Abstract
Microtubules are major components of the cytoskeleton that play important roles in cellular processes such as intracellular transport and cell division. In recent years, it has become evident that microtubule networks play a role in genome maintenance during interphase. In this review, we highlight recent advances in understanding the role of microtubule dynamics in DNA damage response and repair. We first describe how DNA damage checkpoints regulate microtubule organization and stability. We then highlight how microtubule networks are involved in the nuclear remodeling following DNA damage, which leads to changes in chromosome organization. Lastly, we discuss how microtubule dynamics participate in the mobility of damaged DNA and promote consequent DNA repair. Together, the literature indicates the importance of microtubule dynamics in genome organization and stability during interphase.
1. Introduction
Microtubules that form a part of the cytoskeleton are involved in multiple cellular processes, including cell division, intracellular transport, cell motility, and cell shape [1,2]. Microtubules are formed from protein subunits of tubulin, and each tubulin protein consists of two subunits, α-tubulin and β-tubulin. Microtubules are highly dynamic structures that rapidly oscillate between phases of polymerization and depolymerization by the addition or removal of tubulin proteins [2]. Microtubules are nucleated from tubulin subunits at specific subcellular locations, mainly the centrosomes or MTOCs (microtubule organizing centers) [1,3]. Upon nucleation, there are many proteins that bind to microtubules, including the motor proteins dynein and kinesin, and other proteins important for regulating microtubule dynamics [4]. The microtubule cytoskeleton has a central role in cell division by forming the mitotic spindles that segregate chromosomes [5,6]. Besides its function during mitosis, recent publications have shown that microtubules also affect chromosome structure during interphase and play a role in genome maintenance [7,8,9]. Furthermore, it was shown that microtubule stabilization is required for efficient DNA repair [10,11], revealing a link between microtubule dynamics and DNA damage response (DDR).
Microtubules are involved in DNA damage repair at three main levels (Figure 1). First, microtubules are nucleated from the centrosome, which is usually located close to the nucleus in interphase cells. The filamentous network of microtubules extends throughout the cell and has an important role in multiple cellular processes. Cytoplasmic microtubules undergo post-translational modification that may alter their stability and function. These modifications appear to be especially important in the intracellular trafficking of DNA repair proteins. Second, cytoplasmic microtubules physically interact with and exert mechanical forces onto the nuclear envelope, which can impact nuclear shape, leading to changes in chromatin structure. In addition, nuclear localization of microtubule components induces chromatin remodeling. These changes expose DNA damage sites, allowing access to various DNA repair proteins. Lastly, the flexibility of the DNA double-strand break (DSB) end(s) is important for efficient DNA repair. Microtubules mobilize damaged DNA and promote interaction with repair proteins at the site of damage, which is important for DSB repair via both homologous recombination (HR) and non-homologous end joining (NHEJ).
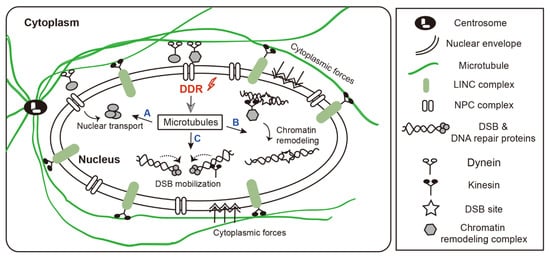
Figure 1.
The role of microtubule dynamics in DNA damage response. Microtubules are involved in DNA damage repair at three main levels. (A) Centrosomes organize microtubules by controlling nucleation and anchoring processes. Cytoplasmic microtubules can mediate the transport of DNA repair factors into the nucleus. (B) Dynamic cytoplasmic microtubules physically interact with and exert mechanical forces onto nuclear envelope, which can impact nuclear morphology, leading to changes in chromatin structure. In addition, nuclear localization of microtubule components induces chromatin reorganization. These changes expose DNA damage sites, allowing access to various DNA repair proteins. (C) Microtubules/LINC complexes increase the mobility of damaged DNA and promote the recruitment of DNA repair proteins at the site of damage, which is important for DSB repair via both HR and NHEJ. Abbreviations: LINC, linker of the nucleoskeleton and cytoskeleton; NPC, nuclear pore complex.
In this review, we present the current state of knowledge in understanding the role of microtubule dynamics in DNA damage repair and discuss some perspectives gained by these discoveries towards genome stability.
2. Microtubule Organization and Stability in Response to DNA Damage
Microtubules are one of the three major cytoskeletal components in eukaryotic cells [2]. The centrosome, a major microtubule-organizing center (MTOC) in animal cells, comprises a pair of centrioles surrounded by pericentriolar material (PCM), which nucleates and anchors microtubules [1,3]. During mitosis, centrosomes form the spindle poles of the bipolar mitotic spindle, and during interphase, they nucleate the formation of the microtubule cytoskeleton [3,5,6]. The DDR proteins such as ataxia-telangectasia and Rad3 related (ATR), checkpoint kinase 1 and 2 (CHK1/CHK2), breast cancer susceptibility gene 1 and 2 (BRCA1/BRCA2), and RAD51 have been shown to localize in the nucleus and the centrosomes [12,13,14,15,16,17,18], implicating the existence of crosstalk between two organelles following DNA damage. Furthermore, recent studies demonstrated that microtubule stabilization is required for efficient DNA repair [10,11], revealing a link between the DDR and microtubule dynamics. In this section, we summarize the molecular mechanisms that regulate microtubule organization and stability in response to DNA damage during interphase.
2.1. NEK2
Centrosome separation is critical for bipolar spindle formation and the accurate segregation of chromosomes during cell division [1]. NIMA-related kinase 2 (NEK2) is a centrosomal kinase required for accurate centrosome separation [19,20,21]. NEK2 activity fluctuates during the cell cycle, which is low in the G1 phase, peaking in the S and G2 phases [22]. The DDR pathway controlling centrosome separation is mechanistically linked to NEK2 [23]. IR-induced DNA damage results in the activation of ataxia-telangiectasia mutated (ATM) and phosphatase 1 (PP1). The increased activity of PP1 dephosphorylates and reduces NEK2 activity, leading to the inhibition of centrosome separation [24,25]. Thus, NEK2 might act as a downstream target of the DDR pathway that regulates centrosome separation and contributes to the G2 arrest under genotoxic stress [23].
2.2. Centrobin
Centrobin is a centriole-associated protein that is required for centriole duplication and elongation [26,27]. Centrobin also has a potential role in microtubule stabilization by interacting with α-tubulin [26,28,29,30]. Two different kinases have been shown to regulate the microtubule-stabilizing activity of centrobin [31,32]. Phosphorylation by PLK1 (polo-like kinase 1) stimulates centrobin to stabilize the microtubules during mitosis [31], while phosphorylation by NEK2 antagonizes the microtubule-stabilizing activity of centrobin during interphase [32]. As discussed earlier, NEK2 activity is decreased upon DNA damage [25], which therefore could increase microtubule-stabilizing activity of centrobin. Furthermore, centrobin was previously identified as a potential ATM/ATR substrate [33], suggesting a potential role of centrobin in DDR. A recent study has demonstrated that centrobin is phosphorylated in an ATR-dependent manner following ultraviolet (UV) exposure, and depletion of centrobin has a defect in UV-induced microtubule stabilization [34]. Within this context, it is proposed that ATM/ATR might be involved in regulating microtubule stability after DNA damage, at least in part, through centrobin and NEK2. However, further study is needed to identify the precise mechanism underlying the role of centrobin in DDR.
