The RNAi Mechanism Regulates a New Exonuclease Gene Involved in the Virulence of Mucorales
Abstract
1. Introduction
2. Results
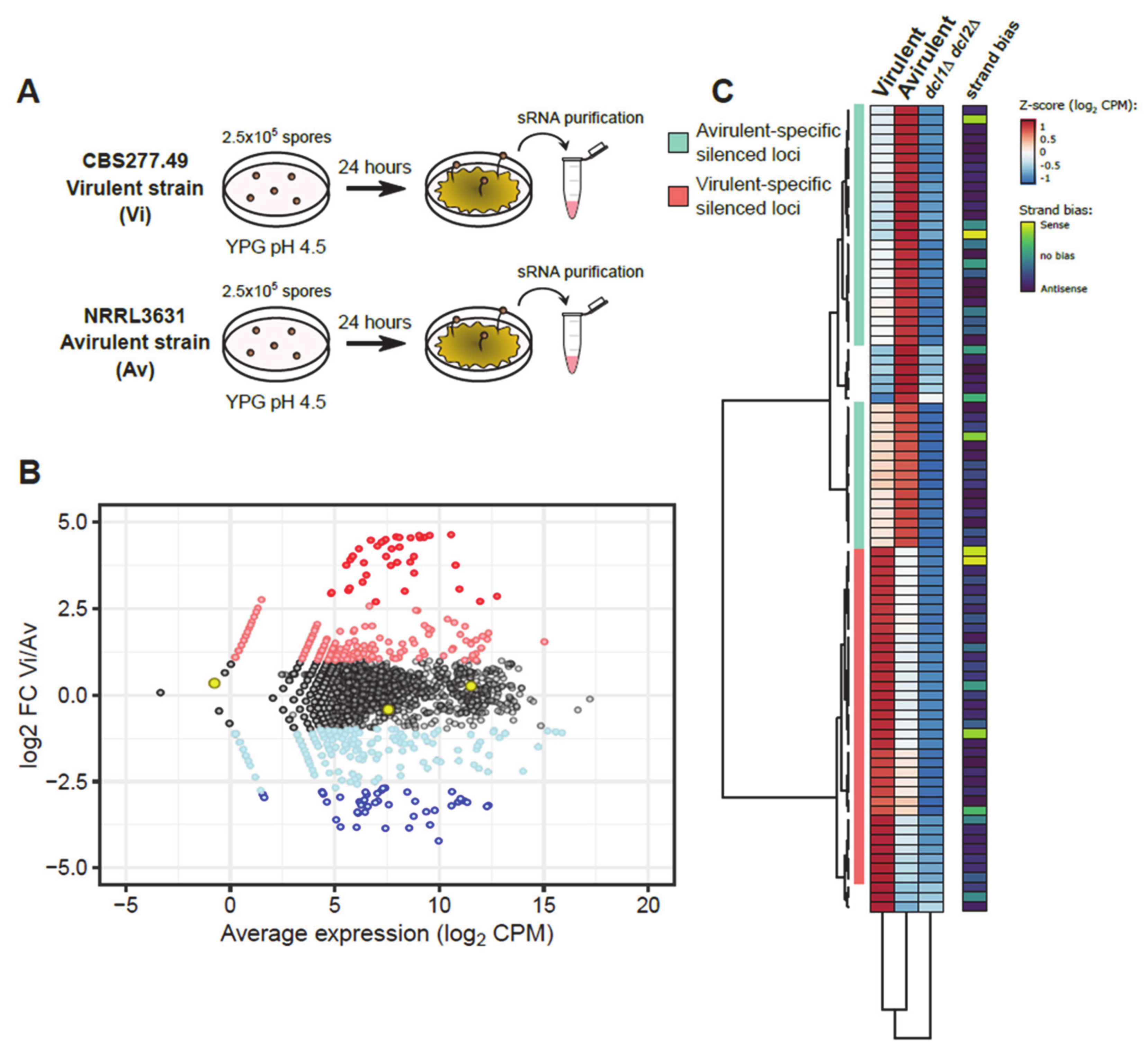
2.1. Comparative Analysis of sRNAs Transcriptomic Profiles between Virulent and Avirulent Pathotypes
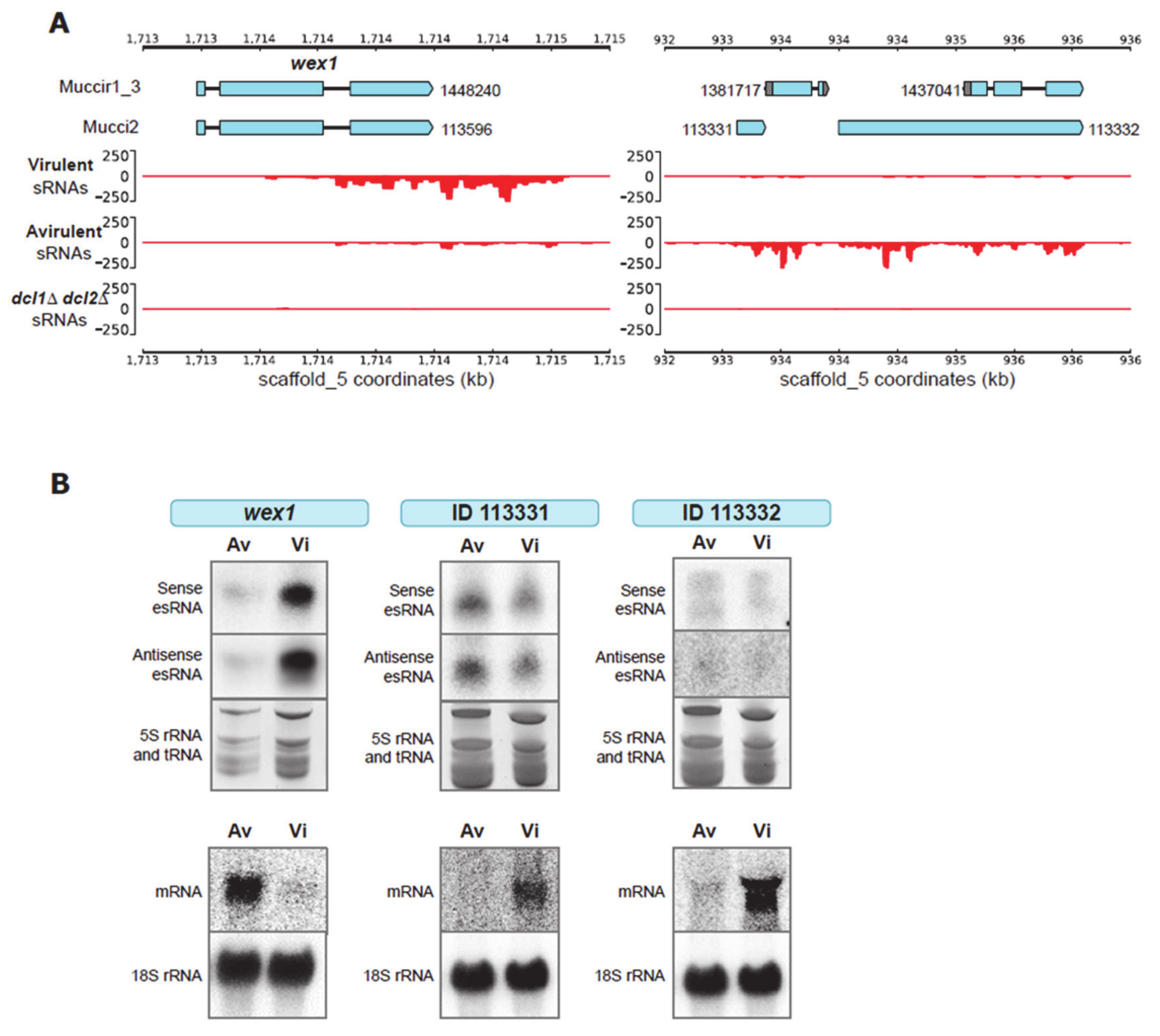
2.2. Validation of Target Genes That Are Differentially Regulated by RNAi
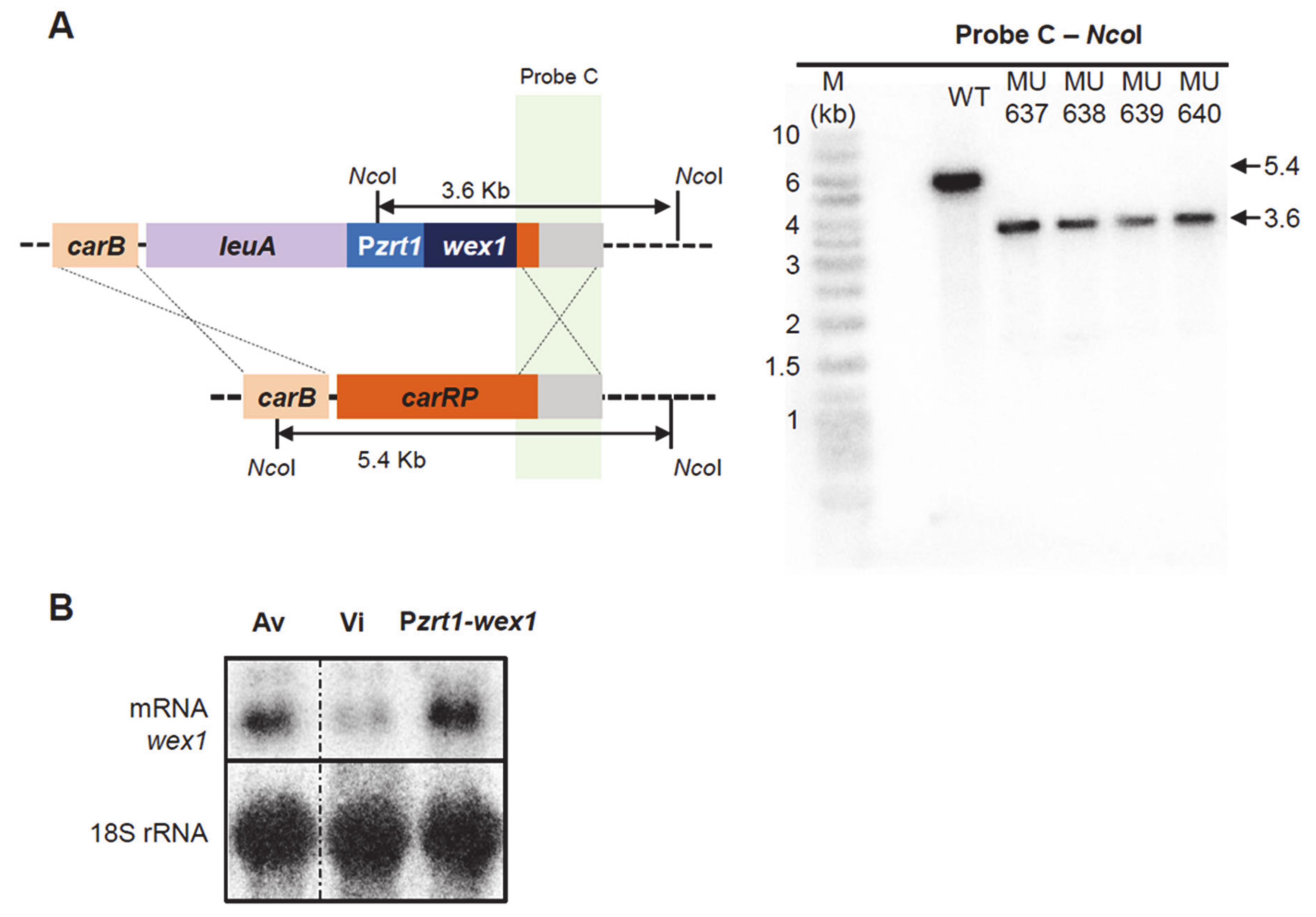
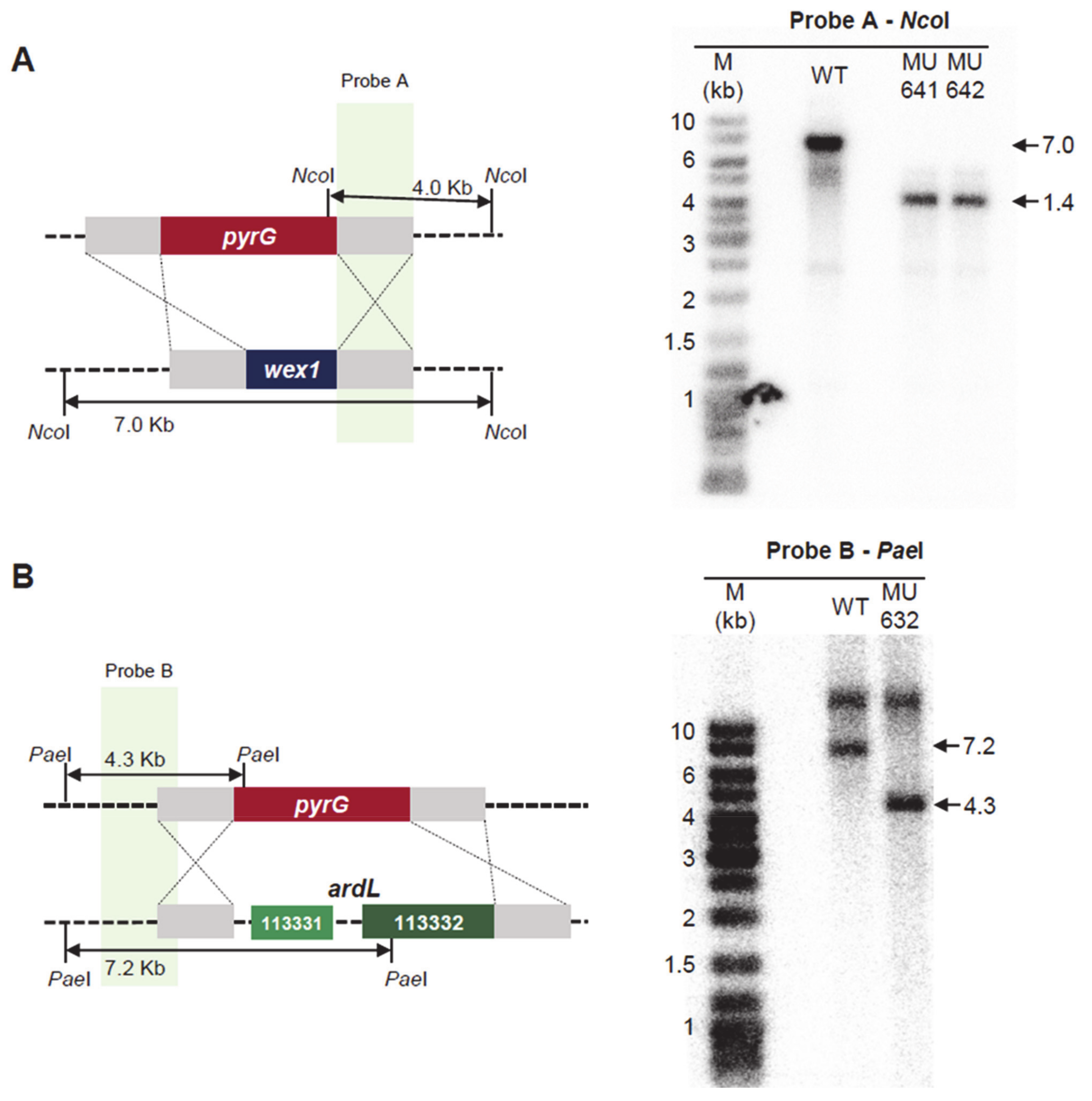
2.3. Generation of Mutant Strains with Altered Expression of the Candidate Genes
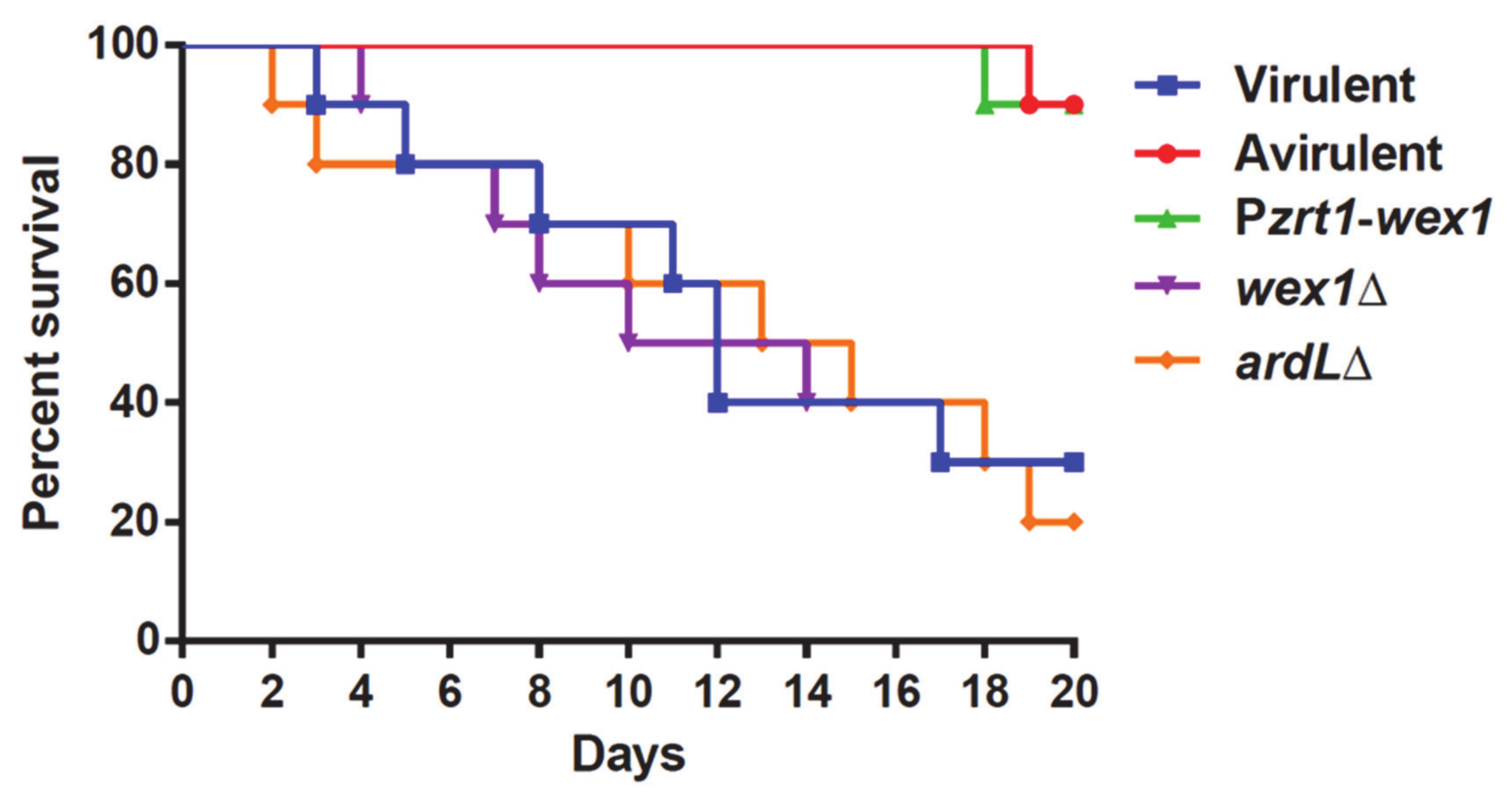
2.4. Functional Analysis of the Genes Regulated by RNAi and Their Role in Virulence
3. Discussion
4. Materials and Methods
4.1. Fungal Strains
4.2. RNA Isolation
4.3. RNA-Sequencing and Analysis for Small RNA Production
4.4. Northern Blot Analyses for mRNA and sRNA
4.5. Genetic Transformation
4.6. Virulence Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dannaoui, E. Antifungal resistance in mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: A retrospective study. BMC Infect. Dis. 2016, 16, 730. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Chakrabarti, A. Global epidemiology of mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic short-tailed azole resistance in mucormycetes is due to an evolutionary conserved aminoacid substitution of the lanosterol 