Abstract
RNase H1s are associated with growth and development in both plants and animals, while the roles of RNase H1s in bryophytes have been rarely reported. Our previous data found that PpRNH1A, a member of the RNase H1 family, could regulate the development of Physcomitrium (Physcomitrella) patens by regulating the auxin. In this study, we further investigated the biological functions of PpRNH1A and found PpRNH1A may participate in response to heat stress by affecting the numbers and the mobilization of lipid droplets and regulating the expression of heat-related genes. The expression level of PpRNH1A was induced by heat stress (HS), and we found that the PpRNH1A overexpression plants (A-OE) were more sensitive to HS. At the same time, A-OE plants have a higher number of lipid droplets but with less mobility in cells. Consistent with the HS sensitivity phenotype in A-OE plants, transcriptomic analysis results indicated that PpRNH1A is involved in the regulation of expression of heat-related genes such as DNAJ and DNAJC. Taken together, these results provide novel insight into the functions of RNase H1s.
1. Introduction
With global warming, heat stress (HS) has gradually become a major limiting factor affecting plant growth, plant geographic distribution, crop yield, and quality [,,]. Research analysis showed that the negative impact of high temperature on crop yield is becoming more severe []. Plants do not move and have evolved a series of self-protective mechanisms against external stresses to adapt to the environment []. Heat shock proteins (HSPs) and molecular chaperones are commonly found to be involved in plants’ responses to HS [,]. DnaJ (HSP40) proteins are important molecular chaperones involved in signal transduction, cellular proteostasis, and tolerance to various stresses in plants [,]. The expression levels of these genes are up-regulated to improve heat tolerance in plants [,]. For example, GmDNJ1 (a major HSP40) is highly induced at high temperature, and Gmdnj1-knockout mutants have more severe browning and lower chlorophyll content, and higher reactive oxygen species (ROS) content under HS, which suggest that GmDNJ1 plays an important role in response to heat stress in soybean [].
Cytosolic lipid droplets play important roles in plant growth and responses to stress. Lipid droplets (or so-called lipid bodies in some cases) are subcellular structures that are capable of storing neutral lipids and various hydrophobic compounds in eukaryotes, which are present in almost all green plant lineages and is widely explored in various kind of tissues such as seeds, pollen, and leaves in different plant species [,,,]. Feeney et al. found that a large ectopic accumulation of lipid droplets in vegetative organs produced abnormal embryogenic structures in plants []. Triacylglycerol (TAG) is one of the main components of lipid droplets. Abnormally accumulating TAG in plant leaves usually affects plant growth and causes cell death [], which was also observed when subjected to various stresses []. Abiotic stresses such as heat stress, high light, drought, and cold induce lipid droplets accumulation in plants [], including not only land plants but also chlorophyte algae such as Haematococcus pluvialis, Chlamydomonas reinhardtii, and cyanobacterium Synechocystis sp. PCC6803, among others [,,]. Furthermore, the accumulation and mobilization of lipid droplets were reported to be positively related to plant stress resistance [,,]. In the salt-sensitive variety, salt stress resulted in lipid droplets accumulation and higher lipid droplets retention, whereas the tolerant variety exhibited faster lipid droplets mobilization []. In P. patens, lipid droplets were found to be present in dehydrated spores and photosynthetic gametophytes []. Previous studies have shown that numerous lipid droplets could be observed after dehydration, which indicates that drought stress also induces the accumulation of lipid droplets in P. patens []. Lipidome analysis revealed that lipid metabolism plays an important role in P. patens to cope with the terrestrial environment stresses, although the composition of monogalactosyldiacylglycerol is different between P. patens and vascular plants [].
RNase H is considered as a class of sequence-nonspecific ribonucleases and was first isolated and identified from calf mammary glands []. RNase Hs were found widespread in archaebacteria, prokaryotes, and eukaryotes [] and are classified into three types (RNase H1, H2, and H3) []. Among them, RNase H3 only exists in certain archaebacteria [,], while RNase H1 and RNase H2, are present in plants and animals [,]. Although the structure of RNase H1s are evolutionarily highly conserved [], RNase H1s were found to play important roles in diverse biological processes such as replication process, RNA processing process, and development of mitochondrial DNA [,]. In addition, they play crucial roles in the maintenance of genomic stability, the repair of DNA damage, and affect the development of organisms as well [,,]. Three AtRNH1s were found in Arabidopsis thaliana, which are localized in the nucleus (AtRNH1A), mitochondria (AtRNH1B), and chloroplasts (AtRNH1C), respectively []. The diversification results of the subcellular localization of RNAH1s in Arabidopsis facilitate us to hypothesize that RNH1s may have multiple functions in different cellular compartments. In addition, AtRNH1B and AtRNH1C are required for viability in Arabidopsis, while AtRNH1A is not []. Moreover, studies showed that loss-of-function mutation of AtRNH1C exhibits a distinct growth phenotype of dwarfism and leaf chlorosis []. Gene functional mechanism studies showed that AtRNH1C and AtGyrases interact to reduce the damage of DNA [], and AtRNH1C promotes the repair of DNA damage in chloroplasts mainly by its synergistic action with ssDNA-binding proteins (WHY1/3) and recombinases (RecA1) as well []. Interestingly, deletion of AtRNH1B allows AtRNH1C, which is located in chloroplasts, to enter the mitochondria, ensuring the integrity of the mitochondrial genome [].
Despite functional research of RNH1s in Arabidopsis in recent years, it remains unclear how RNH1s function in response to abiotic stresses and how RNH1s evolved within land plant lineage. Mosses, which belong to the early land plants, hold an important position in higher plant evolution. Physcomitrium patens (P. patens), has been a model plant to study evolutionary developmental and stress tolerance/adaption questions as a non-seed plant [,,]. Therefore, it is of some far-reaching significance to study the biological functions of the RNase H1 family in P. patens. In our previous research, we found that there are two family members of RNase H1s in P. patens []. PpRNH1A affects shoot growth and branch formation of P. patens by controlling the formation of the R-loop and regulating the transcription level of auxin-related genes in the mutant pprna1a []. However, different from the subcellular location results of AtRNH1A, PpRNH1A was found localized in both nucleus and cytosol, which suggested a species-specific role of RNH1A in P. patens. In this research, we further explore the function of PpRNH1A and found that overexpression of PpRNH1A (A-OE) plants were more sensitive to heat stress by regulation of heat-related gene expressions, such as DNAJ and DNAJC. Chloroplast development but not pigment synthesis was affected by overexpression of PpRNH1A. Ultrastructural and surface structural observations show growth defects in A-OE plants, and abnormal accumulation and mobilization of lipid droplets were found in the cytosol, which is associated with the sensitivity of A-OE plants to heat stress.