2.3. Pericentrin (PCNT)
PCNT is an integral centrosomal component that functions as a scaffold for anchoring numerous proteins in the centrosome [35,36]. Through this anchoring function, PCNT is involved in functional crosstalk between microtubule organization and DDR [37,38]. PCNT contributes to the microtubule organization in both interphase and mitosis. For instance, PCNT anchors the γ-tubulin ring complex (γ-TuRC) at spindle poles in mitotic cells, which is required for proper spindle assembly [39,40]. Loss of this anchoring mechanism induces a checkpoint response that prevents mitotic entry. PCNT is involved in DDR by mediating PCNT-dependent CHK1 localization at the interphase centrosome that regulates mitotic entry [37,38]. In addition, recent studies found that mutations in PCNT cause Seckel syndrome, defects in ATR-dependent DNA damage signaling, which displays mitotic failure and cell death [37,41,42,43], revealing PCNT functions in the DDR. Interestingly, it has been recently shown that chromatin remodeling proteins are involved in centrosome integrity [44]. Silibourne et al. identified chromodomain helicase DNA-binding protein 3 (CHD3), a component of the nucleosome remodeling deacetylase complex, as PCNT-interacting proteins and demonstrated that CHD3-PCNT complex is required for centrosomal localization of PCNT and for centrosome integrity [44].
2.4. CEP Family Proteins
Centrosomal protein of 63 kDa (CEP63) was first identified as a target of ATM/ATR following DNA damage [45]. ATM and ATR phosphorylate Xenopus CEP63 and promote its delocalization from the centrosome, inhibiting spindle assembly and delaying mitotic progression [45]. Furthermore, mutations of human CEP63 were found in a primary microcephaly with defective ATR-dependent DNA damage signaling [46]. It has been also shown that CEP63 forms a complex with CEP152, another centrosomal protein implicated in microcephaly [46,47], and CEP63 deficiency leads to centriole loss due to impaired recruitment of CEP152 to the centrosome [48]. A centrosomal protein of 164 kDa (CEP164) is also phosphorylated by ATM/ATR upon DNA damage [49], which is required for DNA damage-induced CHK1 phosphorylation and G2/M checkpoints, indicating a critical role of CEP164 in ATM/ATR DNA damage signaling pathways.
2.5. αTAT1
The α-tubulin acetyltransferase 1 (αTAT1) catalyzes the acetylation of α-tubulin at lysine 40 (K40) in microtubules [50,51]. α-tubulin K40 acetylation has been shown to be enriched in stable microtubules, such as mitotic spindles and cilia [52,53]. A recent study demonstrated that α-tubulin K40 acetylation is induced following DNA damage and that αTAT1 catalytic activity is required for DNA damage checkpoint response [54], suggesting a potential role of α-tubulin K40 acetylation in DDR. Although the molecular mechanism by which αTAT1 affects DDR remains unclear, as α-tubulin K40 acetylation has been known to enhance the microtubule association of motor proteins and subsequent intracellular transport [55,56], αTAT1 may play a role in DDR, at least in part, by promoting nuclear transport of DNA repair proteins. HDAC6 (histone deacetylase 6) and SIRT2 (Sirtuin 2) are known to negatively regulate α-tubulin K40 acetylation [57,58]. Thus, it will be possible that reduced activity of HDAC6 and SIRT2 could contribute to the increase in α-tubulin K40 acetylation following DNA damage. However, it seems unlikely because it has been shown that deacetylating activity of HDAC6 or SIRT2 is required for efficient DNA repair [59,60], suggesting that αTAT1 is primarily responsible for inducing α-tubulin K40 acetylation in response to DNA damage.
3. Microtubule-Dependent Nuclear Remodeling Following DNA Damage
Recent studies in yeast and mammalian cells suggest that cytoplasmic actin and microtubules induce global changes in chromatin structure in response to DNA damage [61,62]. The microtubule dynamics are particularly important for chromatin segregation during mitosis; however, there is increasing evidence to suggest that microtubules are implicated in chromatin organization during interphase [7]. Recent studies have shown that, upon DNA damage, cytoplasmic microtubules can change the nuclear structure that provides a nuclear environment conducive to repair [8,63,64]. How can microtubule networks influence interphase chromatin upon DNA damage? Several links have been found between the microtubule network and the interphase chromatin. The first mechanism involves force transmission from cytoplasmic microtubules to chromatin through the nuclear envelope. The second mechanism involves nuclear accumulation of microtubule components that bind to chromatin and influence chromatin structure. The third mechanism involves microtubule-dependent nuclear transport of chromatin remodeling complexes. In this section, we highlight the role of microtubule dynamics in nuclear changes following DNA damage.
3.1. Microtubule-Driven Cytoplasmic Forces
The nuclear envelope is in close association with the microtubule networks. In interphase, centrosomes are usually located close to the outer surface of the nuclear envelope and interphase chromosome ends attach to the inner surface of the nuclear envelope [65,66]. Therefore, a physical link is expected to form between cytoplasmic microtubules and chromosomes. The nuclear envelope is connected to the different types of cytoskeletal elements by the linker of nucleoskeleton and cytoskeleton (LINC) complex formed by Sad1 and UNC84 (SUN) and Klarsicht/ANC-1/Syne homology (KASH) domain proteins [67,68,69]. SUN domain proteins span the inner nuclear membrane and interact with nucleoplasm and chromatin. KASH domain proteins are anchored in the outer nuclear membrane and interact with the cytoskeleton [67,68,69]. The interactions of SUN-KASH domain proteins across the nuclear envelope link the microtubule cytoskeleton to the nucleus [69]. The connection between cytoplasmic microtubules and the LINC complexes may allow the microtubule cytoskeleton to influence the nucleus by transmitting mechanical forces across the nuclear envelope. It has been shown that mechanical force applied to the nucleus induces direct stretching of chromatin, resulting in the activation of transcription [70]. Therefore, it is tempting to speculate that in response to DNA damage, microtubule tracks for intracellular transport may generate mechanical forces in the nuclear envelope, which may influence the nucleus and subsequently induce chromatin reorganization for DNA repair.
In eukaryotes, heterochromatin is generally located just beneath the nuclear envelope where it interacts with the nuclear lamina [71,72]. Nuclear lamina-associated heterochromatin has been shown to increase nuclear tension [73], which may provide nuclear stiffness [74]. As a consequence, microtubule-driven cytoplasmic forces to the nuclear envelope could be counteracted by the heterochromatin at the nuclear periphery. It is therefore speculated that balanced force along the nuclear envelope might play an important role in maintaining nuclear structure. Heterochromatin is markedly reorganized in response to DNA damage to control and facilitate DNA repair [75,76,77,78,79]. In particular, decondensation of heterochromatin has been observed in response to DSBs [76,78,80]. Among the mechanisms that may drive heterochromatin reorganization following DNA damage, ATM-dependent phosphorylation of the heterochromatin building factor KRAB-domain associated protein 1 (KAP1) results in dissociation of the chromatin remodeler CHD3, promoting chromatin relaxation [81,82]. This can result in moving away from a region containing heterochromatin, and possibly decreasing nuclear tension from the nuclear envelope-associated heterochromatin. Indeed, most recently, dos Santos et al. showed that DNA damage decreases nuclear tension through chromatin decondensation, which is required for genome stability [63]. Therefore, it would be possible that in the presence of DNA damage, nuclear morphology might be easily affected by the cytoplasmic forces, which in turn induces chromatin reorganization.
3.2. Microtubule and Microtubule-Associated Proteins
Recently, it has been shown that the chromatin remodeling complex has been implicated in microtubule organization [44,83,84,85,86,87]. Because chromatin-remodeling factors affect microtubule polymerization and spindle dynamics [44,85,87], it would be possible that microtubules and/or microtubule-associated proteins could be linked to chromatin reorganization.
