Abstract
Antibiotic resistance is now a global problem, and the lack of effective antimicrobial agents for the treatment of diseases caused by resistant microbes is increasing. The 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines presented in this article may provide a good starting point for the development of potential new effective antimicrobial agents useful in the treatment of bacterial and fungal infections. Particular attention is drawn to the 1,3,4-oxadiazole derivative marked with the number 29 with 5-nitrofuran-2-yl substituent in its chemical structure. This substance showed a strong bactericidal effect, especially against Staphylococcus spp., and no cytotoxicity to the L929 normal cell line.
1. Introduction
The overuse and misuse of antibiotics has caused one of the most serious global threats—antimicrobial resistance [1,2]. Antimicrobial resistance is among the principal factors involved in the persistence of chronic infections [3]. This problem becomes more serious for immunocompromised patients and those who are often disposed to opportunistic infections [4]. The most significant fact associated with this phenomenon is the reduction of the number of effective antimicrobial drugs, which has led to an increase in therapeutic problems, complications and an increase in mortality [5,6,7]. Moreover, there is a huge disproportion between the frequency of the introduction of new antibiotics into treatment and the rate of the development of bacterial and fungal resistance. Therefore, in order to maintain the effectiveness of the treatment of bacterial infections as long as possible, it is necessary to take preventive measures, but to also search for new forms of therapy. If antibiotics with reduced and documented action become ineffective, new substances with high effectiveness that are safe for patients should be discovered [5].
Bearing in mind the above-mentioned problems, we conducted a literature review that showed that 3-acetyl-1,3,4-oxadiazoline derivatives constitute the class of compounds with high biological potential [8]. A simple method of the synthesis and a broad spectrum of activity make them an intensively studied group [9]. Our literature review confirmed their effectiveness. The mechanism of antimicrobial action is likely based on the presence of the -N=CO group in their chemical structure and its influence on the transcription of genes involved in biofilm formation, especially against Staphylococcus aureus [10]. According to the literature, antibacterial [11,12,13,14,15,16], antifungal [17,18,19,20], anticancer [21,22,23], antimycobacterial [24,25,26,27] and antiprotozoal [28,29] effects of 1,3,4-oxadiazoles have been documented. Currently, there are no registered medicines with an acetyl substituent in the 1,3,4-oxadiazole ring, but there are medicines with an 1,3,4-oxadiazole moiety, such as Furamizole, Nesapidil, Zibotentan, Raltegravir or Tiodazosin [8]. The introduction of an acetyl group in the third position of the 1,3,4-oxadiazole ring seems to be an interesting solution, especially with regard to the fact that many studies showed that it can induce antimicrobial activity. This relationship can be observed in the article by Chawla et al. [14], where the authors compare the antimicrobial activity of 1,3,4-oxadiazoles in relation to N-acetyl-1,3,4-oxadiazoles. Compounds with an acetyl group showed significantly greater antimicrobial activity against all tested strains, and two of the synthesized compounds were more active than ciprofloxacin, which was used as the reference substance [14]. Taking into account all these aspects, we decided to synthesize a group of compounds with the 3-acetyl-1,3,4-oxadiazole system and evaluate their antimicrobial activity and cytotoxicity, and we decided to determine their lipophilicity, especially due to the fact that it is one of the most important parameters used in the prediction of the biological activity of a given substance, as well as its toxicity [30,31].
2. Results
2.1. Chemistry
For the purpose of this study, we synthesized new 3-acetyl-2,5-disubstituted-1,3,4-oxadiazoline derivatives in the cyclization reaction with pure acetic anhydride of the previously described acylhydrazones [32] (Scheme 1). Novel 1,3,4-oxadiazole derivatives were obtained with 48–70% yields. All synthesized compounds are solids and can be dissolved in DMSO at room temperature.
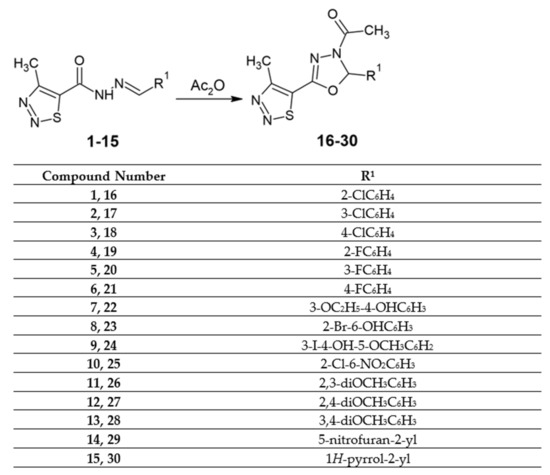
Scheme 1.
