Abstract
Sitosterolemia is a lipid disorder characterized by the accumulation of dietary xenosterols in plasma and tissues caused by mutations in either ABCG5 or ABCG8. ABCG5 ABCG8 encodes a pair of ABC half transporters that form a heterodimer (G5G8), which then traffics to the surface of hepatocytes and enterocytes and promotes the secretion of cholesterol and xenosterols into the bile and the intestinal lumen. We review the literature from the initial description of the disease, the discovery of its genetic basis, current therapy, and what has been learned from animal, cellular, and molecular investigations of the transporter in the twenty years since its discovery. The genomic era has revealed that there are far more carriers of loss of function mutations and likely pathogenic variants of ABCG5 ABCG8 than previously thought. The impact of these variants on G5G8 structure and activity are largely unknown. We propose a classification system for ABCG5 ABCG8 mutants based on previously published systems for diseases caused by defects in ABC transporters. This system establishes a framework for the comprehensive analysis of disease-associated variants and their impact on G5G8 structure–function.
1. Discovery to Therapy
In 1974, Bhattacharyya and Conner described a new lipid storage disorder in two sisters who presented with tendon and tuberous xanthomas and elevated plasma levels of phytosterols, sitosterol, campesterol, and stigmasterol [1]. Absorption of radiolabeled β-sitosterol was reported to be thirty-five times greater than that of normal subjects. They named their new lipid disorder β-sitosterolemia (hereon referred to as sitosterolemia), but it would be another 26 years before the discovery of ABCG5 ABCG8 as the causative gene defect. Subsequent case reports established recessive genetics of the disease and greatly expanded its potential clinical presentation, which may include elevated low density lipoprotein (LDL) cholesterol, premature coronary artery disease and death, hemolytic anemia, macrothrombocytopenia, splenomegaly, adrenal dysfunction, elevated liver function tests, and cirrhosis [2,3,4,5,6,7,8,9,10,11,12,13]. Clinical studies in individuals with sitosterolemia revealed reductions in cholesterol synthesis, biliary cholesterol secretion, plasma clearance, and fecal elimination of neutral sterols [8,9,14,15]. Despite the absorptive phenotype and metabolism of phytosterols to bile acids, the clinical management of these patients with low sterol diets and bile acid binding resins resulted in modest and inconsistent reductions in plasma phytosterols [5,16].
Following the elimination of key genes in the esterification, absorbance, biosynthesis, and regulation of cholesterol metabolism, the sitosterolemia locus was mapped to a 0.5 centimorgan region on chromosome 2p21 [17,18]. The breakthrough would come two years later when investigators were studying agonists for liver X receptors (LXR-α NR1H3, LXR-β NR1H2), sensors of excess cholesterol that promote cholesterol mobilization from macrophages, metabolism to primary bile acids, and the excretion of neutral and acidic sterols [19,20]. A microarray screen of liver and intestinal transcripts from mice treated with an LXR agonist (T0901317) revealed a modest induction (2.5-fold) of the mouse brown gene, so named as it is the ortholog of the ABC transporter that determines brown eye color in Drosophila [21,22]. The human ortholog to mouse brown mapped to 2p21, STSL (OMIM: STSL1: 210250/STSL2:618666). At virtually the same time, independent groups identified individuals in multiple kindreds harboring nonsense mutations in either ABCG5 or ABCG8 with sitosterolemia, but not their unaffected family members [22,23]. STSL1 and STSL2 encode a pair of ATP binding cassette (ABC) half transporters in the G-subfamily. They reside on opposite strands of the DNA with initiation codons separated by a mere 374 base pairs. In these early reports, transcripts were restricted to the liver and intestine, the abundance of which increased in response to dietary cholesterol, suggesting the function of the transporters was to oppose intestinal absorption and promote biliary secretion of neutral sterols.
The discovery of ezetimibe as the inhibitor of Neiman–Pick C-1-Like 1 (NPC1L1), and cholesterol absorption was a breakthrough in the clinical management of sitosterolemia [24,25]. Ezetimibe (10 mg/day) was tested as a cholesterol-lowering agent in healthy subjects with moderate hypercholesterolemia. A detailed analysis of plasma sterols revealed that in addition to cholesterol, plasma phytosterols were also reduced, indicating that phytosterols and cholesterol shared a common, ezetimibe-sensitive pathway for absorption, thus suggesting the drug might be effective in the treatment of sitosterolemia (Figure 1 (1)(2)). Ezetimibe was subsequently shown to reduce plasma phytosterols in sitosterolemic subjects by >20% after eight weeks [26]. In a two-year follow-up study, plasma sitosterol and campesterol levels were reduced by 44% and 51%, respectively [27]. It should be noted that phytosterol levels in these subjects remained well above normal. However, the reductions observed with ezetimibe as a single agent or as an adjunctive therapy resolves many of the clinical manifestations of sitosterolemia (reviewed in [28]).
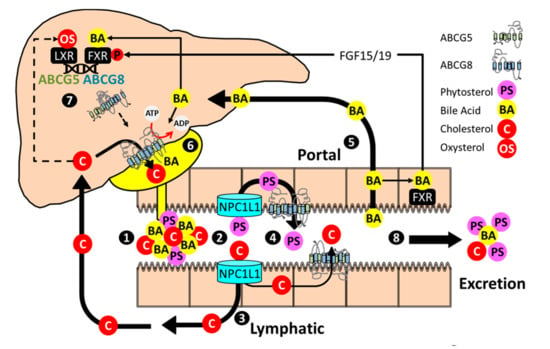
Figure 1.
Enterohepatic sterol flux and regulation of ABCG5 ABCG8. (1) Bile acid micelles facilitate the solubilization of dietary and endogenous sterols in the proximal small intestine. Phospholipids not depicted. (2) NPC1L1 facilitates uptake of cholesterol and phytosterols into intestinal enterocytes (3) Cholesterol is incorporated into chylomicrons, delivered to the plasma compartment through the lymphatic system, and cleared by the liver. ABCG5 ABCG8 also promotes cholesterol secretion into the intestinal lumen. (4) Phytosterols are poorly absorbed and largely returned to the intestinal lumen by ABCG5 ABCG8. (5) Bile acids are reabsorbed in the distal small intestine, stimulate FXR-dependent expression of FGF15/19, and are returned to the liver through the portal system. (6) In the liver, bile acids stimulate ABCG5 ABCG8 catalytic activity and promote the formation of bile acid micelles that serve as acceptors for ABCG5 ABCG8 mediated biliary cholesterol secretion. (7) Cholesterol metabolites (oxysterols), through LXR, and bile acids, through FXR and in cooperation with FGF15/19, activate ABCG5 ABCG8. The half transporters heterodimerize, traffic to the canalicular surface, and promote biliary phytosterol and cholesterol secretion. (8) Excess cholesterol, phytosterols and bile acids that are not absorbed/reabsorbed are eliminated from the body.
2. Animal Models of Sitosterolemia
Three independent mouse models of sitosterolemia have been developed which lack functional Abcg5, Abcg8, or both half transporters [29,30,31]. In each model, the G5G8 heterodimer is absent. These models largely phenocopy one another and share many phenotypes with sitosterolemia in humans. These include elevated plasma and tissue levels of phytosterols, reduced biliary cholesterol, and repression of the cholesterol biosynthetic pathway. Since the discovery of ABCG5 ABCG8, phenotypes in spontaneous rodent models have been attributed to defects in Abcg5 Abcg8 or the accumulation of phytosterols. The Spontaneous hypertensive rat (SHR) harbors a glycine 583 cysteine mutation in Abcg5, which segregates with elevated phytosterol levels in plasma, but not hypertension [32]. Similarly, a premature stop codon in Abcg5 is present in the thrombocytopenia and cardiomyopathy (trac) mouse [33]. Plasma phytosterols and platelet counts were rescued by crossing this strain with mice harboring a human ABCG5 ABCG8 transgene. Mice with a targeted disruption in either Abcg5 or Abcg8 also display platelet dysfunction, effects that are reversed with ezetimibe or low phytosterol-containing diets, respectively [34,35]. Other phenotypes in mice lacking functional G5G8 are reversed by ezetimibe treatment, genetic inactivation of its pharmacological target, Neiman–Pick C1-Like-1 (NPC1L1), or being fed a diet that lacks phytosterols [36,37,38]. Conversely, feeding diets enriched in phytosterols exacerbate phenotypes and results in sudden death [36,39].
Collectively, the available data indicates that the presentation of sitosterolemia in both humans and rodent models is a function of the type and abundance of xenosterols present in the diet that ultimately accumulates in plasma and tissues. This complicates interpretations of ABCG5 ABCG8 physiology with respect to cholesterol metabolism, as phytosterols are known to produce a myriad of biological effects, including disruptions of sterol sensing by sterol receptor element binding protein 2 (SREBP-2), LXR, and the bile acid receptor (farnesoid X receptor, FXR NR1H4) [40,41,42,43].