3.2.1. γ-Tubulin
In eukaryotes, there are five known tubulin isoforms, α-tubulin, β-tubulin, γ-tubulin, δ-tubulin, and ε-tubulin [88,89]. The α- and β-tubulin heterodimers assemble into dynamic microtubules and perform multiple important cellular functions. The γ-tubulin is essential for microtubule function, but it is not a component of microtubules. Rather, it is located at the centrosome and functions in the microtubule nucleation and microtubule polarity from the centrosome [1,3]. The δ-tubulin and ε-tubulin are required for triplet microtubule stability in centrioles and basal bodies [90]. Unlike α-tubulin and β-tubulin, γ-tubulin contains a nuclear localization sequence and a helix–loop–helix DNA-binding motif on the C-terminus [91]. There is growing evidence showing nuclear functions of γ-tubulin including transcription, chromatin remodeling, and DNA damage response. The genetic interaction between γ-Tub23C and SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin-remodeling complex has been shown in Drosophila melanogaster [92], suggesting a potential role of γ-tubulin in chromatin remodeling. In addition, nuclear γ-tubulin interacts with the transcription factor family E2 promoter-binding factor (E2F) and also with E2F DNA binding sites, leading to repression of E2F-induced transcription [93]. This can be similar to that of retinoblastoma protein (Rb), which exerts its tumor suppressor function primarily by inhibiting the E2F transcription factors [94]. Interestingly, besides interacting with E2Fs, Rb inhibits E2F-induced transcription by recruiting chromatin remodeling factors (histone deacetylases and SWI/SNF complexes) and DNA methyltransferase DNMT1 [95,96,97]. It is thus tempting to speculate that γ-tubulin may be involved in the recruitment of DNA-remodeling factors in the nucleus. Furthermore, γ-tubulin interacts with DNA repair protein RAD51 and forms a nuclear complex with BRCA1 after DNA damage [98], suggesting a link between DNA repair and the microtubule networks.
3.2.2. KIF4
A major group of molecular motors involved in intracellular transport are kinesins named KIF (kinesin superfamily protein). There are several dozen KIFs in mammalian cells to constitute at least 14 kinesin families [99,100]. The majority comprises two domains: an ATP hydrolysis domain that allows it to traverse microtubules, and a tail domain that is able to bind to structures and/or cargos [99,100]. KIF4 is a microtubule-bound motor protein that associates with chromosomes and microtubules during mitosis and contributes to faithful chromosome segregation [101]. However, KIF4 is unique among kinesins in that it localizes to the nucleus throughout interphase [102,103], suggesting its non-mitotic function. It has been shown that KIF4 is important for chromatin organization, transcription, and DNA repair. There are several mechanisms for the nuclear functions of KIF4. (1) KIF4 induces chromatin condensation by inhibiting Poly [ADP-ribose] polymerase 1 (PARP1) activity, which maintains an open chromatin architecture through Poly ADP-ribosylation (PARylation) [101,104,105]. (2) KIF4 promotes nucleosome assembly by recruiting histone chaperones and chromatin remodeling complexes to newly synthesized DNA, leading to chromatin compaction [103]. (3) KIF4 negatively regulates transcription by interacting with transcriptional repressive complexes such as DNMT3B and HDAC1 [103,106]. (4) KIF4 is involved in HR repair by interacting with BRCA2, which promotes the recruitment of RAD51 to DSBs sites [107,108]. Taken together, KIF4 performs nuclear functions that can change chromatin structure during interphase, in addition to its known function as a microtubule-bound motor protein during mitosis.
3.2.3. Actin
Actin, one of the cytoskeletal proteins, is also present in the nucleus and is associated with soluble nuclear proteins [109,110,111,112]. It has been shown that actin and actin-related proteins are integral components of several chromatin remodeling complexes, such as SWI/SNF complexes and the INO80-containing complexes [110,111]. Although the roles of actin and actin-related proteins in the complexes are yet unclear, it is speculated that they play crucial roles in maintaining the integrity of the protein complexes in the chromatin and thereby affecting chromatin structure and accessibility.
4. Microtubule-Dependent Chromatin Mobility Following DNA Damage
Recent studies in yeast suggest that DSB repair is thought to involve the broken ends being moved to ‘repair centers’ in the nucleus and indicate that DDR-dependent chromatin mobility promotes HR repair [113,114,115,116]. However, the mechanism by which the DNA damage promotes increased chromatin mobility remains to be elucidated. One clue might be found in the recent observation that DNA damage-dependent phosphorylation of nucleoporins releases the interaction between tethered chromosomes and the pore [117]. Another possible mechanism could involve the chromatin remodeling complex. DSB recruitment of chromatin remodeling factors such as INO80 may be important to promote the increase in chromatin mobility [118]. Whatever the mechanism, DNA damage-induced chromatin mobility is required to increase DNA repair efficiency. However, it should also be noted that DSB mobility is not always positive, because increased mobility might lead to unwanted translocations between chromosomes, increasing genome instability. In this section, we discuss the emerging role of microtubule dynamics in chromatin mobility near the DSB sites.
4.1. Chromatin Mobility during HR and NHEJ
Double-strand breaks can be repaired via either HR, the exchange of genetic material between homologous DNA sequences, or NHEJ, the direct ligation of the broken DNA ends [62,119]. HR is an error-free pathway that predominantly occurs in the late S and G2 phases, whereas NHEJ is an error-prone repair pathway that can occur throughout all cell cycle phases [62,120]. The bacterial genomes experience constant pressure from multiple DNA damaging stresses. The bacterial response to DNA damage is known as the SOS response [121,122]. There are two main proteins involved—one, LexA, to keep the response switched off while the cell is healthy, and the other, RecA, to turn it on when DNA damage occurs [123]. In bacteria, DSB repair mostly relies on HR, which involves the action of bacterial recombinase protein RecA [121,122,124]. Interestingly, a number of bacteria have evolved additional DNA protection mechanisms provided by small bacterial DNA-binding proteins, namely, nucleoid-associated proteins (NAPs; e.g., HU, DNA-binding protein from starved cells (Dps)), small acid-soluble spore proteins (SAAPs), and single-stranded binding proteins (SSBs) [125,126,127,128,129,130,131,132,133,134]. These small DNA-binding proteins are able to protect bacterial genomic DNA by the formation of nonspecific protein–DNA complexes, which could be linked with efficient DNA damage repair. Together, both DNA damage repair and DNA protective binding ensure genome stability in bacteria. Yeasts preferentially use HR [62]. Increased chromatin mobility in response to DNA breaks has been reported in yeast [113,114,115,116]. The mobility of damaged chromatin depends on the Mec1ATR kinase, resection of the DSB ends, and the RAD51 recombinase [135,136]. Increased ability of RAD51-DSB ends to find a homologous sequence promotes an efficient HR repair [113,116,137]. As discussed in the previous section, DNA-damage-induced chromatin reorganization can promote the extrusion of DSB sites from the heterochromatic domain and increase access to repair factors. As a consequence, DSB ends become more mobile, which can facilitate homology search and repair. A recent study in C. elegance has shown that LINC complexes facilitate DSB repair through both the inhibition of NHEJ and the promotion of HR [138]. Based on the data, Lawrence et al. have proposed a model whereby the LINC complex can both directly inhibit the KU70/KU80/DNA dependent protein kinase (DNA-PK) complex through SUN proteins and license HR repair through microtubules [138].
NHEJ that relies on the direct rejoining of broken ends is more predominant in mammalian cells [62]. Compared to yeast, DSBs are thought to be immobile within the mammalian cell nucleus [139,140,141]. However, deprotected telomere ends have increased mobility compared with protected telomeres [142]. This increased mobility depends on both ATM and p53-binding protein 1 (53BP1), and these ends are repaired through NHEJ. Recently, Lottersberger et al. revealed that uncapped telomeres and irradiation-induced DSBs in mouse cells exhibit increased DSB mobility that is dependent on dynamic microtubules, 53BP1, the LINC complex, and the motor protein kinesins [143]. In this study, Lottersberger et al. have proposed a model whereby dynamic cytoplasmic microtubules with the LINC complexes can “poke” the nucleus and increase the mobility of 53BP1-associated damaged DNA for NHEJ repair [143]. A recent investigation employing a single-particle tracking method in yeast further demonstrated the requirement of microtubules in DSB mobility upon DNA damage [144].
4.2. KIF2C
As described in the previous section, there are different kinesin proteins localized inside the nucleus, although their roles are largely unknown. Cytoplasmic microtubules can affect the DDR either by the nuclear transport of repair factors [11] or by association with the LINC complexes [143], whereas nuclear kinesins are likely to be involved in a more direct manner. Kinesin family member 2C (KIF2C), also known as mitotic centromere-associated kinesin, is a microtubule-dependent motor protein with a variety of important cellular regulatory functions, such as the regulation of mitosis and genome stability [145,146,147]. Interestingly, it has been recently demonstrated that KIF2C is required for efficient DSB repair via both HR and NHEJ [148]. Depending on its microtubule depolymerizing activity, KIF2C is recruited to DSB sites, promoting DSB mobility and forming DNA damage foci in an ATM-dependent manner, suggesting that KIF2C serves as an important DDR factor that mediates the local mobility and dynamics of DSB ends.