Synthesis scheme of novel derivatives of 3-acetyl-2,5-disubstituted-1,3,4-oxadiazoline.
In order to confirm the chemical structure of the obtained compounds, elemental analysis as well as 1H NMR, 13C NMR and FT-IR spectra analyses were performed.
On the basis of the characteristic signals presented in the 1H NMR, 13C NMR and FT-IR spectra, we determined the positive course of the cyclization reaction and proper synthesis of new 3-acetyl-2,5-disubstituted-1,3,4-oxadiazoline derivatives.
In the 1H NMR spectra, we observed a singlet signal for the acetyl substituent of the methyl group at δ 2.87–2.99 ppm, and for the CH group of 1,3,4-oxadiazoline, in the range of δ 7.18–8.85 ppm. In the 13C NMR spectra, we confirmed the presence of the carbon atom of CH group and the carbon atom of the 1,3,4-oxadiazole ring at δ 85.01–113.12 and 151.54–160.26 ppm, respectively. Additionally, we observed characteristic signals of the following fragments, such as C=O, C=N and C-OC in the FT-IR spectra.
2.2. Microbiology
The obtained 3-acetyl-2,5-disubstituted-1,3,4-oxadiazole derivatives were subjected to a series of microbiological tests against a panel of Gram-positive bacteria, Gram-negative bacteria and fungi from Candida spp. A panel of reference strains of microorganisms also included some resistant staphylococci—methicillin-resistant Staphylococcus aureus—MRSA ATCC 43300.
The obtained results are presented in Table 1 and they indicate that newly synthesized compounds 16–30 exhibited some antimicrobial activity. Among them, compounds 23 and 29 showed the widest spectrum of activity against all microorganisms, except rods from Pseudomonas aeruginosa ATCC 9027. Gram-positive bacteria were more sensitive to these substances than Gram-negative bacteria and fungi. The substance 29 indicated the highest antibacterial activity with minimal inhibitory concentrations (MIC), ranging from 3.91 to 250 µg/mL, and minimal bactericidal concentrations (MBC) from 15.62 to 1000 µg/mL with a bactericidal effect (MBC/MIC = 1–4) toward all reference Gram-positive bacteria. This compound had strong or very strong activity against staphylococci or bacilli. Compound 29 showed strong activity with MIC = 15.62 µg/mL and MBC = 31.25 µg/mL toward S. aureus ATCC 43300 with bactericidal effect. Staphylococcus epidermidis ATCC 12228 was the most sensitive to this derivative (MIC = 3.91 µg/mL and MBC = 15.62 µg/mL). The activity toward rods from Enterobacterales family belonging to Gram-negative bacteria was slightly weaker (MIC = 62.5–500 µg/mL and MBC = 125 to >2000 µg/mL) with bactericidal or bacteriostatic effect. The activity of compound 29 against most of the strains was comparable to the reference substance—nitrofurantoin. Additionally, in Supplementary Materials, we have presented antimicrobial activity of compound 29 as figures (Figures S1 and S2).

Table 1.
The activity data of compounds 16–30 expressed as MIC (MBC or MFC) (µg/mL) and MBC/MIC or MFC/MIC values against the reference strains of microorganisms.
In the case of compound 23, activity was good against Micrococcus luteus ATCC 10240 and Bordetella bronchiseptica ATCC 4617 (MIC = 62.5–125 µg/mL, MBC = 1000–2000 µg/mL and MBC/MIC = 8–16) and moderate or mild toward other bacteria (MIC = 500–1000 µg/mL and MBC = 1000 or ≥2000 µg/mL) with bactericidal or bacteriostatic effects. The remaining compounds 19, 21, 22, 24, and 25 showed some antimicrobial effect only against certain Gram-positive bacteria.