While sitosterolemia may present with normal or only modestly elevated plasma cholesterol, cholesterol levels are generally lower in mice lacking functional G5G8 than their wild-type counterparts. The precise mechanism accounting for this difference has not been investigated but may be due to the paucity of ApoB containing lipoproteins in plasma in mice compared to humans. An alternative explanation is a species difference in the role of G5G8 in cholesterol absorption. Given its abundant expression in the intestinal epithelium, its role in biliary cholesterol secretion, and the common pathway for cholesterol and phytosterol absorption (NPC1L1), it stands to reason that G5G8 would oppose cholesterol absorption. However, studies in both humans and mice have produced inconsistent results. In both humans and mice, phytosterol absorption is clearly elevated [30,31,44,45]. Sitosterolemics appear on the higher end of the range for cholesterol absorption in humans, a trait that maps to the ABCG5 ABCG8 locus [18,23]. However, the magnitude of the increase is relatively modest compared to the increase in the absorption of phytosterols. Mice lacking G5G8 do not show substantial increases in cholesterol absorption as assessed by the dual isotope method [29,31]. When monitoring the appearance of radiolabeled sterol in lymph, the absence of G5G8 either reduced or increased cholesterol absorption across the intestinal epithelium [46,47,48]. Expression of a human transgene under the control of its own promoter reduced fractional cholesterol absorption by 50% [49]. Consequently, the role of G5G8 in cholesterol absorption remains unclear, and like other phenotypes, may depend on dietary phytosterols.
Deletion of Abcg5 Abcg8 reduces biliary cholesterol secretion by 70–90%. Agents that stimulate biliary cholesterol secretion are generally G5G8-dependent, including LXR and FXR agonists, thyroid hormone, and choleretic agents (diosgenin, tauroursodeoxycholate) [29,48,50,51,52,53,54]. Heterologous expression of Niemann–Pick C2 (NPC2) increases biliary secretion of the protein and promotes G5G8-dependent biliary cholesterol secretion, suggesting a role for this sterol-trafficking protein as a mediator of G5G8 activity or perhaps as a source of, or acceptor for G5G8 substrates [55]. G5G8-independent biliary cholesterol secretion is observed under some experimental conditions, including depletion and overexpression of class B, type-1 scavenger receptor (SR-BI), infusion or feeding high levels of cholate, Atp8b1 deficiency, and in lactating rats [29,56,57,58,59,60]. Some fraction of residual biliary cholesterol secretion in the absence of G5G8 is likely mediated by detergent extraction. The extent to which other enzymes contribute to a G5G8-independent pathway, and if such a pathway might be targeted to increase biliary cholesterol secretion remains unknown.
3. Heterologous Expression of G5G8
The first ABCG5 ABCG8 transgenic strain contained an estimated 14 copies of the human gene, increased biliary cholesterol concentrations five to six-fold, and reduced susceptibility to experimental hypercholesterolemia and atherosclerosis [49,61]. A liver-specific transgenic failed to protect mice from atherosclerosis unless combined with the cholesterol absorption inhibitor ezetimibe [62,63]. The protective effects of G5G8 are presumably due to its role as the mediator of the final step of reverse cholesterol transport (RCT). Pharmacological stimuli of RCT, including ezetimibe and LXR and FXR agonists, require G5G8 to promote fecal neutral sterol loss and macrophage to feces RCT [50,64,65]. However, acute adenoviral-mediated overexpression of hepatic G5G8 fails to stimulate macrophage to feces RCT [56]. Further, adenoviral expression of G5G8 paradoxically increases plasma cholesterol, an effect blocked by ezetimibe [66]. This indicates that a substantial amount of biliary cholesterol is reabsorbed in the small intestine and illustrates the cooperative nature of hepatic and intestinal G5G8 in order to oppose hypercholesterolemia and promote RCT. This, however, is not the case for preventing phytosterolemia as tissue-selective deletion of intestinal or hepatic G5G8 results in only modest elevations in plasma phytosterols [67].
4. Beyond Phytosterols
Given the roles of G5G8 in opposing dietary sterol accumulation and biliary cholesterol secretion, it is unsurprising that both rare and common variants have been associated with plasma cholesterol, non-cholesterol sterols, low-density lipoprotein cholesterol (LDL-C), and atherosclerotic and gallbladder disease across a large number of studies and populations ([68,69,70,71,72,73,74,75,76,77,78,79,80], incomplete list). Other associations are intriguing and include insulin resistance (G8:D19H) and type 2 diabetes (G5:G604E, G8:Y54C) and its renal complications (G8:T400K) [81,82,83]. Mouse models of metabolic syndrome which lack leptin or its receptor have diminished hepatic G5G8 and reduced biliary cholesterol secretion [66,84]. Rescue of G5G8 in these models with chemical chaperones, adenoviral expression of the molecular chaperone GRP78/binding immunoglobulin protein (BiP), or adenoviral expression of G5G8 itself accelerates biliary cholesterol secretion, restores glycemic control, and reduces plasma triglycerides [66,84,85]. Conversely, mice lacking G5G8 are more susceptible to diet-induced obesity, insulin resistance, and hepatic steatosis when maintained on phytosterol-free diets [86]. Collectively, these studies suggest an unappreciated relationship between biliary cholesterol secretion, triglyceride metabolism, and insulin signaling. There also appears to be a role for intestinal G5G8 in the absorption of triglycerides and chylomicron assembly, which may influence metabolic phenotypes, but the underlying mechanism for this phenotype remains unclear [46,47].
Independent of phytosterol accumulation, genome-wide association studies (GWAS) and animal model data support an anti-atherosclerotic role for G5G8. This is presumably due to the combined effects of limiting dietary cholesterol accumulation and promoting RCT. Classically, the final steps of RCT are hepatobiliary secretion of neutral and acidic sterols. However, disruptions in biliary cholesterol secretion do not result in concomitant reductions in cholesterol excretion, indicating the presence of a compensatory, non-biliary pathway which has been labeled transintestinal cholesterol elimination (TICE) [87]. Tissue-specific deletion of G5G8 in the intestine reduced excretion of radiolabeled sterol from the plasma compartment to feces, indicating a role for intestinal G5G8 in cholesterol excretion [67]. The mediators of TICE and the relative contribution of G5G8 to intestinal cholesterol excretion under a variety of pharmacological conditions remain to be fully elucidated. These studies and the potential for therapeutic development have recently been reviewed and are beyond the scope of this discussion [88,89].
5. Transcriptional Regulation of G5G8
ABCG5 ABCG8 is effectively a single gene with a common promoter that regulates expression of both transcripts encoding each half of the transporter. To the best of our knowledge, there are no reports of differential regulation of Abcg5 and Abcg8 transcripts. Transcriptional regulation of Abcg5 Abcg8 by a small molecule LXR agonist (T0901317) precipitated its discovery as the defective gene in sitosterolemia [22]. Expression of both mRNA and protein increases in liver and intestine in response to small molecule LXR agonists and dietary cholesterol, which promotes the accumulation of endogenous LXR agonists, oxysterols (Figure 1 (7)) [22,29,30,31,49,90]. Liver receptor homolog 1 (LRH1), GATA binding factor 4 (GATA-4), and hepatocyte nuclear factor 4α (HNF4α) binding sites map to the 374 base pair intergenic promoter that separates the initiation codons for each protein, the latter of which synergize with LXREs located in distal regions of the gene, to activate the promoter and increase expression of both transcripts [91,92]
Hepatic expression of Abcg5 Abcg8 is also induced by FXR agonists and bile acids, and where examined, in an FXR-dependent fashion (Figure 1 (7)) [54,93,94,95]. However, regulation by bile acid FXR agonists is far more complicated. FXR-mediated activation of Abcg5 Abcg8 requires fibroblast growth factor 15/19 (FGF15/19), which is itself an FXR target gene that is secreted from the ileum in response to bile acids and promotes Src-mediated phosphorylation of hepatic FXR, and FXR binding to the Abcg5 Abcg8 promoter [96]. FNDC5/Irisin is an FXR target gene that increases Abcg5 Abcg8 mRNA in both the livers and intestines of transgenic mice, but the extent to which the endogenous gene plays a role in Abcg5 Abcg8 regulation and sterol homeostasis mice or humans is not known [97]. Whereas cholesterol and its metabolites tend to increase expression of Abcg5 Abcg8 mRNA, agonists for the constitutive androstane receptor (CAR) repress expression of the transporter under conditions of elevated exogenous or endogenously-derived FXR agonists [98,99].