2. Results
2.1. Phylogenetic Analysis of RNases H1s
In our previous study, we identified two family members of RNase H1s in P. patent, named PpRNH1A (Pp3c4_14290) and PpRNH1B (Pp3c2_25170), which contain the RNase H catalytic domain []. To investigate the evolutionary relationship of RNase H1s in plants, we used the full-length protein sequence of PpRNH1A from the Phytozome database to search for the RNase H1s homologs in representative species such as algae, mosses, and higher terrestrial plant groups. Public database Phytozome, Ensembl plants, NCBI, and the klebsormidium genome project were used for BLAST analysis. The phylogenetic tree (Figure 1) showed that three main branches were identified according to their full-length protein sequences, and the development process of RNase H1s was consistent with the evolutionary history of organisms. PpRNH1A and PpRNH1B were grouped together and showed a close evolutionary relationship to Chlamydomonas reinhardii and Klebsormidium nitens. One to three family members of RNase H1s were identified in different plant species, and some of the proteins in the same species were assigned to different sub-branches, which suggested that they may have differentiated functions.
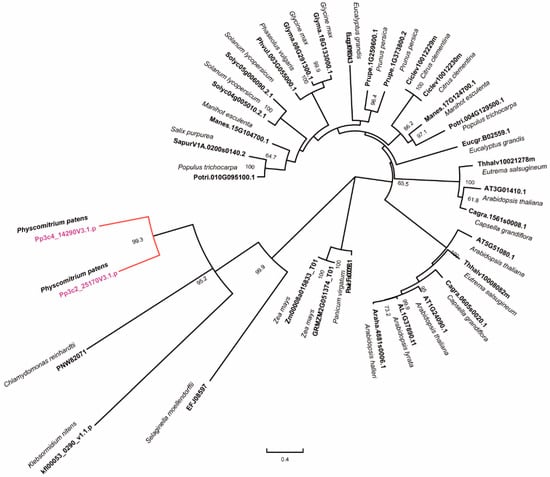
Figure 1.
Phylogenetic analyses of RNase H1 in different species. The tree was constructed with MEGA-X using the maximum likelihood (ML) method with 1000 bootstrap replicates. The numbers above the branches represent the bootstrap support values (>50%) from 1000 replications.
2.2. PpRNH1A Is Involved in Growth Development and Stress Tolerance
To explore the features of the PpRNH1A (Pp3c4_14290), we first investigated the expression pattern of the PpRNH1A in wild type throughout the growth cycle of P. patens. Expression data were downloaded from the public database Physcomitrella eFP Browser (BAR, http://bar.utoronto.ca/, data shown in Figure 2 were accessed on 19 February 2022). The higher expression levels of PpRNH1A were found in spores, rhizoids, archegonia, and at the sporophyte S1 stage, with relatively lower expression levels in chloronema, gametophores, and at the S2, S3, and M stages of sporophyte development (Figure 2A,B). This result suggests that PpRNH1A might be involved in P. patens growth and development.
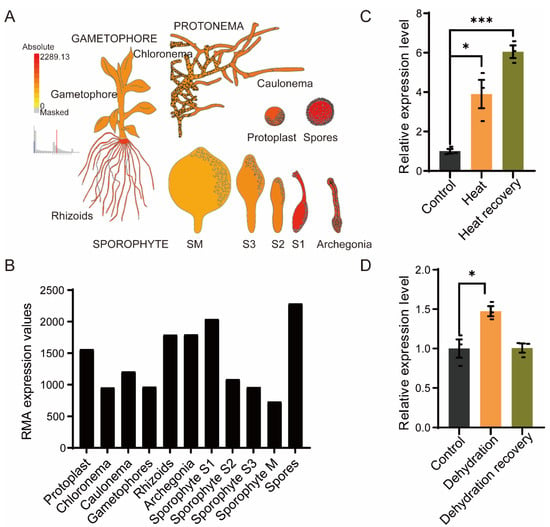
Figure 2.
Gene expression patterns of PpRNH1A throughout the life cycle of P. patens and the expression levels under different stresses. (A) Visualization of the expression of PpRNH1A at different tissues during P. patens growth and development. (B) The RMA expression values of PpRNH1A at different periods of growth and development. Data were downloaded from Physcomitrella eFP Browser. (C) Gene expression levels of PpRNH1A under heat stress and after heat recovery. (D) Gene expression levels of PpRNH1A under dehydrating stress and after recovery. For (C,D), data are presented as means ± SEM of three replicates; t-test was used for statistics; asterisks indicate the significant difference between treatment group and the control group, * p < 0.05, *** p < 0.001.
We next examined the expression levels of PpRNH1A under abiotic stresses. The wild-type gametophores of P. patens were exposed to heat stress (HS) at 40 °C for 18 h and then recovered for 4 days, and samples at three timepoints (control, heated, and recovered) were taken to determine the relative expression level of the PpRNH1A. Our results showed that HS significantly induced the expression of the PpRNH1A (3.91 times induction compared to the control) with a continuous induction (6.33 times induction compared to the control) during the recovery period (Figure 2C). Furthermore, PpRNH1A expression levels under dehydration stress treatment were extracted from our previous dehydrated transcriptome data []. The result showed that PpRNH1A was slightly induced (1.47 times induction compared to the control) by dehydration stress treatment and then returned to a normal level after recovery (Figure 2D). Our results showed that PpRNH1A participates in both development and stress tolerance in P. patens.