4.3. KIF18B
Kinesin family member 18B (KIF18B) is another member of kinesin that localizes to the nucleus and binds to chromatin throughout the interphase [149]. Most recently, KIF18B, which is also recruited to the sites of DSBs, was reported to be required for 53BP1-mediated DSB repair [150]. This study demonstrated that the ability of KIF18B to bind 53BP1, as well as its motor function is required for efficient 53BP1-mediated end-joining of DSBs.
4.4. DNA-PK-AKT
Ma et al. have recently reported the effect of DNA damage on microtubule dynamics. The authors discovered that DSBs promote microtubule dynamics in G1 cells through DSB-induced microtubule dynamics stress response, which occurs in a DNA-PK-AKT-dependent manner [151]. As a consequence, increased microtubule dynamics promote DSB mobility and facilitates NHEJ repair in G1 cells.
5. Conclusions
This review highlights recent studies investigating the role of microtubule dynamics in DNA damage repair with a focus on the connections between DDR and microtubule networks (Figure 2).
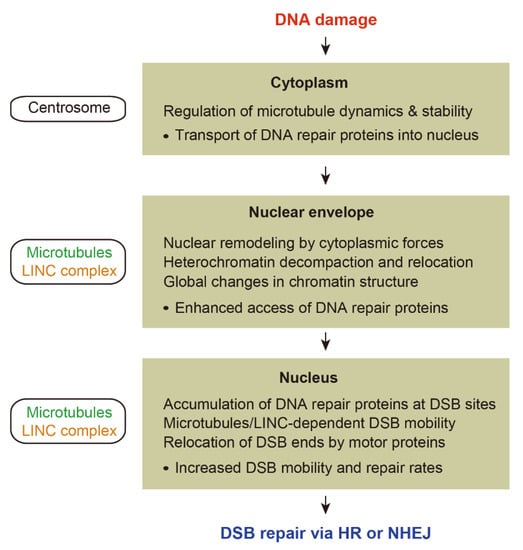
Figure 2.
Overview of the role of microtubule dynamics in DNA damage response.
We first focused on proteins directly related to microtubule organization and stability in response to DNA damage, in which centrosomes play a central role in the regulation of microtubule organization as part of the DDR. We then discussed how cytoplasmic microtubules are involved in the nuclear reorganization following DNA damage. Microtubules can impact nuclear architecture either by generating the microtubule-driven mechanical forces or facilitating the nuclear import of proteins involved in chromatin reorganization. Lastly, we highlighted the molecular mechanisms underlying microtubule-dependent chromatin mobility during DNA repair. Increased mobility of damaged chromatin appears to play an important role for both HR and NHEJ.
Microtubule-targeted agents (MTAs), such as paclitaxel and vinblastine, can induce mitotic catastrophe in cancer cells by disrupting the mitotic spindle [152,153]. Interestingly, MTAs display greater efficacy than mitosis-specific inhibitors, suggesting that MTAs can inhibit both mitotic and non-mitotic functions of microtubules [152,153,154]. MTAs are very effective in cancer treatment when used in combination with DNA-damaging agents [11]. This may be attributed to the ability of MTAs to interfere with microtubule-dependent DNA damage repair. In addition to their antimitotic effects, MTAs can elicit an immune response following the disruption of microtubules [155]. As many cancer cells often overexpress immune checkpoint proteins, thus escaping cancer immune surveillance, immune checkpoint inhibitor (ICI)-based therapy is designed to strengthen cancer immune surveillance [156,157]. As discussed earlier, MTA treatment can stimulate immune responses [155]; thus, combining with ICIs could enhance the antitumor activity of MTAs by stimulating immune surveillance. Indeed, several clinical trials are ongoing to test the combining effect of taxanes and ICIs [158,159,160].
Given their central role in the therapy of cancer, MTAs will continue to be used widely in combination with other anticancer drugs. Thus, there is a critical need to identify novel tubulin-targeting drugs with improved properties that can be used as anticancer agents. In addition, it will be important to select cancer groups that can receive the maximum benefits of a combination with MTAs for cancer therapy, which may provide tumor selectivity.
Funding
This work was supported by Basic science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2020R1F1A1-068668).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank all the lab members for critical reading of the manuscript and helpful discussion.
Conflicts of Interest
The author declares no conflict of interest.
References
- Conduit, P.T.; Wainman, A.; Raff, J.W. Centrosome function and assembly in animal cells. Nat. Rev. Mol. Cell. Biol. 2015, 16, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Kollman, J.M.; Merdes, A.; Mourey, L.; Agard, D.A. Microtubule nucleation by gamma-tubulin complexes. Nat. Rev. Mol. Cell. Biol. 2011, 12, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.; Mandelkow, E.M. Microtubules and microtubule-associated proteins. Curr. Opin. Cell Biol. 1995, 7, 72–81. [Google Scholar] [CrossRef]
- Gadde, S.; Heald, R. Mechanisms and molecules of the mitotic spindle. Curr. Biol. 2004, 14, R797–R805. [Google Scholar] [CrossRef]
- Petry, S. Mechanisms of Mitotic Spindle Assembly. Annu. Rev. Biochem. 2016, 85, 659–683. [Google Scholar] [CrossRef]
- Gerlitz, G.; Reiner, O.; Bustin, M. Microtubule dynamics alter the interphase nucleus. Cell Mol. Life Sci. 2013, 70, 1255–1268. [Google Scholar] [CrossRef]
- Maizels, Y.; Gerlitz, G. Shaping of interphase chromosomes by the microtubule network. FEBS J. 2015, 282, 3500–3524. [Google Scholar] [CrossRef]
- Shokrollahi, M.; Mekhail, K. Interphase microtubules in nuclear organization and genome maintenance. Trends Cell Biol. 2021, 31, 721–731. [Google Scholar] [CrossRef]
- Graml, V.; Studera, X.; Lawson, J.L.D.; Chessel, A.; Geymonat, M.; Bortfeld-Miller, M.; Walter, T.; Wagstaff, L.; Piddini, E.; Carazo Salas, R.E. A genomic Multiprocess survey of machineries that control and link cell shape, microtubule organization, and cell-cycle progression. Dev. Cell 2014, 31, 227–239. [Google Scholar] [CrossRef]
- Poruchynsky, M.S.; Komlodi-Pasztor, E.; Trostel, S.; Wilkerson, J.; Regairaz, M.; Pommier, Y.; Zhang, X.; Kumar Maity, T.; Robey, R.; Burotto, M.; et al. Microtubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteins. Proc. Natl. Acad. Sci. USA 2015, 112, 1571–1576. [Google Scholar] [CrossRef]
- Chouinard, G.; Clement, I.; Lafontaine, J.; Rodier, F.; Schmitt, E. Cell cycle-dependent localization of CHK2 at centrosomes during mitosis. Cell Div. 2013, 8, 7. [Google Scholar] [CrossRef]
- Hsu, L.C.; Doan, T.P.; White, R.L. Identification of a gamma-tubulin-binding domain in BRCA1. Cancer Res. 2001, 61, 7713–7718. [Google Scholar]
- Hsu, L.C.; White, R.L. BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. USA 1998, 95, 12983–12988. [Google Scholar] [CrossRef]
- Kramer, A.; Mailand, N.; Lukas, C.; Syljuasen, R.G.; Wilkinson, C.J.; Nigg, E.A.; Bartek, J.; Lukas, J. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat. Cell Biol. 2004, 6, 884–891. [Google Scholar] [CrossRef]
- Shimada, M.; Komatsu, K. Emerging connection between centrosome and DNA repair machinery. J. Radiat. Res. 2009, 50, 295–301. [Google Scholar] [CrossRef]
- Starita, L.M.; Machida, Y.; Sankaran, S.; Elias, J.E.; Griffin, K.; Schlegel, B.P.; Gygi, S.P.; Parvin, J.D. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol. Cell. Biol. 2004, 24, 8457–8466. [Google Scholar] [CrossRef]