Transcriptional regulation of Abcg5 Abcg8 by LXR and FXR fits well with its central role in opposing the accumulation of excess cholesterol. Expansion of whole body neutral and/or acid sterol pools increases expression [31,54,84,93,94,100,101]. Conversely, blocking absorption of cholesterol or bile acids reduces expression in either liver or intestine [37,101,102,103]. Less clear is the physiological benefit, if any, for alterations in Abcg5 Abcg8 expression by regulators of metabolism. Thyroid hormone increases Abcg5 Abcg8 mRNA, biliary cholesterol secretion, and fecal sterol excretion in both intact and hypophysectomized rats [104]. Hepatic Abcg5 Abcg8 mRNA and protein are upregulated in the absence of insulin signaling in mice, an effect attributed to disinhibition of Forkhead box protein O1 (FOXO-1) [105,106]. The opposite was observed in a type 1 diabetic model in rats [107]. The insulin-sensitizing drug, metformin, increased Abcg5 Abcg8 mRNA and protein, an effect attributed to reduced period 2 occupancy of the ABCG5 ABCG8 promoter and disinhibition of gene expression [108]. Indeed, Abcg5 Abcg8 mRNA exhibits a robust circadian rhythm at the transcriptional level (not observed for protein level, unpublished observation) and hepatic Abcg5 Abcg8 mRNA and biliary cholesterol are reduced in Bmal1-deficient mice [108,109]
Alterations in Abcg5 Abcg8 expression have been reported by a variety of nutritional cues, including upregulation in response elevated n-3 polyunsaturated fatty acids [110,111,112]. While upregulation of ABCG5 ABCG8 is generally observed in high fat diets containing cholesterol and cholesterol-free, high fat diets, a single oral gavage of triacylglycerols robustly repressed intestinal Abcg5 Abcg8 mRNA in mice [113]. In an independent study, suppression of Abcg5 Abcg8 mRNA following high fat, high sucrose feeding was not observed, but the diet used in this study contained both added cholesterol and cholate, and thus hepatic cholesterol was increased five-fold compared to mice fed the control diet [114]. Diets containing high levels of sucrose robustly repressed expression of hepatic, but not intestinal Abcg5 Abcg8 in rats [115]. Collectively, the data suggests that dietary repression of ABCG5 ABCG8 may reflect reductions in the abundance of LXR and FXR agonists rather than active repression by sucrose or triglyceride. Alternatively, differences across studies may be associated with species and strain differences, both of which have been reported for various ligands [116,117]. Diets supplemented with soy protein increased hepatic Abcg5 Abcg8 mRNA in rats [118]. The mechanisms for such an effect is not known but may include modulation of the intestinal microbiota. Germ free mice exhibited elevations in both intestinal and hepatic Abcg5 Abcg8 mRNA relative to specific pathogen free mice in the absence and presence of ezetimibe [119]. Depletion of dietary iron upregulated hepatic Abcg5 Abcg8 mRNA and promotes increased biliary cholesterol secretion [120]. Dietary calcium supplementation was shown to increased intestinal Abcg5 Abcg8 mRNA and fecal neutral sterol excretion in a hamster model of menopause [121].
Female biological sex has long been associated with increased biliary cholesterol. Female mice had modest, but significant increases in biliary cholesterol and ABCG5 ABCG8 mRNA in the human transgenic strain [49]. Ovariectomy was subsequently shown to reduce, and estrogen replacement to increase Abcg5 Abcg8 mRNA across the intestine in independent strains of mice [116,122]. Diosgenin is a choleretic compound with estrogenic properties that increases biliary cholesterol secretion. Its ability to increase biliary cholesterol is largely G5G8-dependent, but reports on its impact on Abcg5 Abcg8 expression are conflicting, showing no change in mice but an increase in both liver and intestine in rats [54,123]
6. Post-Transcriptional Regulation
Less is known about the post-transcriptional regulation of the G5G8. The half transporters are retained within the endoplasmic reticulum (ER) unless co-expressed [124,125]. Formation of the complex appears to be relatively inefficient in cultured cells, is dependent upon the presence of N-linked glycans that reside in the third extracellular loop of each protein, and can be enhanced by the expression of the lectin chaperones, Calnexin and Calreticulin [124,125,126,127]. Using chimeric approaches, the ER-retention motif was localized to the N-terminal, cytosolic domain, but has yet to be defined [128]. Failure to form complexes within the ER results in rapid degradation of each half transporter [127,129]. At the cell surface, the mature G5G8 complex resides within apical membranes of both hepatocytes and enterocytes [124]. There is also evidence of an intracellular, recruitable pool of G5G8 that translocates to the canalicular surface in response to cAMP and in response to diets containing cholate and cholesterol [114,130]. However, the stimuli and signaling pathways involved in intracellular trafficking of G5G8 have yet to be elucidated.
A number of approaches have been utilized to investigate the activity of G5G8. Heterologous expression in HEK293 and dog gall bladder epithelial cells demonstrated G5G8-dependent cholesterol efflux to bile acid micelles, but not HDL or apolipoprotein A1 [131,132]. Native mouse and recombinant human and mouse G5G8 have been purified to varying degrees from liver, rat hepatocytes, Sf9 insect cells, and Pichia pastoris [133,134,135,136]. These studies demonstrated ATP- and magnesium-dependent, vanadate-sensitive ATPase, and sterol transport activity. Various bile acids stimulate ATP hydrolysis. Among the species tested, G5G8 activity was most sensitive to cholate [133]. Perhaps surprisingly, neither cholesterol nor phytosterols stimulated ATPase or sterol transport activity in preparations from Sf9 cells [135,136]. Using inside-out vesicles in this same system, Wang et al. showed that other nucleotides could support sterol transfer, albeit less efficiently [135]
The nucleotide binding sites of G5G8 were proposed, and later confirmed by crystallography, to be comprised of Walker A and B domains of one partner and the signature motif of the other [137,138]. The Walker A and B domains of G8 juxtaposed to the signature motif of G5 were designated nucleotide binding site (NBS) 1. While both NBSs bind 8-Azido ATP, mutations in highly conserved residues within the Walker A and B domain of G5 (NBS2), but not G8 (NBS1), abolished ATP binding and hydrolysis. These findings were confirmed for G5G8-mediated biliary cholesterol secretion by expressing the mutants in G5G8-defecient mice. Domain swapping experiments between G5 and G8 confirmed that ATP hydrolysis in NBS2 is indispensable for activity [139]. G5G8 was crystallized as a heterodimer in lipid bilayers (bicelles) in the presence of cholesterol in the nucleotide free state to a resolution of four angstroms [138]. G5G8 was designated as a Type II Exporter. Key molecular interactions inferred from this structure were validated as essential for cholesterol transport in vivo by expressing recombinant mutants in G5G8-deficient mice. Naturally occurring missense variants and mutants can provide mechanistic insight to protein structure–function. The potential impact of mutations and polymorphisms on G5G8 structure function inferred from the available crystal structure were recently reviewed [140]. However, formal investigations into the impact of missense variants of any type on G5G8 trafficking, stability, and activity have been limited to only a few.
7. Sitosterolemia and/or Familial Hypercholesterolemia
Challenges in the proper diagnosis of sitosterolemia include heterogeneity of the clinical presentation of the disease, the lack of genotype–phenotype correlations, and the inability of clinical laboratory assays to distinguish phytosterols from cholesterol [28,141,142]. Studies among hypercholesterolemic subjects suggest sitosterolemia is significantly underdiagnosed [143,144]. Genome and exome sequence analysis of large populations indicates that carriers of loss of function mutations are far more common than previously thought and are at an elevated risk of coronary artery disease [72,80]. Exome sequence analysis of over 60,000 individuals across multiple populations reveals 57 and 58 predicted loss of function alleles for ABCG5 and ABCG8, respectively (https://gnomad.broadinstitute.org/ (accessed on 3 January 2021) v2.1.1) [145]. This analysis did not include missense variants, 37 of which have been described for sitosterolemia [141]. At the time of the preparation of this review, 619 and 1307 missense variants have been catalogued in dbSNP for ABCG5 and ABCG8, respectively. Most are predicted to be benign, but virtually none of those likely to be pathogenic or of uncertain significance have been experimentally or clinically validated. The number of ABCG5 ABCG8 variants will undoubtedly grow as additional genomes and exomes are sequenced, as will the need for better tools to predict which variants are pathogenic.
An analysis of selected missense mutants of ABCG5 and ABCG8 suggests that the majority of dysfunctional alleles are due to the inability of ABCG5 ABCG8 to heterodimerize and traffic beyond the endoplasmic reticulum [127]. The development of correctors and potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7, OMIM: 602421) and the rescue of mutants of ABCB4, defective in progressive familial intrahepatic cholestasis type 3 (PFIC3, OMIM: 602347), suggest these or perhaps other small molecule chaperones may facilitate maturation and rescue function of G5G8 [146,147]. However, no systematic approach to classify the types of mutations that cause sitosterolemia has been made. We propose a draft of such a classification system for sitosterolemia (Table 1). We based our initial draft on the system established for PFIC3 due to the fact that both are biliary lipid transporters [148]. Our system may need future revision as additional mutations are identified and the impact of these variants/mutants on G5G8 formation, trafficking, and activity are determined. Nonetheless, this classification system provides a framework for characterizing ABCG5 ABCG8 mutants that cause sitosterolemia and a basis for the systematic investigation of compounds that may potentially rescue G5G8 function.

Table 1.
Classification system for experimentally verified sitosterolemia mutations.
