439–449. [Google Scholar] [CrossRef]
- Brovedan, M.A.; Cameranesi, M.M.; Limansky, A.S.; Morán-Barrio, J.; Marchiaro, P.; Repizo, G.D.; World, J. What do we know about plasmids carried by members of the Acinetobacter genus? Microbiol. Biotechnol. 2020, 36, 109. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, P.; Wang, X.; Zong, Z. Characterization of Acinetobacter johnsonii isolate XBB1 carrying nine plasmids and encoding NDM-1, OXA-58 and PER-1 by genome sequencing. J. Antimicrob. Chemother. 2016, 71, 71–75. [Google Scholar] [CrossRef]
- Brovedan, M.; Repizo, G.D.; Marchiaro, P.; Viale, A.M.; Limansky, A. Characterization of the diverse plasmid pool harbored by the blaNDM-1- containing Acinetobacter bereziniae HPC229 clinical strain. PLoS ONE 2019, 14, e0220584. [Google Scholar] [CrossRef]
- Cui, C.Y.; Chen, C.; Liu, B.T.; He, Q.; Wu, X.T.; Sun, R.Y.; Zhang, Y.; Cui, Z.; Guo, W.; Jia, Q.; et al. Co-occurrence of Plasmid-Mediated Tigecycline and Carbapenem Resistance in Acinetobacter spp. from Waterfowls and Their Neighboring Environment. Antimicrob. Agents Chemother. 2020, 64, e02502-19. [Google Scholar] [CrossRef]
- Kurakov, A.; Mindlin, S.; Beletsky, A.; Shcherbatova, N.; Rakitin, A.; Ermakova, A.; Mardanov, A.; Petrova, M. The ancient small mobilizable plasmid pALWED1.8 harboring a new variant of the non-cassette streptomycin/spectinomycin resistance gene aadA27. Plasmid 2016, 84–85, 36–43. [Google Scholar] [CrossRef]
- Hamidian, M.; Nigro, S.J.; Hall, R.M. Variants of the gentamicin and tobramycin resistance plasmid pRAY are widely distributed in Acinetobacter. J. Antimicrob. Chemother. 2012, 67, 2833–2836. [Google Scholar] [CrossRef]
- Ghaly, T.M.; Paulsen, I.T.; Sajjad, A.; Tetu, S.G.; Gillings, M.R. A novel family of Acinetobacter mega-plasmids are disseminating multi-drug resistance across the globe while acquiring location-specific accessory genes. Front. Microbiol. 2020, 11, 605952. [Google Scholar] [CrossRef] [PubMed]
- Mindlin, S.; Beletsky, A.; Rakitin, A.; Mardanov, A.; Petrova, M. Acinetobacter Plasmids: Diversity and Development of Classification Strategies. Front. Microbiol. 2020, 11, 588410. [Google Scholar] [CrossRef] [PubMed]
- Mindlin, S.; Maslova, O.; Beletsky, A.; Nurmukanova, V.; Zong, Z.; Mardanov, A.; Petrova, M. Ubiquitous Conjugative Mega-Plasmids of Acinetobacter Species and Their Role in Horizontal Transfer of Multi-Drug Resistance. Front. Microbiol. 2021, 12, 728644. [Google Scholar] [CrossRef] [PubMed]
- Gillings, M.R. Class 1 integrons as invasive species. Curr. Opin. Microbiol. 2017, 38, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, A.D.; Yin, X.L.; Zhang, T. The Prevalence of Integrons as the Carrier Resistance Genes in Natural and Man-Made Environments. Environ. Sci. Technol. 2017, 51, 5721–5728. [Google Scholar] [CrossRef]
- Ghaly, T.M.; Gillings, M.R.; Penesyan, A.; Qi, Q.; Rajabal, V.; Tetu, S.G. The Natural History of Integrons. Microorganisms 2021, 9, 2212. [Google Scholar] [CrossRef]
- Mazel, D. Integrons: Agents of bacterial evolution. Nat. Rev. Microbiol. 2006, 4, 608–620. [Google Scholar] [CrossRef]
- Nigro, S.J.; Hall, R.M. Structure and Context of acinetobacter transposons carrying the oxa23 carbapenemase gene. J. Antimicrob. Chemother. 2016, 71, 1135–1147. [Google Scholar] [CrossRef]
- Repizo, G.D.; Viale, A.M.; Borges, V.; Cameranesi, M.M.; Taib, N.; Espariz, M.; Brochier-Armanet, C.; Gomes, J.P.; Salcedo, S.P. The environmental Acinetobacter baumannii isolate DSM30011 reveals clues into the preantibiotic era genome diversity, virulence potential, and niche range of a predominant nosocomial pathogen. Genome Biol. Evol. 2017, 9, 2292–2307. [Google Scholar] [CrossRef]
- Ross, K.; Varani, A.M.; Snesrud, E.; Huang, H.; Alvarenga, D.O.; Zhang, J.; Wu, C.; McGann, P.; Chandler, M. TnCentral: A Prokaryotic Transposable Element Database and Web Portal for Transposon Analysis. mBio 2021, 12, e0206021. [Google Scholar] [CrossRef]
- Kholodii, G.; Mindlin, S.; Gorlenko Zh Petrova, M.; Hobman, J.; Nikirofov, V. Translocation of transposition-deficient (TndPKLH2-like) transposons in the natural environment: Mechanistic insights from the study of adjacent DNA sequences. Microbiology 2004, 150, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Kholodii, G. The shuffling function of resolvases. Gene 2001, 269, 121–130. [Google Scholar] [CrossRef]