2.3. Creation of Stable Transgenic P. patens Line Overexpressing PpRNH1A
To detect the functions of PpRNH1A, we obtained the CDS of the PpRNH1A gene by PCR amplification. The CDS fragment without the stop codon was integrated into the overexpression vector (Figure 3A) under the promoter of PpEF1a followed by a PEG-mediated P. patens protoplast transformation. Positive PpRNH1A-overexpression plants were selected using the hygromycin resistant marker and confirmed at both DNA and RNA levels (Figure 3B,C). Seven positive transformants at the DNA level were obtained. RNA was taken from each line and followed by quantitative real-time polymerase chain reaction (qRT-PCR) to confirm the expression levels of PpRNH1A-overexpressing lines (A-OE). One A-OE with a three times overexpression level compared to wild-type plants was shown in Figure 3C. The pigment contents, including Chlorophyll A (Chl a), Chl b, total Chls, and carotenoids in WT and PpRNH1A-overexpression plants were measured under normal growth conditions, and the result showed that no significant changes were found between WT and A-OE in pigment contents (Figure 3D).
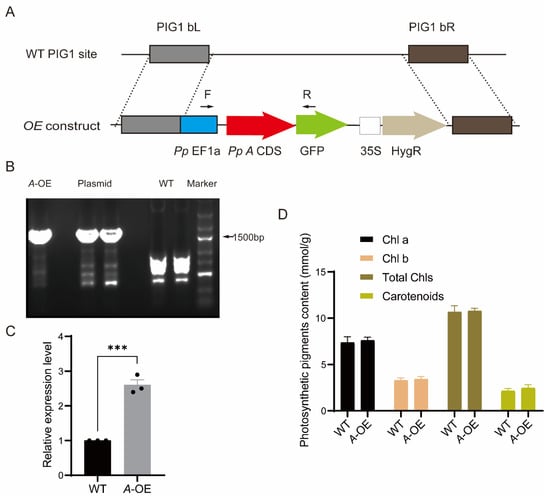
Figure 3.
Stable PpRNH1A-overexpression lines in P. patens. (A) Schematic diagram of pPOG1-A construct. P, plasmid; PIG1 bL, left targeting site; PIG1 bR, right targeting site; PpEF1a, the EF1a promoter from P. patens; HygR, hygromycin selectable marker cassette; GFP, green fluorescent protein. (B) Positive PpRNH1A-overexpression (A-OE) plants were confirmed at DNA level. WT, wild type; A-OE, PpRNH1A-overexpressing plant. (C) Identification of A-OE plant by qRT-PCR at RNA level. (D) Pigment contents in WT and A-OE under normal growth conditions. Chl a: chlorophyll a; Chl b: chlorophyll b; Total Chls: Total chlorophylls. For (C,D), data are presented as means ± SEM of three replicates; t-test was used for statistics; asterisks indicate the significant difference between WT and A-OE, *** p < 0.001.
2.4. Overexpression of PpRNH1A Affects Lipid Droplets Metabolism and Mobilization
The intracellular ultrastructure of A-OE plants was observed by transmission electron microscopy (TEM). We found that the cell walls of the overexpression plants were much thinner than the WT (Figure S1A). Interesting, there was no clear laminal structure of the chloroplast stroma observed in A-OE plants (Figure S1B), although the level of pigments in A-OE seems not to have been affected. Furthermore, in A-OE plants, more plastoglobuli were observed in chloroplasts (Figure S1A,B). The surface of the plant was then observed by scanning electron microscopy (SEM). The results showed that there were protrusions like glandular hairs on the surface of the A-OE plants (Figure S1C). These results lead us to hypothesize that the lipid metabolite was affected in A-OE plants, since the plant cuticle is a lipid membrane covering plant surfaces, and the plastoglobuli is a kind of lipid droplets within the chloroplasts.
To confirm whether the overexpression plants could affect the accumulation of cytosolic lipid droplets, the dye Nile red was used to stain the A-OE plants and wild-type plants to observe the cytosolic lipid droplets under the confocal microscope. As shown in Figure 4, the yellow fluorescence represented the accumulation of cytosolic lipid droplets by Nile red. Chloroplast autofluorescence was marked with red fluorescence in both wild-type and A-OE plants (Figure 4). The results showed that the lipid droplets were hardly seen in the gametophore of wild-type plants under normal growth conditions, however, in contrast, abundant of cytosolic lipid droplets around chloroplasts were observed in A-OE plants (Figure 4). In addition, a proportion of the lipid droplets observed in the overexpression plants had irregular morphology (Figure 4). Although additional experimental evidence is needed, these results revealed that overexpression of PpRNH1A may be associated with the metabolism and mobilization of lipid droplets.
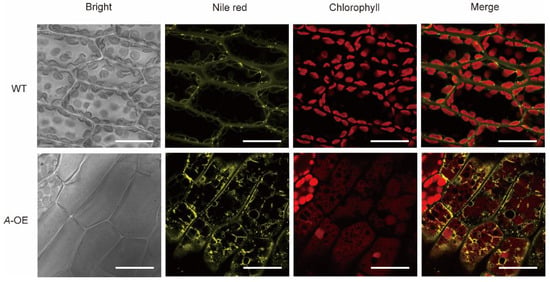
Figure 4.
Accumulation of lipid droplets in WT and A-OE under normal growth conditions. Scale bar = 20 μm.
2.5. PpRNH1A-Overexpression Line Is More Sensitive to Heat Stress
To detect the function of the protein deduced by PpRNH1A, plants were exposed to heat stress. Thirty days old gametophores of the wild-type plants and A-OE plants were treated with 40 ℃ for 18 h. Followed by a recovery at normal temperature (25 °C) for 5 days. Results showed that A-OE plants were more sensitive to heat stress and could not recover from the HS (Figure 5A). Photosynthetic parameters, including the maximal efficiency of PSII photochemistry (Fv/Fm), non-photochemical energy dissipation (NPQ), and electron transport rate (ETR) were measured at the timepoints of before heat stress (the control), after heat stress, and recovered for 4 days. Similar Fv/Fm, NPQ, and ETR levels were observed between A-OE plants and WT before HS. Heat stress severely affects the function of the photosystem in both A-OE plants and WT, whereas the Fv/Fm and NPQ levels in A-OE plants were significantly lower than in WT plants, both under heat stress and after recovery (Figure 5B–D). These results revealed that the overexpression of PpRNH1A resulted in decreasing tolerance to heat stress in P. patens plants.