8. Conclusions and Future Directions
The last twenty years have revealed a great deal about the role of ABCG5 ABCG8 in cholesterol metabolism and in the defense against the accumulation of dietary xenosterols. The master regulators of neutral and acidic sterol metabolism modulate G5G8 abundance and activity as a component of the integrated machinery that maintains sterol homeostasis. Much, however, remains unknown about the hormonal and intracellular signals that promote G5G8 translocation to the biliary surface and G5G8-mediated cholesterol secretion. Beyond G5G8, the hepatocyte orchestrates the clearance of excess cellular cholesterol by metabolism to bile acid or incorporation into very low density and high-density lipoproteins, either on the surface of the particles or in the hydrophobic core following esterification. What regulates and under what conditions does the intracellular flux of cholesterol favor G5G8-dependent biliary secretion? Investigations of intestinal G5G8 regulation and activity have been more limited than in the liver. In what ways does the regulation of G5G8 in the enterocyte differ, if any, from the hepatocyte? Targeting G5G8 to promote TICE is conceptually attractive to promote RCT, but studies have yet to reveal a route to a potential therapeutic and the molecular mechanisms that mediate TICE remain elusive.
Sequencing of large numbers of genomes and exomes reveals that disease-causing mutants in ABCG5 ABCG8 are significantly more common that previously appreciated. The combination of the required instrumentation, expertise, and cost for routine clinical laboratory analysis of plasma xenosterols is presently impractical. Conversely, genetic screening has become substantially less costly and increasingly common across healthcare systems. Genetic testing may offer a more practical means to identify and diagnose sitosterolemics. Such an approach requires the cataloguing and inclusion of ABCG5 ABCG8 mutants among the various genetic testing platforms in use. A major limitation for such an approach is the lack of validation of suspected and likely pathogenic variants that may disrupt G5G8 function. Frameshift mutations in exon 13 of both ABCG5 and ABCG8 cause sitosterolemia, indicating little tolerance for truncation of the protein. Thus, deletions or frameshifts are expected to be Class I mutants. A significant number of splice donor and acceptor variants have also been identified and are likely pathogenic, but have yet to be formally analyzed. Of the 37 known missense variants, only 13 have been analyzed for maturation. Although most failed to form mature complexes and have been designated as Class II, three retained at least some degree of G5G8 maturation (G5:E146Q, G8:R543S, G8:G574R). Future investigations of the structural and functional impact of these and other missense mutants and variants will advance our understanding of sterol transport, the molecular dynamics of the transporter, and potential therapeutics for sitosterolemia as well as gall bladder and cardiovascular risk reduction.
Author Contributions
A.S. and K.W. crafted the initial draft. G.A.G. revised and edited the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health, grant numbers 1R01DK113625, 1P20GM130456, 5P30GM127211.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhattacharyya, A.K.; Connor, W.E. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J. Clin. Investig. 1974, 53, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: A case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur. J. Clin. Investig. 1980, 10, 27–35. [Google Scholar] [CrossRef]
- Kwiterovich, P.O., Jr.; Bachorik, P.S.; Smith, H.H.; McKusick, V.A.; Connor, W.E.; Teng, B.; Sniderman, A.D. Hyperapobetalipoproteinaemia in two families with xanthomas and phytosterolaemia. Lancet 1981, 1, 466–469. [Google Scholar] [CrossRef]
- Shulman, R.S.; Bhattacharyya, A.K.; Connor, W.E.; Fredrickson, D.S. Beta-sitosterolemia and xanthomatosis. N. Engl. J. Med. 1976, 294, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Wang, C.; Salen, G.; Lam, K.C.; Chan, T.K. Sitosterol and cholesterol metabolism in a patient with coexisting phytosterolemia and cholestanolemia. Metab. Clin. Exp. 1983, 32, 126–133. [Google Scholar] [CrossRef]
- Beaty, T.H.; Kwiterovich, P.O., Jr.; Khoury, M.J.; White, S.; Bachorik, P.S.; Smith, H.H.; Teng, B.; Sniderman, A. Genetic analysis of plasma sitosterol, apoprotein B, and lipoproteins in a large Amish pedigree with sitosterolemia. Am. J. Hum. Genet. 1986, 38, 492–504. [Google Scholar]
- Salen, G.; Horak, I.; Rothkopf, M.; Cohen, J.L.; Speck, J.; Tint, G.S.; Shore, V.; Dayal, B.; Chen, T.; Shefer, S. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J. Lipid Res. 1985, 26, 1126–1133. [Google Scholar] [CrossRef]
- Nguyen, L.B.; Salen, G.; Shefer, S.; Tint, G.S.; Shore, V.; Ness, G.C. Decreased cholesterol biosynthesis in sitosterolemia with xanthomatosis: Diminished mononuclear leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and enzyme protein associated with increased low-density lipoprotein receptor function. Metab. Clin. Exp. 1990, 39, 436–443. [Google Scholar] [CrossRef]
- Bhattacharyya, A.K.; Connor, W.E.; Lin, D.S.; McMurry, M.M.; Shulman, R.S. Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler. Thromb. A J. Vasc. Biol./Am. Heart Assoc. 1991, 11, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Iolascon, A.; Carella, M.; O’Marcaigh, A.S.; Kendra, J.R.; Jowitt, S.N.; Wales, J.K.; Vora, A.; Makris, M.; Manning, N.; et al. Stomatocytic haemolysis and macrothrombocytopenia (Mediterranean stomatocytosis/macrothrombocytopenia) is the haematological presentation of phytosterolaemia. Br. J. Haematol. 2005, 130, 297–309. [Google Scholar] [CrossRef]
- Mushtaq, T.; Wales, J.K.; Wright, N.P. Adrenal insufficiency in phytosterolaemia. Eur. J. Endocrinol. 2007, 157 (Suppl. 1), S61–S65. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, L.; Su, Y.; Wang, G.; Wang, R.; Yu, Z.; Bai, X.; Ruan, C. Specific macrothrombocytopenia/hemolytic anemia associated with sitosterolemia. Am. J. Hematol. 2014, 89, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Bazerbachi, F.; Conboy, E.E.; Mounajjed, T.; Watt, K.D.; Babovic-Vuksanovic, D.; Patel, S.B.; Kamath, P.S. Cryptogenic Cirrhosis and Sitosterolemia: A Treatable Disease If Identified but Fatal If Missed. Ann. Hepatol. 2017, 16, 970–978. [Google Scholar] [CrossRef]
- Salen, G.; Shore, V.; Tint, G.S.; Forte, T.; Shefer, S.; Horak, I.; Horak, E.; Dayal, B.; Nguyen, L.; Batta, A.K.; et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J. Lipid Res. 1989, 30, 1319–1330. [Google Scholar] [CrossRef]
- Cobb, M.M.; Salen, G.; Tint, G.S. Comparative effect of dietary sitosterol on plasma sterols and cholesterol and bile acid synthesis in a sitosterolemic homozygote and heterozygote subject. J. Am. Coll. Nutr. 1997, 16, 605–613. [Google Scholar]
- Nguyen, L.B.; Cobb, M.; Shefer, S.; Salen, G.; Ness, G.C.; Tint, G.S. Regulation of cholesterol biosynthesis in sitosterolemia: Effects of lovastatin, cholestyramine, and dietary sterol restriction. J. Lipid Res. 1991, 32, 1941–1948. [Google Scholar] [CrossRef]
- Patel, S.B.; Honda, A.; Salen, G. Sitosterolemia: Exclusion of genes involved in reduced cholesterol biosynthesis. J. Lipid Res. 1998, 39, 1055–1061. [Google Scholar] [CrossRef]
- Patel, S.B.; Salen, G.; Hidaka, H.; Kwiterovich, P.O.; Stalenhoef, A.F.; Miettinen, T.A.; Grundy, S.M.; Lee, M.H.; Rubenstein, J.S.; Polymeropoulos, M.H.; et al. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J. Clin. Investig. 1998, 102, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef]
- Ewart, G.D.; Cannell, D.; Cox, G.B.; Howells, A.J. Mutational analysis of the traffic ATPase (ABC) transporters involved in uptake of eye pigment precursors in Drosophila melanogaster. Implications for structure-function relationships. J. Biol. Chem. 1994, 269, 10370–10377. [Google Scholar] [CrossRef]