On the basis of obtained data, it was shown that some substances also had antifungal activity against yeasts belonging to reference Candida spp. In the case of the compounds 23, 24 and 29, growth of all fungal strains was inhibited at MIC = 62.5–1000 µg/mL. In turn, minimal fungicidal concentrations (MFC) ranged from 62.5 µg/mL to >2000 µg/mL. The activity of substances 23 and 29 was the highest, with good fungicidal effect toward C. albicans ATCC 2091 and C. albicans ATCC 10231. Additionally, mild activity was displayed by the compound 25 against C. parapsilosis ATCC 22019.
Moreover, remaining newly synthesized compounds, namely 16–18, 20, 26–28 and 30, had no activity against all reference microorganisms.
2.3. Cytotoxicity Studies
The most active compounds were tested for cytotoxicity. The 24 and 48 h culture incubation of L929 cells with 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines (24, 25, 29), showed that compound 25 at a concentration of 100 µM (24 h) and 200 µM (48 h) was the most toxic for this line. The remaining compounds did not significantly affect cell cytotoxicity, and sometimes led to an increase in viability—the compound 24 at a concentration of 12 and 6 µM after 24 h and after 48 h at 6 µM, and 29—at a concentration of 50 µM (Table 2). The 24 and 48 h culture incubation of A549 and HepG2 cells with tested substances showed that obtained 1,3,4-oxadiazoline derivatives significantly stimulated cell viability. In both systems tested, values above 100% cell viability were mostly obtained (Table 3 and Table 4).

Table 2.
The cell proliferation in % according to control after 24 and 48 h exposition on studied compounds in L929 cell line.

Table 3.
The cell proliferation in % according to control after 24 and 48 h exposition on studied compounds in A549 cell line.

Table 4.
The cell proliferation in % according to control after 24 and 48 h exposition on studied compounds in HepG2 cell line.
2.4. Lipophilicity
Chromatographic methods are good for determining experimental lipophilicity. Due to their speed and repeatability, they allow to determine the lipophilicity of a wide range of newly synthesized compounds. This study was based on a standardization procedure with the use of six reference substances in the lipophilicity range of 0.46 to 3.8 [33]. As a result, it gave a strong correlation of the log P value with RM0 in solvent systems containing various organic modifiers, i.e., acetone, acetonitrile, 1,4-dioxane and methanol (Table 5), and finally, respective calibration curves for further lipophilicity study were obtained:

Table 5.
The log P values from the literature [31] and the calculated RM0 values for the reference substances.
- (1)
- acetone: log PEXP = 0.8945 × RM0 + 0.1651; r2 = 0.9241;
- (2)
- acetonitrile: log PEXP = 2.2154 × RM0 − 1.6825; r2 = 0.9459;
- (3)
- 1,4-dioxane: log PEXP = 0.9387 × RM0 + 0.6354; r2 = 0.9653;
- (4)
- methanol: log PEXP = 0.9344 × RM0 + 0.2411; r2 = 0.9442.
The correlation coefficients (r2) for presented equations for the reference compounds were above 0.92 for all organic modifiers used, i.e., acetone, acetonitrile, 1,4-dioxane and methanol. Similarly, the correlations between the RF and RM0 for all newly tested 1,3,4-oxadiazoline derivatives were also sufficiently high (r2 > 0.91) for all chromatographic systems. In addition, better correlations, i.e., r2 > 0.96, were obtained for almost all derivatives, providing accuracy for further lipophilicity determination (Table 6).

Table 6.
The RM0 values of the synthesized 1,3,4-oxadiazoline derivatives.
On the basis of the above-presented calibration equations and respective RM0 values, experimental lipophilicity (log PEXP) of fourteen 1,3,4-oxadiazoline derivatives (16–22, 24–30) was calculated. The final results of our lipophilicity experiments are shown in Table 7. For all fourteen compounds, the obtained log PEXP values were close to the RM0 values in the case of solvent systems, which contained acetone or methanol as an organic modifier. As a result, the obtained values of lipophilicity can be considered as reliable. For the solvent systems with acetone or methanol, the highest values of lipophilicity were obtained for the compounds 17, 18 and 24, and among them for the compound 24 containing an iodine atom. In addition, for the compounds 17 and 18, the position of the chlorine atom (meta- or para-) in the phenyl ring did not visibly affect lipophilicity. However, for the ortho- substituted chlorine isomer (the compound 16), the lowest lipophilicity was obtained, not only in acetone and methanol, but in all solvent systems used. For the fluorine substituted compounds (19, 20, 21), lower lipophilicity values were obtained versus for the chlorine derivatives, with no difference for the individual ortho-, meta- and para- isomers. Finally, the additional substitution of the chlorine derivative with the nitro group increased the lipophilicity, which was observed for the pair of compounds 16 and 25. In general, it was observed that the antibacterial activity of synthesized compounds was not dependent on their lipophilicity.