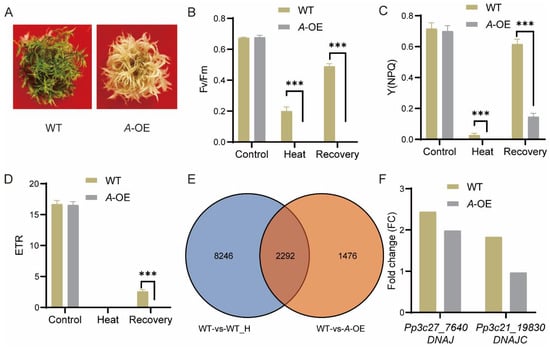
Figure 5.
Response of A-OE plants to heat stress (HS) treatment. (A) A-OE plants were more sensitive to heat stress. (B–D) Chlorophyll fluorescence (Fv/Fm) (B), non-photochemical energy dissipation (NPQ) (C), and electron transport rate (ETR) (D) of WT and A-OE before HS, after HS, and after recovery from the heat stress. Data are presented as means ± SEM of three replicates; t-test was used for statistics; asterisks indicate the significant difference between WT and A-OE, *** p < 0.001. (E) Venn diagram shows the overlapping between HS-regulated DEGs in WT (WT versus WT under heat stress) and PpRNH1A-regulated DEGs (WT versus A-OE under normal growth conditions). (F) Fold change comparison of the expression levels of DNAJ and DNAJC under HS and normal conditions. Each bar represents the ratio of the average expression value of three biological replicates of HS (after heat stress) vs. control (before heat stress).
2.6. PpRNH1A Regulates the Expression of Heat-Responsive Genes including DNAJ and DNAJC
Considering that overexpression plants are more sensitive to heat stress, we then tested if PpRNH1A regulates the heat-related genes. Transcriptomic profiles of WT plants before HS (control) and 18 h after HS were compared to identify the heat-responsive genes. In total, 10,538 DEGs (Fold change > 1.5 or 0 < Fold change < 0.67, p-value < 0.05) were found responding to heat stress in wild-type plants (collection: WT-vs-WT_H). To identify the genes regulated by PpRNH1A, 3768 DEGs were identified from the comparison between WT and A-OE plants under normal growth conditions (collection: WT-vs-A-OE). The overlap of these two collections of DEGs showed that 2292 heat-related genes were regulated by PpRNH1A (Figure 5E). Representative heat-related genes [] such as Pp3c27_7640 (molecular chaperone DNAJ, HSP40) and Pp3c21_19830 (DNAJ homolog subfamily C member DNAJC) were selected for further analysis. Heat stress significantly induced the expression level of DNAJ and DNAJC in WT plants. However, in A-OE plants, DNAJC was not induced by heat (Figure 5F). The fold change of DNAJ induced by HS in A-OE plants was much less than that in the WT plants. These results suggest that PpRNH1A regulates the expression of heat-responsive genes, including DNAJ and DNAJC (Figure 5F).
3. Discussion
RNase H1s are ribonucleases widely present in organisms [], and most studies on RNase H1s are related to growth and development. In Arabidopsis, AtRNH1B and AtRNH1C play important roles in maintaining the normal development of embryos, and AtRNH1C deletion mutants showed severe growth-defective phenotypes []. Our recent progress on P. patens revealed that PpRNH1A regulates the transcription of auxin-related genes by controlling the formation of R-loops, further regulating the development []. In this study, we found novel biological functions of PpRNH1A, which may also participate in heat stress responses, possibly by affecting the numbers and the mobilization of lipid droplets and regulating the expression of heat-related genes.
Phylogeny analysis results in this study suggest that there is functional differentiation of RNase H1s within and among plant species (Figure 1), which confirmed our previous finding that there may be functional differentiation between AtRNH1A and PpRNH1A []. Previously, we found that the development of gametophores of the pprnh1a mutant was affected through the modulation of R-loop formation on auxin-related genes. Overexpression plants of PpRNH1A were obtained (Figure 3A) in this study, and we found that the chloroplasts development was affected and no clear lamellar structure was observed (Figure S1B). Interestingly, although the chloroplast development was impaired, the pigments did not show any difference in A-OE plants compared to WT plants (Figure 3D). Abnormal development of lamellar structure and more plastoglobuli were found in the chloroplasts of the A-OE plants (Figure S1A,B). Furthermore, protrusions like glandular hairs were found on the surface of the A-OE plants as well. In addition, abundant lipid droplets with irregular morphology were accumulated in the cytosol of A-OE plants (Figure 4). These results suggest that overexpression of PpRNH1A resulted in disordered lipid metabolism and mobilization.
TAGs are the primary constituents of lipid droplets, which do not accumulate in vegetative tissues of plants under normal growth conditions but accumulate significantly under stress conditions, such as drought, high temperature, low temperature, and nutrient starvation, especially in leaves [,]. Correspondingly, lipid droplets were reported to be closely associated with plant biotic and abiotic stress as well. Pseudomonas-infected leaves caused an accumulation of lipid droplets and induced hypersensitivity reactions in Arabidopsis []. Seeds with irregular lipid droplets morphology were found susceptible to chilling injury during germination [,]. Consistent with the above, we found that PpRNH1A is involved in plant responses to heat stress, which provided a novel function of RNase H1s in plants. In our study, the expression levels of PpRNH1A were found to be significantly induced by abiotic stresses such as heat (Figure 2C) and drought treatment (Figure 2D). Moreover, A-OE plants were sensitive to heat stress (Figure 5A), further confirmed by the levels of photosynthetic parameters (Figure 5B–D). This is coincident with the findings in sunflower (Helianthus annuus L.), where stress-sensitive lines showed longer retention of the lipid droplets membrane under salt stress, thus exhibiting higher lipid accumulation and faster mobilization than that of stress-tolerant lines []. As a note, the lipid droplets mentioned here in this study are not what had been described as “oil body” in liverworts. Lipid droplets are present in almost all green plants accumulating compounds that are not soluble in the aqueous phase, and oil bodies are present only in liverwort, storing large quantities of toxic sesquiterpenoids [,,,,]. Lipid droplets are subcellular organelles of monolayer membranes with a diameter of about 0.5–2 µm, while oil bodies are an endocrine structure surrounded by a lipid bilayer membrane [,]. The size of small oil bodies is 2–5 × 3–9 µm on average, and the diameter of large oil bodies can reach 70 µm [,]. Although both mosses and liverworts are bryophytes, lipid droplets were found in our experiment.