- Berge, K.E.; Tian, H.; Graf, G.A.; Yu, L.; Grishin, N.V.; Schultz, J.; Kwiterovich, P.; Shan, B.; Barnes, R.; Hobbs, H.H. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 2000, 290, 1771–1775. [Google Scholar] [CrossRef]
- Lee, M.H.; Lu, K.; Hazard, S.; Yu, H.; Shulenin, S.; Hidaka, H.; Kojima, H.; Allikmets, R.; Sakuma, N.; Pegoraro, R.; et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 2001, 27, 79–83. [Google Scholar] [CrossRef]
- Van Heek, M.; France, C.F.; Compton, D.S.; McLeod, R.L.; Yumibe, N.P.; Alton, K.B.; Sybertz, E.J.; Davis, H.R., Jr. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J. Pharm. Exp. Ther. 1997, 283, 157–163. [Google Scholar]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [PubMed]
- Salen, G.; von Bergmann, K.; Lutjohann, D.; Kwiterovich, P.; Kane, J.; Patel, S.B.; Musliner, T.; Stein, P.; Musser, B.; Multicenter Sitosterolemia Study Group. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation 2004, 109, 966–971. [Google Scholar] [CrossRef]
- Lutjohann, D.; von Bergmann, K.; Sirah, W.; Macdonell, G.; Johnson-Levonas, A.O.; Shah, A.; Lin, J.; Sapre, A.; Musliner, T. Long-term efficacy and safety of ezetimibe 10 mg in patients with homozygous sitosterolemia: A 2-year, open-label extension study. Int J. Clin. Pract. 2008, 62, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Escola-Gil, J.C.; Quesada, H.; Julve, J.; Martin-Campos, J.M.; Cedo, L.; Blanco-Vaca, F. Sitosterolemia: Diagnosis, investigation, and management. Curr. Atheroscler. Rep. 2014, 16, 424. [Google Scholar] [CrossRef]
- Plosch, T.; Bloks, V.W.; Terasawa, Y.; Berdy, S.; Siegler, K.; Van Der Sluijs, F.; Kema, I.P.; Groen, A.K.; Shan, B.; Kuipers, F.; et al. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology 2004, 126, 290–300. [Google Scholar] [CrossRef]
- Klett, E.L.; Lu, K.; Kosters, A.; Vink, E.; Lee, M.H.; Altenburg, M.; Shefer, S.; Batta, A.K.; Yu, H.; Chen, J.; et al. A mouse model of sitosterolemia: Absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004, 2, 5. [Google Scholar] [CrossRef]
- Yu, L.; Hammer, R.E.; Li-Hawkins, J.; Von Bergmann, K.; Lutjohann, D.; Cohen, J.C.; Hobbs, H.H. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA 2002, 99, 16237–16242. [Google Scholar] [CrossRef]
- Chen, J.; Batta, A.; Zheng, S.; Fitzgibbon, W.R.; Ullian, M.E.; Yu, H.; Tso, P.; Salen, G.; Patel, S.B. The missense mutation in Abcg5 gene in spontaneously hypertensive rats (SHR) segregates with phytosterolemia but not hypertension. BMC Genet. 2005, 6, 40. [Google Scholar]
- Chase, T.H.; Lyons, B.L.; Bronson, R.T.; Foreman, O.; Donahue, L.R.; Burzenski, L.M.; Gott, B.; Lane, P.; Harris, B.; Ceglarek, U.; et al. The mouse mutation “thrombocytopenia and cardiomyopathy” (trac) disrupts Abcg5: A spontaneous single gene model for human hereditary phytosterolemia/sitosterolemia. Blood 2010, 115, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.K.; Drayer, A.L.; Bloks, V.W.; Blom, N.; Olthof, S.G.; Sauer, P.J.; de Haan, G.; Kema, I.P.; Vellenga, E.; Kuipers, F. Plant sterols cause macrothrombocytopenia in a mouse model of sitosterolemia. J. Biol. Chem. 2008, 283, 6281–6287. [Google Scholar] [CrossRef]
- Kanaji, T.; Kanaji, S.; Montgomery, R.R.; Patel, S.B.; Newman, P.J. Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of sitosterolemia. Blood 2013, 122, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Solca, C.; Tint, G.S.; Patel, S.B. Dietary xenosterols lead to infertility and loss of abdominal adipose tissue in sterolin-deficient mice. J. Lipid Res. 2013, 54, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; von Bergmann, K.; Lutjohann, D.; Hobbs, H.H.; Cohen, J.C. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J. Lipid Res. 2005, 46, 1739–1744. [Google Scholar] [CrossRef]
- Tang, W.; Ma, Y.; Jia, L.; Ioannou, Y.A.; Davies, J.P.; Yu, L. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J. Lipid Res. 2009, 50, 293–300. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, A.L.; Alger, H.M.; Sawyer, J.K.; Kelley, K.L.; Kock, N.D.; Brown, J.M.; Temel, R.E.; Rudel, L.L. Phytosterol feeding causes toxicity in ABCG5/G8 knockout mice. Am. J. Pathol. 2013, 182, 1131–1138. [Google Scholar] [CrossRef]
- Yang, C.; McDonald, J.G.; Patel, A.; Zhang, Y.; Umetani, M.; Xu, F.; Westover, E.J.; Covey, D.F.; Mangelsdorf, D.J.; Cohen, J.C.; et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 2006, 281, 27816–27826. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.A.; Taylor, O.A.; Prendergast, D.R.; Zimmerman, T.L.; Von Furstenberg, R.; Moore, D.D.; Karpen, S.J. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr. Res. 2007, 62, 301–306. [Google Scholar] [CrossRef]
- Sabeva, N.S.; McPhaul, C.M.; Li, X.; Cory, T.J.; Feola, D.J.; Graf, G.A. Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. J. Nutr. Biochem. 2011, 22, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Nichols, J.A.; Mensink, R.P. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef]
- Salen, G.; Tint, G.S.; Shefer, S.; Shore, V.; Nguyen, L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler. Thromb. A J. Vasc. Biol. Am. Heart Assoc. 1992, 12, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D.; Björkhem, I.; Ose, L. Phytosterolaemia in a Norwegian family: Diagnosis and characterization of the first Scandinavian case. Scand. J. Clin. Lab. Investig. 1996, 56, 229–240. [Google Scholar] [CrossRef]
- Zhang, L.S.; Xu, M.; Yang, Q.; Lou, D.; Howles, P.N.; Tso, P. ABCG5/G8 deficiency in mice reduces dietary triacylglycerol and cholesterol transport into the lymph. Lipids 2015, 50, 371–379. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Sawyer, J.K.; Kelley, K.L.; Davis, M.A.; Kent, C.R.; Rudel, L.L. ACAT2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: Evidence from thoracic lymph duct cannulation. J. Lipid Res. 2012, 53, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Patel, S.B.; Carey, M.C.; Wang, D.Q. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(-/-) mice. Hepatology 2007, 45, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li-Hawkins, J.; Hammer, R.E.; Berge, K.E.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Investig. 2002, 110, 671–680. [Google Scholar] [CrossRef]
- Jakulj, L.; Vissers, M.N.; van Roomen, C.P.; van der Veen, J.N.; Vrins, C.L.J.; Kunne, C.; Stellaard, F.; Kastelein, J.J.P.; Groen, A.K. Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett. 2010, 584, 3625–3628. [Google Scholar] [CrossRef]
- de Boer, J.F.; Schonewille, M.; Boesjes, M.; Wolters, H.; Bloks, V.W.; Bos, T.; van Dijk, T.H.; Jurdzinski, A.; Boverhof, R.; Wolters, J.C.; et al. Intestinal Farnesoid X Receptor Controls Transintestinal Cholesterol Excretion in Mice. Gastroenterology 2017, 152, 1126–1138.e6. [Google Scholar] [CrossRef] [PubMed]
- Bonde, Y.; Plosch, T.; Kuipers, F.; Angelin, B.; Rudling, M. Stimulation of murine biliary cholesterol secretion by thyroid hormone is dependent on a functional ABCG5/G8 complex. Hepatology 2012, 56, 1828–1837. [Google Scholar] [CrossRef]
- Kosters, A.; Frijters, R.J.; Kunne, C.; Vink, E.; Schneiders, M.S.; Schaap, F.G.; Nibbering, C.P.; Patel, S.B.; Groen, A.K. Diosgenin-induced biliary cholesterol secretion in mice requires Abcg8. Hepatology 2005, 41, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gupta, S.; Xu, F.; Liverman, A.D.; Moschetta, A.; Mangelsdorf, D.J.; Repa, J.J.; Hobbs, H.H.; Cohen, J.C. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J. Biol. Chem. 2005, 280, 8742–8747. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Takada, T.; Yoshikado, T.; Shoda, J.; Suzuki, H. NPC2 regulates biliary cholesterol secretion via stimulation of ABCG5/G8-mediated cholesterol transport. Gastroenterology 2011, 140, 1664–1674. [Google Scholar] [CrossRef]