Table 7.
The log PEXP values of the synthesized 1,3,4-oxadiazoline derivatives.
3. Material and Methods
3.1. Chemistry
All reagents used in the experiments in this research were purchased from Sigma-Aldrich (Munich, Germany) and Merck Co. (Darmstadt, Germany) and used without further purification. They had class of purity declared by the manufacturer. The purity of the obtained compounds was assessed by means of thin layer chromatography (TLC) on plates covered with silica gel (aluminum oxide 60 F-254) delivered by Merck Co. Chloroform–ethanol mixture in the 10:1 (v/v) ratio was used as the mobile phase. The spots were detected by irradiation with UV light at a wavelength of λ = 254 nm. The FT-IR spectra were recorded on a Nicolet 6700 spectrometer (Thermo Scientific, Madison, WI, USA); in cm−1. The 1H and 13C NMR spectra were recorded on the Bruker Avance 300 and 600 apparatus (Bruker BioSpin GmbH, Ettlingen, Germany). The melting points of the obtained compounds were determined with a Fisher–Johns apparatus (Fisher Scientific, Waltham, MA, USA) and were presented without any correction. The compounds were dissolved in dimethylsulfoxide (DMSO-d6) for the analysis. Tetramethylsilane (TMS) was used as an internal standard. Chemical shift values are given in ppm. The elemental analysis was determined by a Perkin Elmer 2400 series II CHNS/O analyzer (Waltham, MA, USA), and the results were within ± 0.4% of the theoretical values.
Synthesis of 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines
Previously obtained, 0.001 mole of appropriate acylhydrazone (1–15) [32] was dissolved in 3 mL of neat acetic anhydride and heated under reflux for 3 h. Subsequently, the acetic anhydride was removed under reduced pressure. Crushed ice was added to the liquid remaining in the flask and was shaken vigorously for 15 min. The resulting mixture was allowed to stand at room temperature for 24 h. After that, the precipitate formed was filtered under pressure and crystallized from ethanol. The crude solid was transferred to a round bottom flask and heated under reflux until it dissolved in the appropriate amount of ethanol (96%). Then, undissolved impurities were filtered off, and the filtrate was cooled to room temperature to precipitate crystals which were then filtered off under reduced pressure. The product was assessed for purity by TLC chromatography.
Detailed physico-chemical properties of new derivatives of 3-acetyl-2,5-disubstituted-1,3,4-oxadiazoline derivatives (16–30)
1-[2-(2-chlorophenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (16)
White powder, Yield: 54%, M.p.: 100 °C; IR: 3031 (CHarom), 2970 (CHaliph), 1644 (C=O), 1599 (C=N), 1277, 1046 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.30 (s, 3H, CH3), 2.98 (s, 3H, CH3), 7.52–7.54 (m, 1H, ArH), 7.56–7.60 (m, 1H, ArH), 8.13–8.14 (m, 1H, ArH), 8.63 (s, 1H, CHoxadiazole); 13C NMR (75 MHz, DMSO-d6): 11.56 (CH3), 14.95 (CH3), 90.91 (CHoxadiazole), 128.47, 130.72, 132.25, 132.56, 137.38, 141.88, 148.28 (7Car), 155.13 (Coxadiazole), 158.50 (Car), 163.36 (C=O); Anal. calc. for C13H11ClN4O2S (322.77) (%): C 48.37; H 3.44; N 17.36. Found: C 49.25; H 3.31; N 17.50.
1-[2-(3-chlorophenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (17)
White powder, Yield: 62%, M.p.: 196 °C; IR: 3069 (CHarom), 2970 (CHaliph), 1636 (C=O), 1577 (C=N), 1216, 1036 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.50 (s, 3H, CH3), 2.98 (s, 3H, CH3), 7.57–7.59 (m, 2H, ArH), 7.83–7.85 (m, 2H, ArH), 8.22 (s, 1H, CHoxadiazole); 13C NMR (150 MHz, DMSO-d6): 11.66 (CH3), 14.97 (CH3), 91.72 (CHoxadiazole), 126.07, 127.33, 130.66, 131.41, 133.95, 137.44, 138.08 (7Car), 155.24 (Coxadiazole), 158.87 (Car), 163.37 (C=O); Anal. calc. for C13H11ClN4O2S (322.77) (%): C 48.37; H 3.44; N 17.36. Found: C 48.62; H 3.41; N 18.10.