HS affects all aspects of plant growth and elicits responses in a range of genes, including the accumulation of heat shock proteins (HSPs). We found that 60.8% of the genes regulated by overexpression of PpRNH1A are responsive to heat stress (Figure 5E), which is one of the explanations for why A-OE plants were more sensitive to HS than wild-type plants (Figure 5A–D). Heat responsive genes play critical roles in regulating the resistance/tolerance of plants to heat stress. GmDNJ1 (a major HSP40) was induced by heat stress and was responsible for enhanced heat tolerance in soybean []. In tomatoes, the expression levels of LeCDJ1 of Lycopersicon esculentum and SlDnaJ20 of Solanum lycopersicum were induced by heat stress, and overexpression of LeCDJ1 or SlDnaJ20 could improve the heat tolerance of plants [,]. In alfalfa (Medicago sativa), the DnaJ-like protein (MsDJLP) gene was induced by heat stress, and ectopic expression of MsDJLP in tobacco enhances the heat tolerance of tobacco []. All of these indicate that DNAJ could be used as a representative heat responsive marker and its expression level is closely related to heat stress tolerant ability. Our results showed that both DNAJ and DNAJC were induced by heat stress in WT plants, however, on the contrary, the induction folds in the A-OE plants were not as strong as that in the wild type, especially DNAJC, whose induction was totally hampered under heat stress. This is corroborated by the result that our A-OE plants is more susceptible to heat stress.
4. Materials and Methods
4.1. Phylogenetic Analysis
Amino acid sequences used were obtained from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html, accessed on 19 February 2022), Ensembl plants (http://plants.ensembl.org/Cyanidioschyzon_merolae/Tools/Blast, accessed on 19 February 2022) and NCBI (https://www.ncbi.nlm.nih.gov, accessed on 19 February 2022) database. And the RNase H1 of the Klebsormidium nitens was obtained from the klebsormidium genome project website (http://www.plantmorphogenesis.bio.titech.ac.jp/~algae_genome_project/klebsormidium/index.html, accessed on 19 February 2022) []. Multiple sequence alignments of these amino acid sequences were conducted with the ClustalW of MEGA-X, and the phylogenetic tree construction was performed with MEGA-X using the maximum likelihood (ML) method and 1000 bootstrap []. The best-fit model we selected was JTT + G + I [].
4.2. Plant Materials and Growth Conditions
Gransden 2004 (Courtesy Prof. Mitsuyasu Hasebe) was the wild-type (WT) Physcomitrium (Physcomitrella) patens genetic material we used. All plant materials were grown on BCD medium supplemented with 5 mM ammonium tartrate and 1 mM CaCl2. Plants were grown at 25 ℃ under 16 h light photoperiod per 8 h dark photoperiod with light intensity 60–80 µmol photons m−2 s−1.
4.3. Protoplast Transformation
Protoplast of P. patens were prepared from protonema which were continuously disrupted with an electric stirrer every week. The overexpression vector pPOG1-A was linearized using the restriction enzyme MssI (Thermo Fisher Scientific, Waltham, MA, USA). The transformants were obtained by transferring the linearized vector into wild-type using polyethylene glycol (PEG)–mediated protoplast transformation [].
4.4. PCR and Real-Time qRT-PCR Characterization of Overexpression Plants
Stable transgenetic lines were identified by PCR and qRT-PCR at both DNA and RNA level. The transformants were initially screened at the DNA level using primers “F” and “R”. Total RNA from P. patens tissues was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the instructions. cDNA synthesis was performed using a PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China) according to the manufacturer’s instructions. qRT-PCR was performed using SYBR Premix Ex Taq II (Takara, Dalian, China) and carried out on Bio-Rad CFX96. The primer sequences for PCR and qRT-PCR are listed in Supplementary Table S1.
4.5. Analysis of Gene Expression Patterns and Analysis of Expression by Stress Treatment
Expression pattern data were retrieved and visualized from the public database Physcomitrella eFP Browser of BAR (http://bar.utoronto.ca/, accessed on 19 February 2022).
4.6. Observation of Cell Ultrastructure
Leaves were cut into 1 mm2 with a blade and immediately fixed with 3% glutaraldehyde overnight at 4 °C. Rinse the material by adding 0.1 M phosphate buffer (PBS buffer, pH 7.2) every 30 min for 3 times. Subsequently, 1% osmium tetroxide was added for sample fixation at 4 °C for 2 h. Continue rinsing three times for 20 min each. The materials were continuous rinsed for 3 times and dehydrated in a serial ethanol gradient. After embedded in Epon 812 resin, materials were sectioned and stained with 2% uranyl acetate and lead citrate under EM UC7 ultramicrotome (Leica, Weztlar, Germany). The cellular ultrastructure was observed under JEM-1400Plus transmission electron microscopy (JEOL, Tokyo, Japan).
4.7. Observation of Cell Surface Structure
Leaves of the material were cut into 9 mm2 with a blade and immediately fixed with 3% glutaraldehyde overnight at 4 °C. The rinsing, fixation, and dehydration procedures are the same as previous mentioned. Samples were dried with a CO2 critical-point drier. The dried materials were mounted on aluminium stubs using tweezer and sputter-coated with gold. Cell surface structure was observed by Zeiss Sigma 300 scanning electron microscopy (Zeiss, Oberkochen, Germany).
4.8. Nile Red Staining
Gametophytes grown for 30 days were taken and placed in a certain volume of Nile red working solution for staining in dark for 5 mins as previously described, with appropriate modifications []. Lipid droplets were observed under Olympus FV1000 confocal microscope (Olympus, Tokyo, Japan). The emission filters for Nile red were 493–636 nm.