- Zhang, S.; Hemmerich, P.; Grosse, F. Centrosomal localization of DNA damage checkpoint proteins. J. Cell. Biochem. 2007, 101, 451–465. [Google Scholar] [CrossRef]
- Fry, A.M.; Meraldi, P.; Nigg, E.A. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 1998, 17, 470–481. [Google Scholar] [CrossRef]
- Fry, A.M.; O’Regan, L.; Sabir, S.R.; Bayliss, R. Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 2012, 125, 4423–4433. [Google Scholar] [CrossRef]
- Moniz, L.; Dutt, P.; Haider, N.; Stambolic, V. Nek family of kinases in cell cycle, checkpoint control and cancer. Cell Div. 2011, 6, 18. [Google Scholar] [CrossRef]
- Fry, A.M.; Schultz, S.J.; Bartek, J.; Nigg, E.A. Substrate specificity and cell cycle regulation of the Nek2 protein kinase, a potential human homolog of the mitotic regulator NIMA of Aspergillus nidulans. J. Biol. Chem. 1995, 270, 12899–12905. [Google Scholar] [CrossRef]
- Fletcher, L.; Cerniglia, G.J.; Nigg, E.A.; Yend, T.J.; Muschel, R.J. Inhibition of centrosome separation after DNA damage: A role for Nek2. Radiat. Res. 2004, 162, 128–135. [Google Scholar] [CrossRef]
- Andreassen, P.R.; Lacroix, F.B.; Villa-Moruzzi, E.; Margolis, R.L. Differential subcellular localization of protein phosphatase-1 alpha, gamma1, and delta isoforms during both interphase and mitosis in mammalian cells. J. Cell Biol. 1998, 141, 1207–1215. [Google Scholar] [CrossRef]
- Mi, J.; Guo, C.; Brautigan, D.L.; Larner, J.M. Protein phosphatase-1alpha regulates centrosome splitting through Nek2. Cancer Res. 2007, 67, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lee, J.; Kim, K.; Yoo, J.C.; Rhee, K. Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization. J. Cell Sci. 2007, 120, 2106–2116. [Google Scholar] [CrossRef]
- Zou, C.; Li, J.; Bai, Y.; Gunning, W.T.; Wazer, D.E.; Band, V.; Gao, Q. Centrobin: A novel daughter centriole-associated protein that is required for centriole duplication. J. Cell Biol. 2005, 171, 437–445. [Google Scholar] [CrossRef]
- Gudi, R.; Zou, C.; Li, J.; Gao, Q. Centrobin-tubulin interaction is required for centriole elongation and stability. J. Cell Biol. 2011, 193, 711–725. [Google Scholar] [CrossRef]
- Jeffery, J.M.; Urquhart, A.J.; Subramaniam, V.N.; Parton, R.G.; Khanna, K.K. Centrobin regulates the assembly of functional mitotic spindles. Oncogene 2010, 29, 2649–2658. [Google Scholar] [CrossRef]
- Shin, W.; Yu, N.K.; Kaang, B.K.; Rhee, K. The microtubule nucleation activity of centrobin in both the centrosome and cytoplasm. Cell Cycle 2015, 14, 1925–1931. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, Y.; Jeong, S.; Rhee, K. Centrobin/NIP2 is a microtubule stabilizer whose activity is enhanced by PLK1 phosphorylation during mitosis. J. Biol. Chem. 2010, 285, 25476–25484. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Rhee, K. NEK2 phosphorylation antagonizes the microtubule stabilizing activity of centrobin. Biochem. Biophys. Res. Commun. 2013, 431, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef]
- Ryu, N.M.; Kim, J.M. Centrobin plays a role in the cellular response to DNA damage. Cell Cycle 2019, 18, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Delaval, B.; Doxsey, S.J. Pericentrin in cellular function and disease. J. Cell Biol. 2010, 188, 181–190. [Google Scholar] [CrossRef]
- Doxsey, S.J.; Stein, P.; Evans, L.; Calarco, P.D.; Kirschner, M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell 1994, 76, 639–650. [Google Scholar] [CrossRef]
- Griffith, E.; Walker, S.; Martin, C.A.; Vagnarelli, P.; Stiff, T.; Vernay, B.; Al Sanna, N.; Saggar, A.; Hamel, B.; Earnshaw, W.C.; et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 2008, 40, 232–236. [Google Scholar] [CrossRef]
- Tibelius, A.; Marhold, J.; Zentgraf, H.; Heilig, C.E.; Neitzel, H.; Ducommun, B.; Rauch, A.; Ho, A.D.; Bartek, J.; Kramer, A. Microcephalin and pericentrin regulate mitotic entry via centrosome-associated Chk1. J. Cell Biol. 2009, 185, 1149–1157. [Google Scholar] [CrossRef]
- Purohit, A.; Tynan, S.H.; Vallee, R.; Doxsey, S.J. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 1999, 147, 481–492. [Google Scholar] [CrossRef]
- Zimmerman, W.C.; Sillibourne, J.; Rosa, J.; Doxsey, S.J. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 2004, 15, 3642–3657. [Google Scholar] [CrossRef]
- Alderton, G.K.; Joenje, H.; Varon, R.; Borglum, A.D.; Jeggo, P.A.; O’Driscoll, M. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum. Mol. Genet. 2004, 13, 3127–3138. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Ruiz-Perez, V.L.; Woods, C.G.; Jeggo, P.A.; Goodship, J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003, 33, 497–501. [Google Scholar] [CrossRef]
- Rauch, A.; Thiel, C.T.; Schindler, D.; Wick, U.; Crow, Y.J.; Ekici, A.B.; van Essen, A.J.; Goecke, T.O.; Al-Gazali, L.; Chrzanowska, K.H.; et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 2008, 319, 816–819. [Google Scholar] [CrossRef]
- Sillibourne, J.E.; Delaval, B.; Redick, S.; Sinha, M.; Doxsey, S.J. Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity. Mol. Biol. Cell 2007, 18, 3667–3680. [Google Scholar] [CrossRef]
- Smith, E.; Dejsuphong, D.; Balestrini, A.; Hampel, M.; Lenz, C.; Takeda, S.; Vindigni, A.; Costanzo, V. An ATM- and ATR-dependent checkpoint inactivates spindle assembly by targeting CEP63. Nat. Cell Biol. 2009, 11, 278–285. [Google Scholar] [CrossRef]
- Sir, J.H.; Barr, A.R.; Nicholas, A.K.; Carvalho, O.P.; Khurshid, M.; Sossick, A.; Reichelt, S.; D’Santos, C.; Woods, C.G.; Gergely, F. A primary microcephaly protein complex forms a ring around parental centrioles. Nat. Genet. 2011, 43, 1147–1153. [Google Scholar] [CrossRef]
- Kalay, E.; Yigit, G.; Aslan, Y.; Brown, K.E.; Pohl, E.; Bicknell, L.S.; Kayserili, H.; Li, Y.; Tuysuz, B.; Nurnberg, G.; et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat. Genet. 2011, 43, 23–26. [Google Scholar] [CrossRef]
- Brown, N.J.; Marjanovic, M.; Luders, J.; Stracker, T.H.; Costanzo, V. Cep63 and cep152 cooperate to ensure centriole duplication. PLoS ONE 2013, 8, e69986. [Google Scholar] [CrossRef]
- Sivasubramaniam, S.; Sun, X.; Pan, Y.R.; Wang, S.; Lee, E.Y. Cep164 is a mediator protein required for the maintenance of genomic stability through modulation of MDC1, RPA, and CHK1. Genes Dev. 2008, 22, 587–600. [Google Scholar] [CrossRef]
- Akella, J.S.; Wloga, D.; Kim, J.; Starostina, N.G.; Lyons-Abbott, S.; Morrissette, N.S.; Dougan, S.T.; Kipreos, E.T.; Gaertig, J. MEC-17 is an alpha-tubulin acetyltransferase. Nature 2010, 467, 218–222. [Google Scholar] [CrossRef]
- Kalebic, N.; Sorrentino, S.; Perlas, E.; Bolasco, G.; Martinez, C.; Heppenstall, P.A. alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat. Commun. 2013, 4, 1962. [Google Scholar] [CrossRef]