1-[2-(4-chlorophenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (18)
White powder, Yield: 48%, M.p.: 94 °C; IR: 3034 (CHarom), 2970 (CHaliph), 1667 (C=O), 1520 (C=N), 1206, 1090 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.28 (s, 3H, CH3), 2.92 (s, 3H, CH2), 7.22–7.25 (d, 1H, ArH, J = 18 Hz), 7.52–7.55 (m, 2H, ArH), 7.57 (s, 1H, CHoxadiazole), 7.56–7.58 (m, 1H, ArH); 13C NMR (75 MHz, DMSO-d6): 14.33 (CH3), 14.98 (CH3), 92.61 (CHoxadiazole), 129.42, 135.17, 135.32, 148.49, 155.07 (7Car), 155.24 (Coxadiazole), 159.63 (Car), 163.35 (C=O); Anal. calc. for C13H11ClN4O2S (322.77) (%): C 48.37; H 3.44; N 17.36. Found: C 48.92; H 3.51; N 18.21.
1-[2-(2-fluorophenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (19)
White powder, Yield: 67%, M.p.: 134 °C; IR: 3008 (CHarom), 2938 (CHaliph), 1636 (C=O), 1517 (C=N), 1205, 1092 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.22 (s, 3H, CH3), 2.92 (s, 3H, CH3), 7.30–7.36 (m, 2H, ArH), 7.54–7.59 (m, 2H, ArH), 8.44 (s, 1H, CHoxadiazole); 13C NMR (75 MHz, DMSO-d6): 11.54 (CH3), 14.95 (CH3), 89.02 (CHoxadiazole), 116.51, 122.57, 125.41, 130.07, 137.37, 141.89, 154.91 (7Car), 158.57 (Coxadiazole), 160.23 (Car), 163.34 (C=O); Anal. calc. for C13H11FN4O2S (306.32) (%): C 50.97; H 3.62; N 18.29. Found: C 49.85; H 3.31; N 19.50.
1-[2-(3-fluorophenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (20)
White powder, Yield: 78%, M.p.: 130 °C; IR: 3062 (CHarom), 2929 (CHaliph), 1672 (C=O), 1522 (C=N), 1215, 1061 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.24 (s, 3H, CH3), 2.86 (s, 3H, CH3), 7.22 (s, 1H, CHoxadiazole), 7.31–7.35 (m, 1H, ArH), 7.49–7.43 (m, 2H, ArH), 7.51–7.55 (m, 1H, ArH); 13C NMR (75 MHz, DMSO-d6): 11.67 (CH3), 15.00 (CH3), 91.73 (CHoxadiazole), 114.49, 115.79, 123.51, 131.53, 137.44, 138.83, 155.19 (7Car), 158.83 (Coxadiazole), 161.01 (Car), 163.37 (C=O); Anal. calc. for C13H11FN4O2S (306.32) (%): C 50.97; H 3.62; N 18.29 Found: C 51.15; H 3.51; N 18.54.
1-[2-(4-fluorophenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (21)
White powder, Yield: 59%, M.p.: 94 °C; IR: 3060 (CHarom), 2970 (CHaliph), 1645 (C=O), 1510 (C=N), 1211, 1038 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.24 (s, 3H, CH3), 2.93 (s, 3H, CH3), 7.22 (s, 1H, CHoxadiazole), 7.29–7.32 (m, 2H, ArH), 7.59–7.62 (m, 2H, ArH); 13C NMR (150 MHz, DMSO-d6): 11.56 (CH3), 15.46 (CH3), 91.96 (CHoxadiazole), 116.37, 129.82, 130.48, 145.38 (6Car), 155.07 (Coxadiazole), 158.78, 160.44 (2Car), 163.81 (C=O); Anal. calc. for C13H11FN4O2S (306.32) (%): C 50.97; H 3.62; N 18.29. Found: C 50.25; H 3.70; N 18.59.