14α-demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Ghannoum, M.A.; Perfect, J.R. Zygomycosis: The re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Calo, S.; Shertz-Wall, C.; Lee, S.C.; Bastidas, R.J.; Nicolás, F.E.; Granek, J.A.; Mieczkowski, P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Cardenas, M.E.; et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature 2014, 513, 555–558. [Google Scholar] [CrossRef]
- Li, C.H.; Cervantes, M.; Springer, D.J.; Boekhout, T.; Ruiz-Vazquez, R.M.; Torres-Martinez, S.R.; Heitman, J.; Lee, S.C. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 2011, 7, e1002086. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Walsh, T.J.; Roilides, E. Why is mucormycosis more difficult to cure than more common mycoses? Clin. Microbiol. Infect. 2014, 20, 74–81. [Google Scholar] [CrossRef]
- Nishimoto, A.T.; Sharma, C.; Rogers, P.D. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J. Antimicrob. Chemother. 2019, 75. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef]
- Corrochano, L.M.; Kuo, A.; Marcet-Houben, M.; Polaino, S.; Salamov, A.; Villalobos-Escobedo, J.M.; Grimwood, J.; Álvarez, M.I.; Avalos, J.; Bauer, D.; et al. Expansion of Signal Transduction Pathways in Fungi by Extensive Genome Duplication. Curr. Biol. 2016, 26, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Panchal, S.; Nicolás, F.E.; Mondo, S.J.; Ganguly, P.; Pangilinan, J.; Grigoriev, I.V.; Heitman, J.; Sanyal, K.; et al. Early Diverging Fungus Mucor circinelloides Lacks Centromeric Histone CENP-A and Displays a Mosaic of Point and Regional Centromeres. Curr. Biol. 2019, 29, 3791–3802.e6. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.E.; Vila, A.; Moxon, S.; Cascales, M.D.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Garre, V. The RNAi machinery controls distinct responses to environmental signals in the basal fungus Mucor circinelloides. BMC Genom. 2015, 16, 237. [Google Scholar] [CrossRef]
- Lax, C.; Tahiri, G.; Patiño-Medina, J.A.; Cánovas-Márquez, J.T.; Pérez-Ruiz, J.A.; Osorio-Concepción, M.; Navarro, E.; Calo, S. The evolutionary significance of rnai in the fungal kingdom. Int. J. Mol. Sci. 2020, 21, 348. [Google Scholar] [CrossRef]
- Lax, C.; Pérez-arques, C.; Navarro-mendoza, M.I.; Cánovas-márquez, J.T.; Tahiri, G.; Pérez-ruiz, J.A.; Osorio-concepción, M.; Murcia-flores, L.; Navarro, E.; Garre, V.; et al. Genes, pathways, and mechanisms involved in the virulence of mucorales. Genes 2020, 11, 317. [Google Scholar] [CrossRef]
- Nicolás, F.; Ruiz-Vázquez, R. Functional Diversity of RNAi-Associated sRNAs in Fungi. Int. J. Mol. Sci. 2013, 14, 15348–15360. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.E.; Moxon, S.; de Haro, J.P.; Calo, S.; Grigoriev, I.V.; Torres-Martinez, S.; Moulton, V.; Ruiz-Vazquez, R.M.; Dalmay, T. Endogenous short RNAs generated by Dicer 2 and RNA-dependent RNA polymerase 1 regulate mRNAs in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010, 38, 5535–5541. [Google Scholar] [CrossRef]