- Dikkers, A.; de Boer, J.F.; Groen, A.K.; Tietge, U.J. Hepatic ABCG5/G8 overexpression substantially increases biliary cholesterol secretion but does not impact in vivo macrophage-to-feces RCT. Atherosclerosis 2015, 243, 402–406. [Google Scholar] [CrossRef]
- Coy, D.J.; Wooton-Kee, C.R.; Yan, B.; Sabeva, N.; Su, K.; Graf, G.; Vore, M. ABCG5/ABCG8-independent biliary cholesterol excretion in lactating rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G228–G235. [Google Scholar] [CrossRef] [PubMed]
- Groen, A.; Kunne, C.; Jongsma, G.; van den Oever, K.; Mok, K.S.; Petruzzelli, M.; Vrins, C.L.; Bull, L.; Paulusma, C.C.; Oude Elferink, R.P. Abcg5/8 independent biliary cholesterol excretion in Atp8b1-deficient mice. Gastroenterology 2008, 134, 2091–2100. [Google Scholar] [CrossRef]
- Wiersma, H.; Gatti, A.; Nijstad, N.; Oude Elferink, R.P.; Kuipers, F.; Tietge, U.J. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology 2009, 50, 1263–1272. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, X.; Patel, S.B.; Wang, D.Q. Evidence that the adenosine triphosphate-binding cassette G5/G8-independent pathway plays a determinant role in cholesterol gallstone formation in mice. Hepatology 2016, 64, 853–864. [Google Scholar] [CrossRef]
- Wilund, K.R.; Yu, L.; Xu, F.; Hobbs, H.H.; Cohen, J.C. High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr-/-mice. J. Lipid Res. 2004, 45, 1429–1436. [Google Scholar] [CrossRef]
- Wu, J.E.; Basso, F.; Shamburek, R.D.; Amar, M.J.; Vaisman, B.; Szakacs, G.; Joyce, C.; Tansey, T.; Freeman, L.; Paigen, B.J.; et al. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J. Biol. Chem. 2004, 279, 22913–22925. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.; Freeman, L.A.; Ko, C.; Joyce, C.; Amar, M.J.; Shamburek, R.D.; Tansey, T.; Thomas, F.; Wu, J.; Paigen, B.; et al. Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J. Lipid Res. 2007, 48, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Rotllan, N.; Fievet, C.; Roig, R.; Blanco-Vaca, F.; Escola-Gil, J.C. Liver X receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J. Lipid Res. 2008, 49, 1904–1911. [Google Scholar] [CrossRef]
- Altemus, J.B.; Patel, S.B.; Sehayek, E. Liver-specific induction of Abcg5 and Abcg8 stimulates reverse cholesterol transport in response to ezetimibe treatment. Metab. Clin. Exp. 2014, 63, 1334–1341. [Google Scholar] [CrossRef]
- Su, K.; Sabeva, N.S.; Wang, Y.; Liu, X.; Lester, J.D.; Liu, J.; Liang, S.; Graf, G.A. Acceleration of biliary cholesterol secretion restores glycemic control and alleviates hypertriglyceridemia in obese db/db mice. Arter. Thromb. Vasc. Biol. 2014, 34, 26–33. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Mitsche, M.A.; Lutjohann, D.; Cohen, J.C.; Xie, X.S.; Hobbs, H.H. Relative roles of ABCG5/ABCG8 in liver and intestine. J. Lipid Res. 2015, 56, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.E.; von Bergmann, K.; Lutjohann, D.; Guerra, R.; Grundy, S.M.; Hobbs, H.H.; Cohen, J.C. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 2002, 43, 486–494. [Google Scholar] [CrossRef]
- Buch, S.; Schafmayer, C.; Volzke, H.; Becker, C.; Franke, A.; von Eller-Eberstein, H.; Kluck, C.; Bassmann, I.; Brosch, M.; Lammert, F.; et al. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat. Genet. 2007, 39, 995–999. [Google Scholar] [CrossRef]
- Grunhage, F.; Acalovschi, M.; Tirziu, S.; Walier, M.; Wienker, T.F.; Ciocan, A.; Mosteanu, O.; Sauerbruch, T.; Lammert, F. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology 2007, 46, 793–801. [Google Scholar] [CrossRef]
- Stender, S.; Frikke-Schmidt, R.; Nordestgaard, B.G.; Tybjaerg-Hansen, A. The ABCG5/8 cholesterol transporter and myocardial infarction versus gallstone disease. J. Am. Coll. Cardiol. 2014, 63, 2121–2128. [Google Scholar] [CrossRef]
- Helgadottir, A.; Thorleifsson, G.; Alexandersson, K.F.; Tragante, V.; Thorsteinsdottir, M.; Eiriksson, F.F.; Gretarsdottir, S.; Bjornsson, E.; Magnusson, O.; Sveinbjornsson, G.; et al. Genetic variability in the absorption of dietary sterols affects the risk of coronary artery disease. Eur. Heart J. 2020, 41, 2618–2628. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Cai, Q.; Chen, E.Z. Association of three common single nucleotide polymorphisms of ATP binding cassette G8 gene with gallstone disease: A meta-analysis. PLoS ONE 2014, 9, e87200. [Google Scholar]
- Viturro, E.; de Oya, M.; Lasuncion, M.A.; Gorgojo, L.; Moreno, J.M.; Benavente, M.; Cano, B.; Garces, C. Cholesterol and saturated fat intake determine the effect of polymorphisms at ABCG5/ABCG8 genes on lipid levels in children. Genet. Med. 2006, 8, 594–599. [Google Scholar] [CrossRef]
- Jakulj, L.; Vissers, M.N.; Tanck, M.W.; Hutten, B.A.; Stellaard, F.; Kastelein, J.J.; Dallinga-Thie, G.M. ABCG5/G8 polymorphisms and markers of cholesterol metabolism: Systematic review and meta-analysis. J. Lipid Res. 2010, 51, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- von Kampen, O.; Buch, S.; Nothnagel, M.; Azocar, L.; Molina, H.; Brosch, M.; Erhart, W.; von Schonfels, W.; Egberts, J.; Seeger, M.; et al. Genetic and functional identification of the likely causative variant for cholesterol gallstone disease at the ABCG5/8 lithogenic locus. Hepatology 2013, 57, 2407–2417. [Google Scholar] [CrossRef]
- Ma, L.; Yang, J.; Runesha, H.B.; Tanaka, T.; Ferrucci, L.; Bandinelli, S.; Da, Y. Genome-wide association analysis of total cholesterol and high-density lipoprotein cholesterol levels using the Framingham heart study data. BMC Med. Genet. 2010, 11, 55. [Google Scholar] [CrossRef]
- Li, Q.; Yin, R.X.; Wei, X.L.; Yan, T.T.; Aung, L.H.; Wu, D.F.; Wu, J.Z.; Lin, W.X.; Liu, C.W.; Pan, S.L. ATP-binding cassette transporter G5 and G8 polymorphisms and several environmental factors with serum lipid levels. PLoS ONE 2012, 7, e37972. [Google Scholar] [CrossRef]
- Wu, G.; Li, G.B.; Yao, M.; Zhang, D.Q.; Dai, B.; Ju, C.J.; Han, M. ABCG5/8 variants are associated with susceptibility to coronary heart disease. Mol. Med. Rep. 2014, 9, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Emdin, C.A.; Won, H.H.; Peloso, G.M.; Natarajan, P.; Ardissino, D.; Danesh, J.; Schunkert, H.; Correa, A.; Bown, M.J.; et al. Heterozygous ATP-binding Cassette Transporter G5 Gene Deficiency and Risk of Coronary Artery Disease. Circ. Genom. Precis. Med. 2020, 13, 417–423. [Google Scholar] [CrossRef]
- Chen, Z.C.; Shin, S.J.; Kuo, K.K.; Lin, K.D.; Yu, M.L.; Hsiao, P.J. Significant association of ABCG8:D19H gene polymorphism with hypercholesterolemia and insulin resistance. J. Hum. Genet. 2008, 53, 757–763. [Google Scholar] [CrossRef][Green Version]
- Gok, O.; Karaali, Z.E.; Acar, L.; Kilic, U.; Ergen, A. ABCG5 and ABCG8 gene polymorphisms in type 2 diabetes mellitus in the Turkish population. Can. J. Diabetes 2015, 39, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, A.; Fatima, S.; Lamri, A.; Bellili-Munoz, N.; Halimi, J.M.; Saulnier, P.J.; Hadjadj, S.; Velho, G.; Marre, M.; Roussel, R.; et al. ABCG8 polymorphisms and renal disease in type 2 diabetic patients. Metab. Clin. Exp. 2015, 64, 713–719. [Google Scholar] [CrossRef]
- Sabeva, N.S.; Rouse, E.J.; Graf, G.A. Defects in the leptin axis reduce abundance of the ABCG5-ABCG8 sterol transporter in liver. J. Biol. Chem. 2007, 282, 22397–22405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, K.; Sabeva, N.S.; Ji, A.; van der Westhuyzen, D.R.; Foufelle, F.; Gao, X.; Graf, G.A. GRP78 rescues the ABCG5 ABCG8 sterol transporter in db/db mice. Metab. Clin. Exp. 2015, 64, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Sabeva, N.S.; Liu, J.; Wang, Y.; Bhatnagar, S.; van der Westhuyzen, D.R.; Graf, G.A. The ABCG5 ABCG8 sterol transporter opposes the development of fatty liver disease and loss of glycemic control independently of phytosterol accumulation. J. Biol. Chem. 2012, 287, 28564–28575. [Google Scholar] [CrossRef]