4.9. Heat Stress Assay
Thirty-days old gametophytes of P. patens were used to perform a heat stress assay. The wild-type plants and overexpression plants were treated at 40 °C for 18 h, followed by a recovery at 25 °C for 4–5 days.
4.10. Measurement of Chlorophyll Fluorescence (Fv/Fm), Non-Photochemical Energy Dissipation (NPQ), Electron Transport Rate (ETR), and Pigments Content
The plants to be tested were placed in the dark for 30 min. Relevant photosynthetic parameters were determined using IMAGING-PAM (Walz, Effeltrich, Germany) and the Imaging Win software (Walz, Effeltrich, Germany) as described previously []. Pigment content was determined using the N, N-dimethylformamide (DMF) method, as described previously [].
4.11. Bioinformatics and Data Analysis
Wild-type and A-OE plants before and after heat treatment were used to extract RNA. Samples for RNA sequencing (RNA-seq) were treated as previous description []. Differentially expressed genes (DEGs) between two samples were identified with the criteria of “Fold change > 1.5” and “p value < 0.05”. Differentially expressed genes were visualized with TBtools []. The dehydrated transcriptome data used in this study were obtained from Dong et al. [].
4.12. Statistical Analysis
All experiments were performed with three biological replicates. Student’s t-test was used for hypothesis testing in statistics between two samples. Significant differences were defined and indicated by asterisks *, **, and ***, corresponding to p-values < 0.05, <0.01, and <0.001, respectively.
5. Conclusions
In conclusion, we revealed that PpRNH1A not only participates in the regulation of growth development of P. patens plants, it also plays a crucial role in plant response and tolerance to abiotic stresses such as heat, possibly by regulating the expression of heat-related genes and causing the abnormal accumulation and the mobilization of lipid droplets in the cytosol. Our data highlights the important role played by PpRNH1A in plant heat stress response, providing a novel insight into the function of RNase H1s.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23169270/s1.
Author Contributions
Z.Y. and T.T. performed experiments. L.L. designed the experiment. Z.Y., L.D., H.Y. and L.L. together contributed to the writing of the original draft. Z.Y., L.D. and L.L. analyzed the data and revised the manuscript. H.L., L.C., K.H. and H.Y. provided experiment support. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31971410).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA-seq data reported in this paper have been uploaded in National Genomics Data Center (accession no. PRJCA011244).
Acknowledgments
The authors thank Mitsuyasu Hasebe (National Institute for Basic Biology) for providing Gransden 2004 and plasmid pPOG1.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Rizwan, M.; Ali, S.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci. Rep. 2018, 8, 17086. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sirhindi, G.; Bhardwaj, R.; Alyemeni, M.N.; Siddique, K.H.M.; Ahmad, P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci. Rep. 2018, 8, 8735. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Hayes, S.; Schachtschabel, J.; Mishkind, M.; Munnik, T.; Arisz, S.A. Hot topic: Thermosensing in plants. Plant Cell Environ. 2021, 44, 2018–2033. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Vierling, E. The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Rajan, V.B.V.; D’Silva, P. Arabidopsis thaliana J-class heat shock proteins: Cellular stress sensors. Funct. Integr. Genom. 2009, 9, 433. [Google Scholar] [CrossRef]
- Kong, F.; Deng, Y.; Wang, G.; Wang, J.; Liang, X.; Meng, Q. LeCDJ1, a chloroplast DnaJ protein, facilitates heat tolerance in transgenic tomatoes. J. Integr. Plant Biol. 2014, 56, 63–74. [Google Scholar] [CrossRef]
- Li, K.P.; Wong, C.H.; Cheng, C.C.; Cheng, S.S.; Li, M.W.; Mansveld, S.; Bergsma, A.; Huang, T.; van Eijk, M.J.T.; Lam, H.M. GmDNJ1, a type-I heat shock protein 40 (HSP40), is responsible for both Growth and heat tolerance in soybean. Plant Direct 2021, 5, e00298. [Google Scholar] [CrossRef]
- Shao, Q.; Liu, X.; Su, T.; Ma, C.; Wang, P. New insights into the role of seed oil body proteins in metabolism and plant development. Front. Plant Sci. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, F.K.; Doner, N.M.; Krawczyk, H.E.; Scholz, P.; Schmitt, K.; Valerius, O.; Braus, G.H.; Mullen, R.T.; Ischebeck, T. Identification of low-abundance lipid droplet proteins in seeds and seedlings. Plant Physiol. 2020, 182, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-L.; Huang, M.-D.; Chen, T.-L.L.; Huang, A.H.C. Oleosin of subcellular lipid droplets evolved in green algae. Plant Physiol. 2013, 161, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, R.; Izquierdo, Y.; López, A.; Muñiz, L.; Martínez, M.; Cascón, T.; Hamberg, M.; Castresana, C. Protein profiles of lipid droplets during the hypersensitive defense response of Arabidopsis against Pseudomonas infection. Plant Cell Physiol. 2020, 61, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Feeney, M.; Frigerio, L.; Cui, Y.; Menassa, R. Following vegetative to embryonic cellular changes in leaves of Arabidopsis overexpressing LEAFY COTYLEDON2. Plant Physiol. 2013, 162, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The role of triacylglycerol in plant stress response. Plants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Ischebeck, T. Ties between stress and lipid droplets pre-date seeds. Trends Plant Sci. 2020, 25, 1203–1214. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef]