1-[2-(3-ethoxy-4-hydroxyphenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (22)
White powder, Yield: 64%, M.p.: 94 °C; IR: 3078 (CHarom), 2970 (CHaliph), 1636 (C=O), 1512 (C=N), 1213, 1042 (C-OC); 1H NMR (600 MHz, DMSO-d6): 1.28–1.30 (t, 3H, CH3, J = 6 Hz), 2.27 (s, 3H, CH3), 2.87 (s, 3H, CH3), 4.05–4.09 (q, 2H, CH2, J = 12 Hz, J = 6 Hz), 8.08–7.10 (m, 1H, ArH), 7.18 (s, 1H, CHoxadiazole), 7.26–7.27 (m, 1H, ArH), 7.53–7.59 (m, 1H, ArH), 9.97 (s, 1H, OH); 13C NMR (150 MHz, DMSO-d6): 14.88 (CH3), 15.00 (CH3), 20.78 (CH3), 64.68 (CH2), 92.30 (CHoxadiazole), 112.87, 119.40, 123.78, 134.56, 137.53, 149.72, 150.81 (7Car), 155.24 (Coxadiazole), 158.76 (Car), 163.34 (C=O); Anal. calc. for C15H16N4O4S (348.38) (%): C 51.71; H 4.63; N 16.08. Found: C 50.85; H 4.31; N 16.50.
1-[2-(2-bromo-6-hydroxyphenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (23)
White powder, Yield: 70%, M.p.: 110 °C; IR: 3074 (CHarom), 2939 (CHaliph), 1683 (C=O), 1598 (C=N), 1202, 1012 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.23 (s, 3H, CH3), 2.91 (s, 3H, CH3), 7.20 (s, 1H, CHoxadiazole), 7.24–7.26 (m, 1H, ArH), 7.74–7.76 (m, 1H, ArH), 7.86–7.91 (m, 1H, ArH), 10.03 (s, 1H, OH); 13C NMR (150 MHz, DMSO-d6): 14.53 (CH3), 23.16 (CH3), 96.46 (CHoxadiazole), 115.72, 115.95, 119.36, 123.60, 125.24, 133.28, 142.24, 148.40 (7Car), 156.52 (Coxadiazole), 158.18 (C=O); Anal. calc. for C13H11BrN4O3S (383.22) (%): C 40.74; H 2.89; N 14.62. Found: C 40.25; H 3.10; N 14.50.
1-[2-(3-iodo-4-hydroxy-5-metoxyphenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (24)
White powder, Yield: 71%, M.p.: 120 °C; IR: 3078 (CHarom), 2970 (CHaliph), 1683 (C=O), 1592 (C=N), 1212, 1042 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.24 (s, 3H, CH3), 2.93 (s, 3H, CH3), 3.87 (s, 3H, OCH3), 7.15–7.17 (d, 1H, ArH, J = 12 Hz), 7.56–7.60 (m, 1H, ArH), 8.00 (CHoxadiazole), 9.93 (s, 1H, OH); 13C NMR (150 MHz, DMSO-d6): 15.01 (CH3), 20.94 (CH3), 56.92 (OCH3), 112.25 (CHoxadiazole), 128.40, 132.82, 135.50, 141.98, 145.38 (5Car), 152.40 (Coxadiazole), 158.69, 160.21, 167.67 (3Car), 168.89 (C=O); Anal. calc. for C14H13IN4O4S (460.25) (%): C 36.53; H 2.85; N 12.17. Found: C 37.27; H 3.11; N 12.54.
1-[5-(4-methyl-1,2,3-thiadiazol-5-yl)-2-(2-chloro-6-nitrophenyl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (25)
White powder, Yield: 66%, M.p.: 120 °C; IR: 3025 (CHarom), 2970 (CHaliph), 1677 (C=O), 1525 (C=N), 1211, 1059 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.32 (s, 3H, CH3), 2.93 (s, 3H, CH3), 7.48–7.50 (d, 1H, ArH, J = 12 Hz), 7.91–7.93 (m, 1H, ArH), 8.33–8.36 (m, 1H, ArH), 8.40 (s, 1H, CHoxadiazole), 8.40–8.41 (m, 1H, ArH); 13C NMR (75 MHz, DMSO-d6): 14.31 (CH3), 14.95 (CH3), 90.40 (CHoxadiazole), 125.62, 127.19, 132.57, 134.07, 139.40, 147.14, 155.62; (7Car), 155.24 (Coxadiazole), 159.79 (Car), 163.47 (C=O); Anal. calc. for C13H10ClN5O4S (367.77) (%): C 42.46; H 2.74; N 19.04. Found: C 42.25; H 2.31; N 19.50.