- Van der Velde, A.E.; Vrins, C.L.J.; van den Oever, K.; Kunne, C.; Oude Elferink, R.P.J.; Kuipers, F.; Groen, A.K. Direct Intestinal Cholesterol Secretion Contributes Significantly to Total Fecal Neutral Sterol Excretion in Mice. Gastroenterology 2007, 133, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Grefhorst, A.; Verkade, H.J.; Groen, A.K. The TICE Pathway: Mechanisms and Lipid-Lowering Therapies. Methodist Debakey Cardiovasc. J. 2019, 15, 70–76. [Google Scholar]
- Nakano, T.; Inoue, I.; Murakoshi, T. A Newly Integrated Model for Intestinal Cholesterol Absorption and Efflux Reappraises How Plant Sterol Intake Reduces Circulating Cholesterol Levels. Nutrients 2019, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Berge, K.E.; Pomajzl, C.; Richardson, J.A.; Hobbs, H.; Mangelsdorf, D.J. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002, 277, 18793–18800. [Google Scholar] [CrossRef]
- Freeman, L.A.; Kennedy, A.; Wu, J.; Bark, S.; Remaley, A.T.; Santamarina-Fojo, S.; Brewer, H.B., Jr. The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J. Lipid Res. 2004, 45, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Sumi, K.; Tanaka, T.; Uchida, A.; Magoori, K.; Urashima, Y.; Ohashi, R.; Ohguchi, H.; Okamura, M.; Kudo, H.; Daigo, K.; et al. Cooperative interaction between hepatocyte nuclear factor 4 alpha and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Mol. Cell Biol. 2007, 27, 4248–4260. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Matozel, M.; Boehme, S.; Kong, B.; Nilsson, L.M.; Guo, G.; Ellis, E.; Chiang, J.Y. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 2011, 53, 996–1006. [Google Scholar] [CrossRef]
- Wang, J.; Einarsson, C.; Murphy, C.; Parini, P.; Bjorkhem, I.; Gafvels, M.; Eggertsen, G. Studies on LXR- and FXR-mediated effects on cholesterol homeostasis in normal and cholic acid-depleted mice. J. Lipid Res. 2006, 47, 421–430. [Google Scholar] [CrossRef]
- Liu, J.; Lu, H.; Lu, Y.F.; Lei, X.; Cui, J.Y.; Ellis, E.; Strom, S.C.; Klaassen, C.D. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol. Sci. 2014, 141, 538–546. [Google Scholar] [CrossRef]
- Byun, S.; Jung, H.; Chen, J.; Kim, Y.C.; Kim, D.H.; Kong, B.; Guo, G.; Kemper, B.; Kemper, J.K. Phosphorylation of hepatic farnesoid X receptor by FGF19 signaling-activated Src maintains cholesterol levels and protects from atherosclerosis. J. Biol. Chem. 2019, 294, 8732–8744. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, J.; Wu, T.; Kuang, J.; Liu, Q.; Cheng, S.; Pu, S.; Chen, L.; Li, R.; Li, Y.; et al. Irisin Is Controlled by Farnesoid X Receptor and Regulates Cholesterol Homeostasis. Front. Pharm. 2019, 10, 548. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, M.; Liu, Q.; Kuang, J.; Shen, J.; Pu, S.; Chen, L.; Li, H.; Wu, T.; Li, R.; et al. Activation of Constitutive Androstane Receptor Prevents Cholesterol Gallstone Formation. Am. J. Pathol. 2017, 187, 808–818. [Google Scholar] [CrossRef]
- Sberna, A.L.; Assem, M.; Gautier, T.; Grober, J.; Guiu, B.; Jeannin, A.; Pais de Barros, J.P.; Athias, A.; Lagrost, L.; Masson, D. Constitutive androstane receptor activation stimulates faecal bile acid excretion and reverse cholesterol transport in mice. J. Hepatol. 2011, 55, 154–161. [Google Scholar] [CrossRef]
- Gooijert, K.E.; Havinga, R.; Wolters, H.; Wang, R.; Ling, V.; Tazuma, S.; Verkade, H.J. The mechanism of increased biliary lipid secretion in mice with genetic inactivation of bile salt export pump. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G450–G457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Pijut, S.S.; Li, J.; Horn, J.; Bradford, E.M.; Leggas, M.; Barrett, T.A.; Graf, G.A. The combination of ezetimibe and ursodiol promotes fecal sterol excretion and reveals a G5G8-independent pathway for cholesterol elimination. J. Lipid Res. 2015, 56, 810–820. [Google Scholar] [CrossRef]
- Wang, H.H.; Portincasa, P.; Mendez-Sanchez, N.; Uribe, M.; Wang, D.Q. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 2008, 134, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Kamisako, T.; Ogawa, H.; Yamamoto, K. Effect of cholesterol, cholic acid and cholestyramine administration on the intestinal mRNA expressions related to cholesterol and bile acid metabolism in the rat. J. Gastroenterol. Hepatol. 2007, 22, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Galman, C.; Bonde, Y.; Matasconi, M.; Angelin, B.; Rudling, M. Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by thyroid hormone. Gastroenterology 2008, 134, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Aleksunes, L.M.; Xu, J.; Lin, E.; Wen, X.; Goedken, M.J.; Slitt, A.L. Pregnancy represses induction of efflux transporters in livers of type I diabetic mice. Pharm. Res. 2013, 30, 2209–2220. [Google Scholar] [CrossRef]
- Biddinger, S.B.; Haas, J.T.; Yu, B.B.; Bezy, O.; Jing, E.; Zhang, W.; Unterman, T.G.; Carey, M.C.; Kahn, C.R. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 2008, 14, 778–782. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Liu, H.; Guo, H.; Zhang, M.; Mei, D.; Liu, C.; He, L.; Liu, L.; Liu, X. Impaired hepatic and intestinal ATP-binding cassette transporter G5/8 was associated with high exposure of beta-sitosterol and the potential risks to blood-brain barrier integrity in diabetic rats. J. Pharm. Pharm. 2014, 66, 428–436. [Google Scholar] [CrossRef]
- Molusky, M.M.; Hsieh, J.; Lee, S.X.; Ramakrishnan, R.; Tascau, L.; Haeusler, R.A.; Accili, D.; Tall, A.R. Metformin and AMP Kinase Activation Increase Expression of the Sterol Transporters ABCG5/8 (ATP-Binding Cassette Transporter G5/G8) With Potential Antiatherogenic Consequences. Arter. Thromb. Vasc. Biol. 2018, 38, 1493–1503. [Google Scholar] [CrossRef]
- Pan, X.; Bradfield, C.A.; Hussain, M.M. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat. Commun. 2016, 7, 13011. [Google Scholar] [CrossRef]
- Nishimoto, T.; Pellizzon, M.A.; Aihara, M.; Stylianou, I.M.; Billheimer, J.T.; Rothblat, G.; Rader, D.J. Fish oil promotes macrophage reverse cholesterol transport in mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1502–1508. [Google Scholar] [CrossRef]
- Kamisako, T.; Tanaka, Y.; Ikeda, T.; Yamamoto, K.; Ogawa, H. Dietary fish oil regulates gene expression of cholesterol and bile acid transporters in mice. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2012, 42, 321–326. [Google Scholar] [CrossRef]
- Kim, E.H.; Bae, J.S.; Hahm, K.B.; Cha, J.Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem. Pharm. 2012, 84, 1359–1365. [Google Scholar] [CrossRef]
- De Vogel-van den Bosch, H.M.; de Wit, N.J.; Hooiveld, G.J.; Vermeulen, H.; van der Veen, J.N.; Houten, S.M.; Kuipers, F.; Muller, M.; van der Meer, R. A cholesterol-free, high-fat diet suppresses gene expression of cholesterol transporters in murine small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1171–G1180. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Hashizume, T.; Morioka, H.; Sadamitsu, S.; Ikari, A.; Miwa, M.; Sugatani, J. Diet-induced lipid accumulation in liver enhances ATP-binding cassette transporter g5/g8 expression in bile canaliculi. Drug Metab. Pharm. 2011, 26, 442–450. [Google Scholar] [CrossRef]
- Apro, J.; Beckman, L.; Angelin, B.; Rudling, M. Influence of dietary sugar on cholesterol and bile acid metabolism in the rat: Marked reduction of hepatic Abcg5/8 expression following sucrose ingestion. Biochem. Biophys. Res. Commun. 2015, 461, 592–597. [Google Scholar] [CrossRef]
- Duan, L.P.; Wang, H.H.; Ohashi, A.; Wang, D.Q. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1l1 in cholesterol absorption in mice: Gender and age effects. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G269–G276. [Google Scholar] [CrossRef] [PubMed]