- Janke, C.; Bulinski, J.C. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat. Rev. Mol. Cell. Biol. 2011, 12, 773–786. [Google Scholar] [CrossRef]
- Janke, C.; Montagnac, G. Causes and Consequences of Microtubule Acetylation. Curr. Biol. 2017, 27, R1287–R1292. [Google Scholar] [CrossRef]
- Ryu, N.M.; Kim, J.M. The role of the alpha-tubulin acetyltransferase alphaTAT1 in the DNA damage response. J. Cell Sci. 2020, 133, jcs246702. [Google Scholar] [CrossRef]
- Magiera, M.M.; Janke, C. Post-translational modifications of tubulin. Curr. Biol. 2014, 24, R351–R354. [Google Scholar] [CrossRef]
- Reed, N.A.; Cai, D.; Blasius, T.L.; Jih, G.T.; Meyhofer, E.; Gaertig, J.; Verhey, K.J. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 2006, 16, 2166–2172. [Google Scholar] [CrossRef]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.F.; Yao, T.P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Zhang, H.; Head, P.E.; Daddacha, W.; Park, S.H.; Li, X.; Pan, Y.; Madden, M.Z.; Duong, D.M.; Xie, M.; Yu, B.; et al. ATRIP Deacetylation by SIRT2 Drives ATR Checkpoint Activation by Promoting Binding to RPA-ssDNA. Cell Rep. 2016, 14, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, C.; Moses, N.; Haakenson, J.; Xiang, S.; Quan, D.; Fang, B.; Yang, Z.; Bai, W.; Bepler, G.; et al. HDAC6 regulates DNA damage response via deacetylating MLH1. J. Biol. Chem. 2019, 294, 5813–5826. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.; Toseland, C.P. Regulation of Nuclear Mechanics and the Impact on DNA Damage. Int. J. Mol. Sci. 2021, 22, 3178. [Google Scholar] [CrossRef]
- Hauer, M.H.; Gasser, S.M. Chromatin and nucleosome dynamics in DNA damage and repair. Genes Dev. 2017, 31, 2204–2221. [Google Scholar] [CrossRef]
- Dos Santos, A.; Cook, A.W.; Gough, R.E.; Schilling, M.; Olszok, N.A.; Brown, I.; Wang, L.; Aaron, J.; Martin-Fernandez, M.L.; Rehfeldt, F.; et al. DNA damage alters nuclear mechanics through chromatin reorganization. Nucleic Acids Res. 2021, 49, 340–353. [Google Scholar] [CrossRef]
- Spichal, M.; Fabre, E. The Emerging Role of the Cytoskeleton in Chromosome Dynamics. Front. Genet. 2017, 8, 60. [Google Scholar] [CrossRef]
- Bolhy, S.; Bouhlel, I.; Dultz, E.; Nayak, T.; Zuccolo, M.; Gatti, X.; Vallee, R.; Ellenberg, J.; Doye, V. A Nup133-dependent NPC-anchored network tethers centrosomes to the nuclear envelope in prophase. J. Cell Biol. 2011, 192, 855–871. [Google Scholar] [CrossRef]
- Zuleger, N.; Robson, M.I.; Schirmer, E.C. The nuclear envelope as a chromatin organizer. Nucleus 2011, 2, 339–349. [Google Scholar] [CrossRef]
- Chang, W.; Worman, H.J.; Gundersen, G.G. Accessorizing and anchoring the LINC complex for multifunctionality. J. Cell Biol. 2015, 208, 11–22. [Google Scholar] [CrossRef]
- Rothballer, A.; Kutay, U. The diverse functional LINCs of the nuclear envelope to the cytoskeleton and chromatin. Chromosoma 2013, 122, 415–429. [Google Scholar] [CrossRef]
- Starr, D.A.; Fridolfsson, H.N. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 2010, 26, 421–444. [Google Scholar] [CrossRef]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef]
- Solovei, I.; Wang, A.S.; Thanisch, K.; Schmidt, C.S.; Krebs, S.; Zwerger, M.; Cohen, T.V.; Devys, D.; Foisner, R.; Peichl, L.; et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013, 152, 584–598. [Google Scholar] [CrossRef]
- van Steensel, B.; Belmont, A.S. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 2017, 169, 780–791. [Google Scholar] [CrossRef]
- Furusawa, T.; Rochman, M.; Taher, L.; Dimitriadis, E.K.; Nagashima, K.; Anderson, S.; Bustin, M. Chromatin decompaction by the nucleosomal binding protein HMGN5 impairs nuclear sturdiness. Nat. Commun. 2015, 6, 6138. [Google Scholar] [CrossRef]
- Schreiner, S.M.; Koo, P.K.; Zhao, Y.; Mochrie, S.G.; King, M.C. The tethering of chromatin to the nuclear envelope supports nuclear mechanics. Nat. Commun. 2015, 6, 7159. [Google Scholar] [CrossRef]
- Amaral, N.; Ryu, T.; Li, X.; Chiolo, I. Nuclear Dynamics of Heterochromatin Repair. Trends Genet. 2017, 33, 86–100. [Google Scholar] [CrossRef]
- Chiolo, I.; Minoda, A.; Colmenares, S.U.; Polyzos, A.; Costes, S.V.; Karpen, G.H. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 2011, 144, 732–744. [Google Scholar] [CrossRef]
- Fortuny, A.; Polo, S.E. The response to DNA damage in heterochromatin domains. Chromosoma 2018, 127, 291–300. [Google Scholar] [CrossRef]
- Jakob, B.; Splinter, J.; Conrad, S.; Voss, K.O.; Zink, D.; Durante, M.; Lobrich, M.; Taucher-Scholz, G. DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucleic Acids Res. 2011, 39, 6489–6499. [Google Scholar] [CrossRef]
- Janssen, A.; Breuer, G.A.; Brinkman, E.K.; van der Meulen, A.I.; Borden, S.V.; van Steensel, B.; Bindra, R.S.; LaRocque, J.R.; Karpen, G.H. A single double-strand break system reveals repair dynamics and mechanisms in heterochromatin and euchromatin. Genes Dev. 2016, 30, 1645–1657. [Google Scholar] [CrossRef]
- Tsouroula, K.; Furst, A.; Rogier, M.; Heyer, V.; Maglott-Roth, A.; Ferrand, A.; Reina-San-Martin, B.; Soutoglou, E. Temporal and Spatial Uncoupling of DNA Double Strand Break Repair Pathways within Mammalian Heterochromatin. Mol. Cell 2016, 63, 293–305. [Google Scholar] [CrossRef]
- Goodarzi, A.A.; Kurka, T.; Jeggo, P.A. KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat. Struct. Mol. Biol. 2011, 18, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, A.A.; Noon, A.T.; Deckbar, D.; Ziv, Y.; Shiloh, Y.; Lobrich, M.; Jeggo, P.A. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol. Cell 2008, 31, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Cairns, B.R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009, 78, 273–304. [Google Scholar] [CrossRef] [PubMed]
- Papamichos-Chronakis, M.; Watanabe, S.; Rando, O.J.; Peterson, C.L. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 2011, 144, 200–213. [Google Scholar] [CrossRef]
- Park, E.J.; Hur, S.K.; Lee, H.S.; Lee, S.A.; Kwon, J. The human Ino80 binds to microtubule via the E-hook of tubulin: Implications for the role in spindle assembly. Biochem. Biophys. Res. Commun. 2011, 416, 416–420. [Google Scholar] [CrossRef]
- Yokoyama, H.; Gruss, O.J. New mitotic regulators released from chromatin. Front. Oncol. 2013, 3, 308. [Google Scholar] [CrossRef]
- Yokoyama, H.; Rybina, S.; Santarella-Mellwig, R.; Mattaj, I.W.; Karsenti, E. ISWI is a RanGTP-dependent MAP required for chromosome segregation. J. Cell Biol. 2009, 187, 813–829. [Google Scholar] [CrossRef]
- Dutcher, S.K. The tubulin fraternity: Alpha to eta. Curr. Opin. Cell Biol. 2001, 13, 49–54. [Google Scholar] [CrossRef]