- Chang, Z.; Billmyre, R.B.; Lee, S.C.; Heitman, J. Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides. PLoS Genet. 2019, 15, e1007957. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Heitman, J. Drug-resistant epimutants exhibit organ-specific stability and induction during murine infections caused by the human fungal pathogen Mucor circinelloides. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Trieu, T.A.; Calo, S.; Nicolás, F.E.; Vila, A.; Moxon, S.; Dalmay, T.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M. A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi. PLOS Genet. 2015, 11, e1005168. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Navarro, E.; Garre, V.; Nicolás, F.E. A non-canonical RNAi pathway controls virulence and genome stability in Mucorales. PLoS Genet. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Calo, S.; Nicolás, F.E.; Lee, S.C.; Vila, A.; Cervantes, M.; Torres-Martinez, S.; Ruiz-Vazquez, R.M.; Cardenas, M.E.; Heitman, J. A non-canonical RNA degradation pathway suppresses RNAi-dependent epimutations in the human fungal pathogen Mucor circinelloides. PLoS Genet. 2017, 13, e1006686. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Murcia, L.; Navarro, E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Garre, V. Mucorales species and macrophages. J. Fungi 2020, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Lax, C.; Martínez-García, P.; Heitman, J.; Nicolás, F.E.; Garre, V. Mucor circinelloides Thrives inside the Phagosome through an Atf-Mediated Germination Pathway. MBio 2019, 10. [Google Scholar] [CrossRef]
- López-Fernández, L.; Sanchis, M.; Navarro-Rodríguez, P.; Nicolás, F.E.; Silva-Franco, F.; Guarro, J.; Garre, V.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Capilla, J. Understanding Mucor circinelloides pathogenesis by comparative genomics and phenotypical studies. Virulence 2018, 9, 707–720. [Google Scholar] [CrossRef]
- Nicolas, F.E.; de Haro, J.P.; Torres-Martinez, S.; Ruiz-Vazquez, R.M. Mutants defective in a Mucor circinelloides dicer-like gene are not compromised in siRNA silencing but display developmental defects. Fungal. Genet. Biol. 2007, 44, 504–516. [Google Scholar] [CrossRef] [PubMed]
- de Haro, J.P.; Calo, S.; Cervantes, M.; Nicolas, F.E.; Torres-Martinez, S.; Ruiz-Vazquez, R.M. A single dicer gene is required for efficient gene silencing associated with two classes of small antisense RNAs in Mucor circinelloides. Eukaryot. Cell 2009, 8, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Deutscher, M.P. Exoribonuclease superfamilies: Structural analysis and phylogenetic distribution. Nucleic Acids Res. 2001, 29, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Frómeta, R.A.; Gutiérrez, A.; Torres-Martínez, S.; Garre, V. Malic enzyme activity is not the only bottleneck for lipid accumulation in the oleaginous fungus Mucor circinelloides. Appl. Microbiol. Biotechnol. 2013, 97, 3063–3072. [Google Scholar] [CrossRef]
- Binder, U.; Navarro-Mendoza, M.I.; Naschberger, V.; Bauer, I.; Nicolas, F.E.; Pallua, J.D.; Lass-Flörl, C.; Garre, V. Generation of a mucor circinelloides reporter strain—A promising new tool to study antifungal drug efficacy and mucormycosis. Genes 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A. Determinants of virulence in the pathogenic fungi. Fungal. Biol. Rev. 2007, 21, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Trieu, T.A.; Navarro-Mendoza, M.I.; Perez-Arques, C.; Sanchis, M.; Capilla, J.; Navarro-Rodriguez, P.; Lopez-Fernandez, L.; Torres-Martinez, S.; Garre, V.; Ruiz-Vazquez, R.M.; et al. RNAi-Based Functional Genomics Identifies New Virulence Determinants in Mucormycosis. PLoS Pathog. 2017, 13, e1006150. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.E.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. Two classes of small antisense RNAs in fungal RNA silencing triggered by non-integrative transgenes. EMBO J. 2003, 22, 3983–3991. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. Loss and Retention of RNA Interference in Fungi and Parasites. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef]