- Moon, H.S.; Abercrombie, L.L.; Eda, S.; Blanvillain, R.; Thomson, J.G.; Ow, D.W.; Stewart, C.N., Jr. Transgene excision in pollen using a codon optimized serine resolvase CinH-RS2 site-specific recombination system. Plant Mol. Biol. 2011, 5, 621–631. [Google Scholar] [CrossRef]
- Thomson, J.G.; Ow, D.W. Site-specific recombination systems for the genetic manipulation of eukaryotic genomes. Genesis 2006, 44, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Balalovski, P.; Grainge, I. Mobilization of pdif modules in Acinetobacter: A novel mechanism for antibiotic resistance gene shuffling? Mol. Microbiol. 2020, 114, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Blakely, G.W.; Sherratt, D.J. Interactions of the site-specific recombinases XerC and XerD with the recombination site dif. Nucleic Acids Res. 1994, 22, 5613–5620. [Google Scholar] [CrossRef]
- Hayes, F.; Sherratt, D.J. Recombinase binding specificity at the chromosome dimer resolution site dif of Escherichia coli. J. Mol. Biol. 1997, 266, 525–537. [Google Scholar] [CrossRef]
- Merino, M.; Acosta, J.; Poza, M.; Sanz, F.; Beceiro, A.; Chaves, F.; Bou, G. OXA-24 carbapenemase gene flanked by XerC/XerD-like recombination sites in different plasmids from different Acinetobacter species isolated during a nosocomial outbreak. Antimicrob. Agents Chemother. 2010, 54, 2724–2727. [Google Scholar] [CrossRef]
- Blackwell, G.A.; Hall, R.M. The tet39 determinant and the msrE-mphE genes in Acinetobacter plasmids are each part of discrete modules flanked by inversely oriented pdif (XerC-XerD) sites. Antimicrob. Agents Chemother. 2017, 61, e780-17. [Google Scholar] [CrossRef]
- Mindlin, S.; Beletsky, A.; Mardanov, A.; Petrova, M. Adaptive dif Modules in Permafrost Strains of Acinetobacter lwoffii and Their Distribution and Abundance Among Present Day Acinetobacter Strains. Front. Microbiol. 2019, 10, 632. [Google Scholar] [CrossRef]
- Mindlin, S.; Petrenko, A.; Petrova, M. Chromium resistance genetic element flanked by XerC/XerD recombination sites and its distribution in environmental and clinical Acinetobacter strains. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.M.; Giani, T.; D’Arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P.; Luzzaro, F.; Rossolini, G.M. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Grosso, F.; Quinteira, S.; Poirel, L.; Novais, A.; Peixe, L. Role of common blaOXA-24/OXA-40-carrying platforms and plasmids in the spread of OXA-24/OXA-40 among Acinetobacter species clinical isolates. Antimicrob. Agents Chemother. 2012, 56, 3969–3972. [Google Scholar] [CrossRef] [PubMed]
- Povilonis, J.; Seputiene, V.; Krasauskas, R.; Juskaite, R.; Miskinyte, M.; Suziedelis, K.; Suziedeliene, E. Spread of carbapenem-resistant Acinetobacter baumannii carrying a plasmid with two genes encoding OXA-72 carbapenemase in Lithuanian hospitals. J. Antimicrob. Chemother. 2013, 68, 1000–1006. [Google Scholar] [CrossRef]
- Liu, H.; Moran, R.A.; Chen, Y.; Doughty, E.L.; Hua, X.; Jiang, Y.; Xu, Q.; Zhang, L.; Blair, J.M.A.; McNally, A.; et al. Transferable Acinetobacter baumannii plasmid pDETAB2 encodes OXA-58 and NDM-1 and represents a new class of antibiotic resistance plasmids. J. Antimicrob. Chemother. 2021, 76, 1130–1134. [Google Scholar] [CrossRef]
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef]
- Hamidian, M.; Ambrose, S.J.; Blackwell, G.A.; Nigro, S.J.; Hall, R.M. An outbreak of multiply antibiotic-resistant ST49:ST128:KL11:OCL8 Acinetobacter baumannii isolates at a Sydney hospital. J. Antimicrob. Chemother. 2021, 7, 893–900. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhang, J.; Wang, X.; Zhang, Y.; Wang, H. Identification of a novel plasmid-mediated tigecycline resistance-related gene, tet(Y), in Acinetobacter baumannii. J. Antimicrob. Chemother. 2021, 77, 58–68. [Google Scholar] [CrossRef]
- Hernández-González, I.L.; Mateo-Estrada, V.; Castillo-Ramirez, S. The promiscuous and highly mobile resistome of Acinetobacter baumannii. Microb. Genom. 2022, 8, 000762. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).