- Dieter, M.Z.; Maher, J.M.; Cheng, X.; Klaassen, C.D. Expression and regulation of the sterol half-transporter genes ABCG5 and ABCG8 in rats. Comp. Biochem. Physiol. C Toxicol. Pharm. 2004, 139, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Kudo, M.; Hamada, T.; Nagao, K.; Oshiro, Y.; Kato, M.; Sugawara, T.; Yamahira, T.; Ito, H.; Tamaru, S.; et al. Dietary soy protein isolate and its undigested high molecular fraction upregulate hepatic ATP-binding cassette transporter G5 and ATP-binding cassette transporter G8 mRNA and increase biliary secretion of cholesterol in rats. J. Nutr. Sci. Vitaminol. 2009, 55, 252–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, C.Y.; Sun, W.W.; Ma, Y.; Zhu, H.; Yang, P.; Wei, H.; Zeng, B.H.; Zhang, Q.; Liu, Y.; Li, W.X.; et al. Microbiota prevents cholesterol loss from the body by regulating host gene expression in mice. Sci. Rep. 2015, 5, 10512. [Google Scholar] [CrossRef]
- Prasnicka, A.; Cermanova, J.; Hroch, M.; Dolezelova, E.; Rozkydalova, L.; Smutny, T.; Carazo, A.; Chladek, J.; Lenicek, M.; Nachtigal, P.; et al. Iron depletion induces hepatic secretion of biliary lipids and glutathione in rats. Biochim. Biophys. Acta 2017, 1862, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.Y.; Yang, N.; Jiao, R.; Peng, C.; Guan, L.; Huang, Y.; Chen, Z.Y. Dietary calcium decreases plasma cholesterol by down-regulation of intestinal Niemann-Pick C1 like 1 and microsomal triacylglycerol transport protein and up-regulation of CYP7A1 and ABCG 5/8 in hamsters. Mol. Nutr. Food Res. 2011, 55, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, J.; Chao, Y.; Bi, Y.; Zhang, W.; Zhang, Y.; Ji, T.; Fu, Y.; Chen, Q.; Zhang, Q.; et al. beta-estradiol adjusts intestinal function via ERbeta and GPR30 mediated PI3K/AKT signaling activation to alleviate postmenopausal dyslipidemia. Biochem. Pharm. 2020, 180, 114134. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Shi, J.; Yu, Y.; Lu, H.; Yu, L.; Liu, Y.; Zhang, F. Diosgenin regulates cholesterol metabolism in hypercholesterolemic rats by inhibiting NPC1L1 and enhancing ABCG5 and ABCG8. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1124–1133. [Google Scholar] [CrossRef]
- Graf, G.A.; Li, W.P.; Gerard, R.D.; Gelissen, I.; White, A.; Cohen, J.C.; Hobbs, H.H. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J. Clin. Investig. 2002, 110, 659–669. [Google Scholar] [CrossRef]
- Graf, G.A.; Yu, L.; Li, W.P.; Gerard, R.; Tuma, P.L.; Cohen, J.C.; Hobbs, H.H. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 2003, 278, 48275–48282. [Google Scholar] [CrossRef] [PubMed]
- Okiyoneda, T.; Kono, T.; Niibori, A.; Harada, K.; Kusuhara, H.; Takada, T.; Shuto, T.; Suico, M.A.; Sugiyama, Y.; Kai, H. Calreticulin facilitates the cell surface expression of ABCG5/G8. Biochem. Biophys. Res. Commun. 2006, 347, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Graf, G.A.; Cohen, J.C.; Hobbs, H.H. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J. Biol. Chem. 2004, 279, 24881–24888. [Google Scholar] [CrossRef]
- Hirata, T.; Okabe, M.; Kobayashi, A.; Ueda, K.; Matsuo, M. Molecular mechanisms of subcellular localization of ABCG5 and ABCG8. Biosci. Biotechnol. Biochem. 2009, 73, 619–626. [Google Scholar] [CrossRef]
- Suzuki, S.; Shuto, T.; Sato, T.; Kaneko, M.; Takada, T.; Suico, M.A.; Cyr, D.M.; Suzuki, H.; Kai, H. Inhibition of post-translational N-glycosylation by HRD1 that controls the fate of ABCG5/8 transporter. Sci. Rep. 2014, 4, 4258. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Yasui, K.; Hashizume, T.; Suto, A.; Mori, A.; Murata, Y.; Yamaguchi, M.; Ikari, A.; Sugatani, J. Involvement of a cyclic adenosine monophosphate-dependent signal in the diet-induced canalicular trafficking of adenosine triphosphate-binding cassette transporter g5/g8. Hepatology 2015, 62, 1215–1226. [Google Scholar] [CrossRef]
- Tachibana, S.; Hirano, M.; Hirata, T.; Matsuo, M.; Ikeda, I.; Ueda, K.; Sato, R. Cholesterol and plant sterol efflux from cultured intestinal epithelial cells is mediated by ATP-binding cassette transporters. Biosci. Biotechnol. Biochem. 2007, 71, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Vrins, C.; Vink, E.; Vandenberghe, K.E.; Frijters, R.; Seppen, J.; Groen, A.K. The sterol transporting heterodimer ABCG5/ABCG8 requires bile salts to mediate cholesterol efflux. FEBS Lett. 2007, 581, 4616–4620. [Google Scholar] [CrossRef]
- Johnson, B.J.; Lee, J.Y.; Pickert, A.; Urbatsch, I.L. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry 2010, 49, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, D.W.; Lei, Y.; Xu, F.; Cohen, J.C.; Hobbs, H.H.; Xie, X.S. Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG8. Biochemistry 2008, 47, 5194–5204. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Sun, F.; Zhang, D.W.; Ma, Y.; Xu, F.; Belani, J.D.; Cohen, J.C.; Hobbs, H.H.; Xie, X.S. Sterol transfer by ABCG5 and ABCG8: In vitro assay and reconstitution. J. Biol. Chem. 2006, 281, 27894–27904. [Google Scholar] [CrossRef]
- Muller, M.; Klein, I.; Kopacsi, S.; Remaley, A.T.; Rajnavolgyi, E.; Sarkadi, B.; Varadi, A. Co-expression of human ABCG5 and ABCG8 in insect cells generates an androstan stimulated membrane ATPase activity. FEBS Lett. 2006, 580, 6139–6144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, D.W.; Graf, G.A.; Gerard, R.D.; Cohen, J.C.; Hobbs, H.H. Functional asymmetry of nucleotide-binding domains in ABCG5 and ABCG8. J. Biol. Chem. 2006, 281, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kinch, L.N.; Borek, D.M.; Wang, J.; Wang, J.; Urbatsch, I.L.; Xie, X.S.; Grishin, N.V.; Cohen, J.C.; Otwinowski, Z.; et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 2016, 533, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Grishin, N.; Kinch, L.; Cohen, J.C.; Hobbs, H.H.; Xie, X.S. Sequences in the nonconsensus nucleotide-binding domain of ABCG5/ABCG8 required for sterol transport. J. Biol. Chem. 2011, 286, 7308–7314. [Google Scholar] [CrossRef]
- Patel, S.B.; Graf, G.A.; Temel, R.E. ABCG5 and ABCG8: More than a defense against xenosterols. J. Lipid Res. 2018, 59, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.M.; Benito, R.; Gonzalez-Porras, J.R.; Rivera, J. ABCG5 and ABCG8 gene variations associated with sitosterolemia and platelet dysfunction. Platelets 2020. [Google Scholar] [CrossRef]
- Wang, J.; Joy, T.; Mymin, D.; Frohlich, J.; Hegele, R.A. Phenotypic heterogeneity of sitosterolemia. J. Lipid Res. 2004, 45, 2361–2367. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, D.Y.; Jun, S.H.; Song, S.H.; Shin, C.H.; Ki, C.S.; Lee, K.; Song, J. High prevalence of increased sitosterol levels in hypercholesterolemic children suggest underestimation of sitosterolemia incidence. PLoS ONE 2020, 15, e0238079. [Google Scholar] [CrossRef]
- Tada, H.; Okada, H.; Nomura, A.; Yashiro, S.; Nohara, A.; Ishigaki, Y.; Takamura, M.; Kawashiri, M.A. Rare and Deleterious Mutations in ABCG5/ABCG8 Genes Contribute to Mimicking and Worsening of Familial Hypercholesterolemia Phenotype. Circ. J. 2019, 83, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Vauthier, V.; Ben Saad, A.; Elie, J.; Oumata, N.; Durand-Schneider, A.M.; Bruneau, A.; Delaunay, J.L.; Housset, C.; Ait-Slimane, T.; Meijer, L.; et al. Structural analogues of roscovitine rescue the intracellular traffic and the function of ER-retained ABCB4 variants in cell models. Sci. Rep. 2019, 9, 6653. [Google Scholar] [CrossRef]
- Davis, P.B.; Yasothan, U.; Kirkpatrick, P. Ivacaftor. Nat. Rev. Drug Discov. 2012, 11, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, J.L.; Durand-Schneider, A.M.; Dossier, C.; Falguieres, T.; Gautherot, J.; Davit-Spraul, A.; Ait-Slimane, T.; Housset, C.; Jacquemin, E.; Maurice, M. A functional classification of ABCB4 variations causing progressive familial intrahepatic cholestasis type 3. Hepatology 2016, 63, 1620–1631. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).