- Stead, J.A.; Costello, J.L.; Livingstone, M.J.; Mitchell, P. The PMC2NT domain of the catalytic exosome subunit Rrp6p provides the interface for binding with its cofactor Rrp47p, a nucleic acid-binding protein. Nucleic Acids Res. 2007, 35, 5556–5567. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wollschlaeger, C.; Trevijano-Contador, N.; Wang, X.; Legrand, M.; Zaragoza, O.; Heitman, J.; Janbon, G. Distinct and redundant roles of exonucleases in Cryptococcus neoformans: Implications for virulence and mating. Fungal. Genet. Biol. 2014, 73, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; López-García, S.; Navarro, E.; Torres-Martínez, S.; Garre, V. Molecular tools for carotenogenesis analysis in the mucoral Mucor circinelloides. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2018; Volume 1852, pp. 221–237. [Google Scholar]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Ramírez, F.; Ryan, D.P.; Grüning, B.; Bhardwaj, V.; Kilpert, F.; Richter, A.S.; Heyne, S.; Dündar, F.; Manke, T. deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016, 44, W160–W165. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomollon, S.; Nicolas, F.E. Purification of DNA oligos by denaturing polyacrylamide gel electrophoresis (PAGE). Methods Enzymol. 2013, 529, 65–83. [Google Scholar] [PubMed]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Murcia, L.; Martínez-García, P.; Lax, C.; Sanchis, M.; Capilla, J.; Nicolás, F.E.; Garre, V. Components of a new gene family of ferroxidases involved in virulence are functionally specialized in fungal dimorphism. Sci. Rep. 2018, 8, 7660. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Navarro, E.; Garre, V.; Nicolás, F.E. The RNAi Mechanism Regulates a New Exonuclease Gene Involved in the Virulence of Mucorales. Int. J. Mol. Sci. 2021, 22, 2282. https://doi.org/10.3390/ijms22052282
Pérez-Arques C, Navarro-Mendoza MI, Murcia L, Navarro E, Garre V, Nicolás FE. The RNAi Mechanism Regulates a New Exonuclease Gene Involved in the Virulence of Mucorales. International Journal of Molecular Sciences. 2021; 22(5):2282. https://doi.org/10.3390/ijms22052282
Chicago/Turabian StylePérez-Arques, Carlos, María Isabel Navarro-Mendoza, Laura Murcia, Eusebio Navarro, Victoriano Garre, and Francisco Esteban Nicolás. 2021. "The RNAi Mechanism Regulates a New Exonuclease Gene Involved in the Virulence of Mucorales" International Journal of Molecular Sciences 22, no. 5: 2282. https://doi.org/10.3390/ijms22052282
APA StylePérez-Arques, C., Navarro-Mendoza, M. I., Murcia, L., Navarro, E., Garre, V., & Nicolás, F. E. (2021). The RNAi Mechanism Regulates a New Exonuclease Gene Involved in the Virulence of Mucorales. International Journal of Molecular Sciences, 22(5), 2282. https://doi.org/10.3390/ijms22052282