- Légeret, B.; Schulz-Raffelt, M.; Nguyen, H.M.; Auroy, P.; Beisson, F.; Peltier, G.; Blanc, G.; Li-Beisson, Y. Lipidomic and transcriptomic analyses of Chlamydomonas reinhardtii under heat stress unveil a direct route for the conversion of membrane lipids into storage lipids. Plant Cell Environ. 2016, 39, 834–847. [Google Scholar] [CrossRef]
- Aizouq, M.; Peisker, H.; Gutbrod, K.; Melzer, M.; Hölzl, G.; Dörmann, P. Triacylglycerol and phytyl ester synthesis in Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 2020, 117, 6216–6222. [Google Scholar] [CrossRef]
- Gogna, M.; Bhatla, S. Biochemical mechanisms regulating salt tolerance in sunflower. Plant Signal. Behav. 2019, 14, 1670597. [Google Scholar] [CrossRef] [PubMed]
- Gogna, M.; Bhatla, S. Salt-tolerant and -sensitive seedlings exhibit noteworthy differences in lipolytic events in response to salt stress. Plant Signal. Behav. 2020, 15, 1737451. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Liu, L.; Wang, Q.; Zhao, J.; Li, P.; Hu, J.; Yang, Z.; Running, M.P.; Sun, H.; Huang, J. Gene refashioning through innovative shifting of reading frames in mosses. Nat. Commun. 2018, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhai, J.; Qi, Z.; Liu, W.; Cui, J.; Zhang, X.; Bai, S.; Li, L.; Shui, G.; Cui, S. The specific glycerolipid composition is responsible for maintaining the membrane stability of Physcomitrella patens under dehydration stress. J. Plant Physiol. 2021, 268, 153590. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Hausen, P. Enzyme from calf thymus degrading the RNA moiety of DNA-RNA Hybrids: Effect on DNA-dependent RNA polymerase. Science 1969, 166, 393–395. [Google Scholar] [CrossRef]
- Amon, J.D.; Koshland, D. RNase H enables efficient repair of R-loop induced DNA damage. eLife 2016, 5, e20533. [Google Scholar] [CrossRef]
- Ohtani, N.; Haruki, M.; Morikawa, M.; Crouch, R.J.; Itaya, M.; Kanaya, S. Identification of the genes encoding Mn2+-dependent RNase HII and Mg2+-dependent RNase HIII from Bacillus subtilis: Classification of RNases H into three families. Biochemistry 1999, 38, 605–618. [Google Scholar] [CrossRef]
- Chon, H.; Matsumura, H.; Koga, Y.; Takano, K.; Kanaya, S. Crystal Structure and Structure-based Mutational Analyses of RNase HIII from Bacillus stearothermophilus: A New Type 2 RNase H with TBP-like Substrate-binding Domain at the N Terminus. J. Mol. Biol. 2006, 356, 165–178. [Google Scholar] [CrossRef]
- Jongruja, N.; You, D.J.; Angkawidjaja, C.; Kanaya, E.; Koga, Y.; Kanaya, S. Structure and characterization of RNase H3 from Aquifex aeolicus. FEBS J. 2012, 279, 2737–2753. [Google Scholar] [CrossRef]
- Kalhorzadeh, P.; Hu, Z.; Cools, T.; Amiard, S.; Willing, E.-M.; De Winne, N.; Gevaert, K.; De Jaeger, G.; Schneeberger, K.; White, C.I. Arabidopsis thaliana RNase H2 deficiency counteracts the needs for the WEE1 checkpoint kinase but triggers genome instability. Plant Cell 2014, 26, 3680–3692. [Google Scholar] [CrossRef]
- Eekhout, T.; Kalhorzadeh, P.; De Veylder, L. Lack of RNase H2 activity rescues HU-sensitivity of WEE1 deficient plants. Plant Signal. Behav. 2015, 10, e1001226. [Google Scholar] [PubMed]
- Hyjek, M.; Figiel, M.; Nowotny, M. RNases H: Structure and mechanism. DNA Repair 2019, 84, 102672. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.B.; Akman, G.; Wood, S.R.; Sakhuja, K.; Cerritelli, S.M.; Moss, C.; Bowmaker, M.R.; Jacobs, H.T.; Crouch, R.J.; Holt, I.J. Primer retention owing to the absence of RNase H1 is catastrophic for mitochondrial DNA replication. Proc. Natl. Acad. Sci. USA 2015, 112, 9334–9339. [Google Scholar] [CrossRef]
- Akman, G.; Desai, R.; Bailey, L.J.; Yasukawa, T.; Dalla Rosa, I.; Durigon, R.; Holmes, J.B.; Moss, C.F.; Mennuni, M.; Houlden, H.; et al. Pathological ribonuclease H1 causes R-loop depletion and aberrant DNA segregation in mitochondria. Proc. Natl. Acad. Sci. USA 2016, 113, E4276–E4285. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, S.M.; Frolova, E.G.; Feng, C.; Grinberg, A.; Love, P.E.; Crouch, R.J. Failure to Produce Mitochondrial DNA Results in Embryonic Lethality in Rnaseh1 Null Mice. Mol. Cell 2003, 11, 807–815. [Google Scholar] [CrossRef]
- Kramara, J.; Osia, B.; Malkova, A. Break-Induced Replication: The Where, The Why, and The How. Trends Genet. 2018, 34, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.L.; Lei, Y.N.; Liu, X.Q.; Xue, W.; Zhang, Y.; Nan, F.; Gao, X.; Zhang, J.; Wei, J.; et al. Linking circular intronic RNA degradation and function in transcription by RNase H1. Sci. China Life Sci. 2021, 64, 1795–1809. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, Q.; Cheng, L.; Xu, W.; Hong, Y.; Li, S.; Sun, Q. RNase H1 cooperates with DNA gyrases to restrict R-loops and maintain genome integrity in Arabidopsis chloroplasts. Plant Cell 2017, 29, 2478–2497. [Google Scholar] [CrossRef]