- Stathatos, G.G.; Dunleavy, J.E.M.; Zenker, J.; O’Bryan, M.K. Delta and epsilon tubulin in mammalian development. Trends Cell Biol. 2021, 31, 774–787. [Google Scholar] [CrossRef]
- Chang, P.; Stearns, T. Delta-tubulin and epsilon-tubulin: Two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat. Cell Biol. 2000, 2, 30–35. [Google Scholar] [CrossRef]
- Alvarado-Kristensson, M. Gamma-tubulin as a signal-transducing molecule and meshwork with therapeutic potential. Signal. Transduct. Target. Ther. 2018, 3, 24. [Google Scholar] [CrossRef]
- Vazquez, M.; Cooper, M.T.; Zurita, M.; Kennison, J.A. GammaTub23C interacts genetically with brahma chromatin-remodeling complexes in Drosophila melanogaster. Genetics 2008, 180, 835–843. [Google Scholar] [CrossRef]
- Hoog, G.; Zarrizi, R.; von Stedingk, K.; Jonsson, K.; Alvarado-Kristensson, M. Nuclear localization of gamma-tubulin affects E2F transcriptional activity and S-phase progression. FASEB J. 2011, 25, 3815–3827. [Google Scholar] [CrossRef]
- Harbour, J.W.; Dean, D.C. The Rb/E2F pathway: Expanding roles and emerging paradigms. Genes Dev. 2000, 14, 2393–2409. [Google Scholar] [CrossRef]
- Brehm, A.; Miska, E.A.; McCance, D.J.; Reid, J.L.; Bannister, A.J.; Kouzarides, T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 1998, 391, 597–601. [Google Scholar] [CrossRef]
- Dunaief, J.L.; Strober, B.E.; Guha, S.; Khavari, P.A.; Alin, K.; Luban, J.; Begemann, M.; Crabtree, G.R.; Goff, S.P. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell 1994, 79, 119–130. [Google Scholar] [CrossRef]
- Robertson, K.D.; Ait-Si-Ali, S.; Yokochi, T.; Wade, P.A.; Jones, P.L.; Wolffe, A.P. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 2000, 25, 338–342. [Google Scholar] [CrossRef]
- Lesca, C.; Germanier, M.; Raynaud-Messina, B.; Pichereaux, C.; Etievant, C.; Emond, S.; Burlet-Schiltz, O.; Monsarrat, B.; Wright, M.; Defais, M. DNA damage induce gamma-tubulin-RAD51 nuclear complexes in mammalian cells. Oncogene 2005, 24, 5165–5172. [Google Scholar] [CrossRef]
- Hirokawa, N.; Noda, Y.; Tanaka, Y.; Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell. Biol. 2009, 10, 682–696. [Google Scholar] [CrossRef]
- Miki, H.; Setou, M.; Kaneshiro, K.; Hirokawa, N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc. Natl. Acad. Sci. USA 2001, 98, 7004–7011. [Google Scholar] [CrossRef]
- Mazumdar, M.; Sundareshan, S.; Misteli, T. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J. Cell Biol. 2004, 166, 613–620. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, S.; Lee, E.; Shin, H.; Hahn, H.; Choi, W.; Kim, W. Human kinesin superfamily member 4 is dominantly localized in the nuclear matrix and is associated with chromosomes during mitosis. Biochem. J. 2001, 360, 549–556. [Google Scholar] [CrossRef]
- Mazumdar, M.; Sung, M.H.; Misteli, T. Chromatin maintenance by a molecular motor protein. Nucleus 2011, 2, 591–600. [Google Scholar] [CrossRef]
- Krishnakumar, R.; Kraus, W.L. The PARP side of the nucleus: Molecular actions, physiological outcomes, and clinical targets. Mol. Cell 2010, 39, 8–24. [Google Scholar] [CrossRef]
- Midorikawa, R.; Takei, Y.; Hirokawa, N. KIF4 motor regulates activity-dependent neuronal survival by suppressing PARP-1 enzymatic activity. Cell 2006, 125, 371–383. [Google Scholar] [CrossRef]
- Geiman, T.M.; Sankpal, U.T.; Robertson, A.K.; Chen, Y.; Mazumdar, M.; Heale, J.T.; Schmiesing, J.A.; Kim, W.; Yokomori, K.; Zhao, Y.; et al. Isolation and characterization of a novel DNA methyltransferase complex linking DNMT3B with components of the mitotic chromosome condensation machinery. Nucleic Acids Res. 2004, 32, 2716–2729. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, W. Association of human kinesin superfamily protein member 4 with BRCA2-associated factor 35. Biochem. J. 2003, 374, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhou, L.; Khidr, L.; Guo, X.E.; Kim, W.; Lee, Y.M.; Krasieva, T.; Chen, P.L. A novel role of the chromokinesin Kif4A in DNA damage response. Cell Cycle 2008, 7, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Caridi, C.P.; D’Agostino, C.; Ryu, T.; Zapotoczny, G.; Delabaere, L.; Li, X.; Khodaverdian, V.Y.; Amaral, N.; Lin, E.; Rau, A.R.; et al. Nuclear F-actin and myosins drive relocalization of heterochromatic breaks. Nature 2018, 559, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Farrants, A.K. Chromatin remodelling and actin organisation. FEBS Lett. 2008, 582, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Shen, X. Mechanisms of nuclear actin in chromatin-remodeling complexes. Trends Cell Biol. 2014, 24, 238–246. [Google Scholar] [CrossRef]
- Treisman, R. Shedding light on nuclear actin dynamics and function. Trends Biochem. Sci. 2013, 38, 376–377. [Google Scholar] [CrossRef]
- Dion, V.; Gasser, S.M. Chromatin movement in the maintenance of genome stability. Cell 2013, 152, 1355–1364. [Google Scholar] [CrossRef]
- Hauer, M.H.; Seeber, A.; Singh, V.; Thierry, R.; Sack, R.; Amitai, A.; Kryzhanovska, M.; Eglinger, J.; Holcman, D.; Owen-Hughes, T.; et al. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nat. Struct. Mol. Biol. 2017, 24, 99–107. [Google Scholar] [CrossRef]
- Herbert, S.; Brion, A.; Arbona, J.M.; Lelek, M.; Veillet, A.; Lelandais, B.; Parmar, J.; Fernandez, F.G.; Almayrac, E.; Khalil, Y.; et al. Chromatin stiffening underlies enhanced locus mobility after DNA damage in budding yeast. EMBO J. 2017, 36, 2595–2608. [Google Scholar] [CrossRef]
- Mine-Hattab, J.; Rothstein, R. Increased chromosome mobility facilitates homology search during recombination. Nat. Cell Biol. 2012, 14, 510–517. [Google Scholar] [CrossRef]
- Bermejo, R.; Capra, T.; Jossen, R.; Colosio, A.; Frattini, C.; Carotenuto, W.; Cocito, A.; Doksani, Y.; Klein, H.; Gomez-Gonzalez, B.; et al. The replication checkpoint protects fork stability by releasing transcribed genes from nuclear pores. Cell 2011, 146, 233–246. [Google Scholar] [CrossRef]
- Neumann, F.R.; Dion, V.; Gehlen, L.R.; Tsai-Pflugfelder, M.; Schmid, R.; Taddei, A.; Gasser, S.M. Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes Dev. 2012, 26, 369–383. [Google Scholar] [CrossRef]
- Brandsma, I.; Gent, D.C. Pathway choice in DNA double strand break repair: Observations of a balancing act. Genome Integr. 2012, 3, 9. [Google Scholar] [CrossRef]
- Shrivastav, M.; De Haro, L.P.; Nickoloff, J.A. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008, 18, 134–147. [Google Scholar] [CrossRef]
- Burby, P.E.; Simmons, L.A. Regulation of Cell Division in Bacteria by Monitoring Genome Integrity and DNA Replication Status. J. Bacteriol. 2020, 202, e00408-19. [Google Scholar] [CrossRef]
- Lenhart, J.S.; Schroeder, J.W.; Walsh, B.W.; Simmons, L.A. DNA repair and genome maintenance in Bacillus subtilis. Microbiol Mol. Biol. Rev. 2012, 76, 530–564. [Google Scholar] [CrossRef]