1-[2-(2,3-dimethoxyphenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (26)
White powder, Yield: 67%, M.p.: 110 °C; IR: 3018 (CHarom), 2970 (CHaliph), 1661 (C=O), 1575 (C=N), 1218, 1005 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.27 (s, 3H, CH3), 2.93 (s, 3H, CH3), 3.73 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 6.97–6.99 (m, 1H, ArH), 7.11–7.14 (t, 1H, ArH, J = 12 Hz, J = 6 Hz), 7.717–7.18 (m, 1H, ArH), 7.25 (s, 1H, CHoxadiazole); 13C NMR (75 MHz, DMSO-d6): 15.48 (CH3), 21.63 (CH3), 56.24 (OCH3), 56.36 (OCH3), 104.96 (CHoxadiazole), 115.36, 120.29, 124.86, 127.28, 135.81, 142.25, 148.91 (7Car), 153.06 (Coxadiazole), 160.31 (Car), 163.24 (C=O); Anal. calc. for C15H16N4O4S (348.38) (%): C 51.71; H 4.63; N 16.08. Found: C 49.95; H 4.51; N 16.40.
1-[2-(2,4-dimethoxyphenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (27)
White powder, Yield: 69%, M.p.: 110 °C; IR 3014 (CHarom), 2970 (CHaliph), 1721 (C=O), 1588 (C=N), 1280, 1031 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.09 (s, 3H, CH3), 2.98 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 6.68–6.69 (m, 1H, ArH), 6.76–6.78 (m, 1H, ArH), 7.91–7.92 (d, 1H, ArH, J = 6 Hz), 8.47 (s, 1H, CHoxadiazole); 13C NMR (75 MHz, DMSO-d6): 15.51 (CH3), 20.95 (CH3), 56.31 (OCH3), 56.40 (OCH3), 107.46 (CHoxadiazole), 114.61, 118.54, 122.10, 128.14, 130.17, 135.93, 142.11 (7Car), 154.02 (Coxadiazole), 160.08 (Car), 163.42 (C=O); Anal. calc. for C15H16N4O4S (348.38) (%): C 51.71; H 4.63; N 16.08. Found: C 50.25; H 4.71; N 16.35.
1-[2-(3,4-dimethoxyphenyl)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (28)
White powder, Yield: 71%, M.p.: 130 °C; IR: 3031 (CHarom), 2970 (CHaliph), 1739 (C=O), 1578 (C=N), 1217, 1037 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.66 (s, 3H, CH3), 2.99 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 7.10–7.11 (d, 1H, ArH, J = 6 Hz), 7.34–7.35 (m, 1H, ArH), 7.43–7.44 (d, 1H, ArH, J = 6 Hz), 8.14 (s, 1H, CHoxadiazole); 13C NMR (150 MHz, DMSO-d6): 15.52 (CH3), 23.17 (CH3), 56.93 (OCH3), 56.12 (OCH3), 109.8 (CHoxadiazole), 109.80, 112.28, 122.62, 126.52, 135.56, 146.32, 149.59 (7Car), 151.54 (Coxadiazole), 160.24 (Car), 163.72 (C=O); Anal. calc. for C15H16N4O4S (348.38) (%): C 51.71; H 4.63; N 16.08. Found: C 50.05; H 4.82; N 16.80.
1-[5-(4-methyl-1,2,3-thiadiazol-5-yl)-2-(5-nitrofuran-2-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (29)
White powder, Yield: 70%, M.p.: 170 °C; IR: 3016 (CHarom), 2970 (CHaliph), 1739 (C=O), 1537 (C=N), 1229, 1032 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.25 (s, 3H, CH3), 2.88 (s, 3H, CH3), 7.32–7.33 (d, 1H, ArH, J = 6 Hz), 7.40 (s, 1H, CHoxadiazole), 7.74–7.75 (d, 1H, ArH, J = 6 Hz); 13C NMR (75 MHz, DMSO-d6): 11.55 (CH3), 14.95 (CH3), 85.01 (CHoxadiazole), 99.74, 115.59, 137.15, 149.83, 155.52 (5Car), 158.60 (Coxadiazole), 163.56 (C=O); Anal. calc. for C11H9N5O5S (323.28) (%): C 40.87; H 2.81; N 21.66. Found: C 40.25; H 3.31; N 21.50.