- Kuciński, J.; Chamera, S.; Kmera, A.; Rowley, M.J.; Fujii, S.; Khurana, P.; Nowotny, M.; Wierzbicki, A.T. Evolutionary history and activity of RNase H1-like proteins in Arabidopsis thaliana. Plant Cell Physiol. 2020, 61, 1107–1119. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Yang, Z.; Hou, Q.; Zhao, W.W.; Sun, Q. RNase H1C collaborates with ssDNA binding proteins WHY1/3 and recombinase RecA1 to fulfill the DNA damage repair in Arabidopsis chloroplasts. Nucleic Acids Res. 2021, 49, 6771–6787. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, W.; Yao, Y.; Sun, Q. Mitochondrial RNase H1 activity regulates R-loop homeostasis to maintain genome integrity and enable early embryogenesis in Arabidopsis. PLoS Biol. 2021, 19, e3001357. [Google Scholar] [CrossRef] [PubMed]
- Cove, D. The moss Physcomitrella patens. Annu. Rev. Genet. 2005, 39, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.-F.; Lindquist, E.A.; Kamisugi, Y. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Goffinet, B.; Meyberg, R.; Wu, S.-Z.; Bezanilla, M. The moss Physcomitrium (Physcomitrella) patens: A model organism for non-seed plants. Plant Cell 2020, 32, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dong, X.; Yang, Z.; Hou, X.; Liu, L. Regulation of the Development in Physcomitrium (Physcomitrella) patens implicates the functional differentiation of plant RNase H1s. Plant Sci. 2021, 313, 111070. [Google Scholar] [CrossRef]
- Dong, X.M.; Pu, X.J.; Zhou, S.Z.; Li, P.; Luo, T.; Chen, Z.X.; Chen, S.L.; Liu, L. Orphan gene PpARDT positively involved in drought tolerance potentially by enhancing ABA response in Physcomitrium (Physcomitrella) patens. Plant Sci. 2022, 319, 11122. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Lee, H.G.; Park, M.-E.; Park, B.Y.; Kim, H.U.; Seo, P.J. The Arabidopsis MYB96 transcription factor mediates ABA-dependent triacylglycerol accumulation in vegetative tissues under drought stress conditions. Plants 2019, 8, 296. [Google Scholar] [CrossRef]
- Angkawijaya, A.E.; Nguyen, V.C.; Nakamura, Y. LYSOPHOSPHATIDIC ACID ACYLTRANSFERASES 4 and 5 are involved in glycerolipid metabolism and nitrogen starvation response in Arabidopsis. New Phytol. 2019, 224, 336–351. [Google Scholar] [CrossRef]
- Shimada, T.L.; Shimada, T.; Takahashi, H.; Fukao, Y.; Hara-Nishimura, I. A novel role for oleosins in freezing tolerance of oilseeds in Arabidopsis thaliana. Plant J. 2008, 55, 798–809. [Google Scholar] [CrossRef]
- Miquel, M.; Ghassen, T.; d’Andréa, S.; Kelemen, Z.; Baud, S.; Berger, A.; Deruyffelaere, C.; Trubuil, A.; Lepiniec, L.; Dubreucq, B. Specialization of oleosins in oil body dynamics during seed development in Arabidopsis Seeds. Plant Physiol. 2014, 164, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, P.K.; Shivaiah, K.-K.; Espinoza-Corral, R. Lipid droplets throughout the evolutionary tree. Prog. Lipid Res. 2020, 78, 101029. [Google Scholar] [CrossRef] [PubMed]
- Duckett, J.G.; Ligrone, R. The formation of catenate foliar gemmae and the origin of oil bodies in the liverwort Odontoschisma denudatum (Mart.) Dum. (Jungermanniales): A light and electron microscope study. Ann. Bot. 1995, 76, 405–419. [Google Scholar] [CrossRef]
- Scholz, P.; Chapman, K.D.; Mullen, R.T.; Ischebeck, T. Finding new friends and revisiting old ones—How plant lipid droplets connect with other subcellular structures. New Phytol. 2022. [Google Scholar] [CrossRef]
- Romani, F.; Flores, J.; Tolopka, J.; Suarez, G.; He, X.; Moreno, J. Liverwort oil bodies: Diversity, biochemistry, and molecular cell biology of the earliest secretory structure of land plants. J. Exp. Bot. 2022, 73, 4427–4439. [Google Scholar] [CrossRef]
- Schuster, R.M. The oil-bodies of the Hepaticae. I. Introduction. J. Hattori Bot. Lab. 1992, 72, 151–162. [Google Scholar]
- Schuster, R.M.; Konstantinova, N. Studies on Treubiales, I. On Apotreubia Hatt. et al. and A. hortonae Schust. & Konstantinova, sp. n. J. Hattori Bot. Lab. 1995, 78, 41–61. [Google Scholar]
- Wang, G.; Cai, G.; Xu, N.; Zhang, L.; Sun, X.; Guan, J.; Meng, Q. Novel DnaJ protein facilitates thermotolerance of transgenic tomatoes. Int. J. Mol. Sci. 2019, 20, 367. [Google Scholar] [CrossRef]
- Lee, K.-W.; Rahman, M.A.; Kim, K.-Y.; Choi, G.J.; Cha, J.-Y.; Cheong, M.S.; Shohael, A.M.; Jones, C.; Lee, S.-H. Overexpression of the alfalfa DnaJ-like protein (MsDJLP) gene enhances tolerance to chilling and heat stresses in transgenic tobacco plants. Turk. J. Biol. 2018, 42, 12–22. [Google Scholar] [CrossRef]
- Hori, K.; Maruyama, F.; Fujisawa, T.; Togashi, T.; Yamamoto, N.; Seo, M.; Sato, S.; Yamada, T.; Mori, H.; Tajima, N. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 2014, 5, 3978. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Liu, L.; McNeilage, R.T.; Shi, L.-x.; Theg, S.M. ATP requirement for chloroplast protein import is set by the Km for ATP hydrolysis of stromal Hsp70 in Physcomitrella patens. Plant Cell 2014, 26, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.F.; Liu, Z.; Rochfort, S.; Mouradov, A. Lipid production in aquatic plant Azolla at vegetative and reproductive stages and in response to abiotic stress. Plant Physiol. Biochem. 2018, 124, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, H.; Liu, G.; Ma, W.; Li, C.; Huo, H.; He, J.; Liu, L. PpSARK regulates moss senescence and salt tolerance through ABA related pathway. Int. J. Mol. Sci. 2018, 19, 2609. [Google Scholar] [CrossRef]
- Pu, X.; Yang, L.; Liu, L.; Dong, X.; Chen, S.; Chen, Z.; Liu, G.; Jia, Y.; Yuan, W.; Liu, L. Genome-wide analysis of the MYB transcription factor superfamily in Physcomitrella patens. Int. J. Mol. Sci. 2020, 21, 975. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).