- Butala, M.; Zgur-Bertok, D.; Busby, S.J. The bacterial LexA transcriptional repressor. Cell Mol. Life Sci. 2009, 66, 82–93. [Google Scholar] [CrossRef]
- Puig, J.; Knodlseder, N.; Quera, J.; Algara, M.; Guell, M. DNA Damage Protection for Enhanced Bacterial Survival Under Simulated Low Earth Orbit Environmental Conditions in Escherichia coli. Front. Microbiol. 2021, 12, 789668. [Google Scholar] [CrossRef]
- Almiron, M.; Link, A.J.; Furlong, D.; Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992, 6, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.R.; Barton, J.K. DNA protection by the bacterial ferritin Dps via DNA charge transport. J. Am. Chem. Soc. 2013, 135, 15726–15729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dillon, S.C.; Dorman, C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010, 8, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Holowka, J.; Zakrzewska-Czerwinska, J. Nucleoid Associated Proteins: The Small Organizers That Help to Cope With Stress. Front. Microbiol. 2020, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Karas, V.O.; Westerlaken, I.; Meyer, A.S. The DNA-Binding Protein from Starved Cells (Dps) Utilizes Dual Functions To Defend Cells against Multiple Stresses. J. Bacteriol. 2015, 197, 3206–3215. [Google Scholar] [CrossRef]
- Lee, K.S.; Bumbaca, D.; Kosman, J.; Setlow, P.; Jedrzejas, M.J. Structure of a protein-DNA complex essential for DNA protection in spores of Bacillus species. Proc. Natl. Acad. Sci. USA 2008, 105, 2806–2811. [Google Scholar] [CrossRef]
- Molan, K.; Zgur Bertok, D. Small Prokaryotic DNA-Binding Proteins Protect Genome Integrity throughout the Life Cycle. Int. J. Mol. Sci. 2022, 23, 4008. [Google Scholar] [CrossRef]
- Pallares, M.C.; Marcuello, C.; Botello-Morte, L.; Gonzalez, A.; Fillat, M.F.; Lostao, A. Sequential binding of FurA from Anabaena sp. PCC 7120 to iron boxes: Exploring regulation at the nanoscale. Biochim. Biophys. Acta 2014, 1844, 623–631. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Shereda, R.D.; Kozlov, A.G.; Lohman, T.M.; Cox, M.M.; Keck, J.L. SSB as an organizer/mobilizer of genome maintenance complexes. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 289–318. [Google Scholar] [CrossRef]
- Rudin, N.; Haber, J.E. Efficient repair of HO-induced chromosomal breaks in Saccharomyces cerevisiae by recombination between flanking homologous sequences. Mol. Cell. Biol. 1988, 8, 3918–3928. [Google Scholar] [CrossRef]
- Weiner, A.; Zauberman, N.; Minsky, A. Recombinational DNA repair in a cellular context: A search for the homology search. Nat. Rev. Microbiol. 2009, 7, 748–755. [Google Scholar] [CrossRef]
- Forget, A.L.; Kowalczykowski, S.C. Single-molecule imaging of DNA pairing by RecA reveals a three-dimensional homology search. Nature 2012, 482, 423–427. [Google Scholar] [CrossRef]
- Lawrence, K.S.; Tapley, E.C.; Cruz, V.E.; Li, Q.; Aung, K.; Hart, K.C.; Schwartz, T.U.; Starr, D.A.; Engebrecht, J. LINC complexes promote homologous recombination in part through inhibition of nonhomologous end joining. J. Cell Biol. 2016, 215, 801–821. [Google Scholar] [CrossRef]
- Kruhlak, M.J.; Celeste, A.; Dellaire, G.; Fernandez-Capetillo, O.; Muller, W.G.; McNally, J.G.; Bazett-Jones, D.P.; Nussenzweig, A. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J. Cell Biol. 2006, 172, 823–834. [Google Scholar] [CrossRef]
- Nelms, B.E.; Maser, R.S.; MacKay, J.F.; Lagally, M.G.; Petrini, J.H. In situ visualization of DNA double-strand break repair in human fibroblasts. Science 1998, 280, 590–592. [Google Scholar] [CrossRef]
- Soutoglou, E.; Dorn, J.F.; Sengupta, K.; Jasin, M.; Nussenzweig, A.; Ried, T.; Danuser, G.; Misteli, T. Positional stability of single double-strand breaks in mammalian cells. Nat. Cell Biol. 2007, 9, 675–682. [Google Scholar] [CrossRef]
- Dimitrova, N.; Chen, Y.C.; Spector, D.L.; de Lange, T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 2008, 456, 524–528. [Google Scholar] [CrossRef]
- Lottersberger, F.; Karssemeijer, R.A.; Dimitrova, N.; de Lange, T. 53BP1 and the LINC Complex Promote Microtubule-Dependent DSB Mobility and DNA Repair. Cell 2015, 163, 880–893. [Google Scholar] [CrossRef]
- Lawrimore, J.; Barry, T.M.; Barry, R.M.; York, A.C.; Friedman, B.; Cook, D.M.; Akialis, K.; Tyler, J.; Vasquez, P.; Yeh, E.; et al. Microtubule dynamics drive enhanced chromatin motion and mobilize telomeres in response to DNA damage. Mol. Biol. Cell 2017, 28, 1701–1711. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Thompson, S.L.; Manning, A.L.; Compton, D.A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009, 11, 27–35. [Google Scholar] [CrossRef]
- Gwon, M.R.; Cho, J.H.; Kim, J.R. Mitotic centromere-associated kinase (MCAK/Kif2C) regulates cellular senescence in human primary cells through a p53-dependent pathway. FEBS Lett. 2012, 586, 4148–4156. [Google Scholar] [CrossRef]
- Manning, A.L.; Ganem, N.J.; Bakhoum, S.F.; Wagenbach, M.; Wordeman, L.; Compton, D.A. The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Mol. Biol. Cell 2007, 18, 2970–2979. [Google Scholar] [CrossRef]
- Zhu, S.; Paydar, M.; Wang, F.; Li, Y.; Wang, L.; Barrette, B.; Bessho, T.; Kwok, B.H.; Peng, A. Kinesin Kif2C in regulation of DNA double strand break dynamics and repair. eLife 2020, 9, e53402. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, E.; Park, M.; Moon, E.; Ahn, S.M.; Kim, W.; Hwang, K.B.; Kim, Y.K.; Choi, W.; Kim, W. Cell cycle-regulated expression and subcellular localization of a kinesin-8 member human KIF18B. Gene 2010, 466, 16–25. [Google Scholar] [CrossRef]
- Luessing, J.; Sakhteh, M.; Sarai, N.; Frizzell, L.; Tsanov, N.; Ramberg, K.O.; Maretto, S.; Crowley, P.B.; Lowndes, N.F. The nuclear kinesin KIF18B promotes 53BP1-mediated DNA double-strand break repair. Cell Rep. 2021, 35, 109306. [Google Scholar] [CrossRef]
- Ma, S.; Rong, Z.; Liu, C.; Qin, X.; Zhang, X.; Chen, Q. DNA damage promotes microtubule dynamics through a DNA-PK-AKT axis for enhanced repair. J. Cell Biol. 2021, 220, e201911025. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Zheng, L.L.; Wang, D.; Liang, X.X.; Gao, F.; Zhou, X.L. Recent advances in microtubule-stabilizing agents. Eur. J. Med. Chem. 2018, 143, 806–828. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Haschka, M.; Karbon, G.; Fava, L.L.; Villunger, A. Perturbing mitosis for anti-cancer therapy: Is cell death the only answer? EMBO Rep. 2018, 19, e45440. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.J.; Pineda, J.; Shi, J.; Florian, S. Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug? Open Biol. 2017, 7, 170182. [Google Scholar] [CrossRef]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).