1-[5-(4-methyl-1,2,3-thiadiazol-5-yl)-2-(1H-pyrrol-2-yl)-1,3,4-oxadiazol-3(2H)-yl]ethan-1-one (30)
White powder, Yield: 59%, M.p.: 150 °C; IR: 3016 (CHarom), 2970 (CHaliph), 1735 (C=O), 1555 (C=N), 1202, 1068 (C-OC); 1H NMR (600 MHz, DMSO-d6): 2.29 (s, 3H, CH3), 2.93 (s, 3H, CH3), 6.57–6.58 (m, 1H, ArH), 7.32–7.33 (m, 1H, ArH), 7.86–7.87 (m, 1H, ArH), 8.85 (s, 1H, CHoxadiazole), 9.18 (s, 1H, NH); 13C NMR (150 MHz, DMSO-d6): 15.49 (CH3), 21.50 (CH3), 113.12 (CHoxadiazole), 116.33, 126.13, 129.59, 135.88, 139.65 (5Car), 160.26 (Coxadiazole), 163.62 (Car), 172.53 (C=O); Anal. calc. for C11H11N5O2S (277.30) (%): C 47.64; H 4.00; N 25.26. Found: C 48.75; H 3.91; N 25.90.
3.2. Microbiology
The methodology for microbiological tests has been previously described by our research group [32,34] and is consistent with the standards of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute guidelines [35,36]. We used strains from the American Type Culture Collection (ATCC) as a panel of reference and clinical or saprophytic microbial strains. All compound stocks were prepared by dissolving them in DMSO. Tests were repeated in triplicate and representative results are shown.
3.3. Cytotoxicity Studies
Cytotoxicity studies were performed with the use of the normal cell line L929 (murine fibroblasts and neoplastic cells), HepG2 (human liver cancer) and, A549 (human lung cancer). All stock solutions of the test compounds were dissolved in DMSO. The methodology of testing was described by our team in the previous article [37].
3.4. Lipophilicity
Experimental lipophilicity of the synthesized compounds 16–22 and 24–30 (Table 6 and Table 7) was determined with the use of reversed-phase thin-layer chromatography on 10 × 20 cm RP18 F254 plates from Merck (Darmstadt, Germany). The methodology of the process was described in our earlier work [32].
4. Discussion
S. aureus is of great clinical importance, as it causes infections ranging from superficial skin symptoms to systemic sepsis. Initially, penicillin was the drug of choice for staphylococcal infections. However, the increased incidence of resistance to this antibiotic led to the introduction of methicillin—the first semisynthetic penicillin. Unfortunately, methicillin-resistant S. aureus (MRSA) was discovered shortly after, now belonging to multi-drug resistant organisms (MDRO) [38,39]. It is one of the most important serious opportunistic human pathogens involved in nosocomial infections. Resistance to methicillin primarily derives from acquisition of the mecA gene, which encodes a modified penicillin-binding protein (PBP2a) with low affinity for beta-lactams [40] The treatment of MRSA infection is greatly challenging since it has developed the resistance to almost all types of antibiotics, especially from the beta-lactam group, including penicillins, cephalosporins, monobactams and carbapenems. MRSA strains have also developed resistance to various other clinically used antibiotics such as fluoroquinolones, macrolides, aminoglycosides, daptomycin, and clindamycin creating a great threat to global healthcare [38,39]. Moreover, recent studies indicates that MRSA is continuously evolving as a superbug. Therefore, the development of new therapies for eradication of this microorganism is of great importance. Due to this high activity of compound 29 against S. aureus, ATCC 43300 is significant.
Additionally, on the basis of our microbiological tests, both these presented in this article and those already published [32], it can be concluded that acylhydrazones, compared to 3-acetyl-1,3,4-oxadizolines, show greater activity against Gram-positive and Gram-negative bacterial strains, but lower activity against fungi.
A similar situation was observed in the article by Zorzi et al. [41], where the cyclization reaction was performed with [N-(5-nitrofuran-2-yl)methylene]benzhydrazide, which resulted in the synthesis of a series of 3-acetyl-5-(substitutedphenyl)-2-(5-nitrofuran-2-yl)-2,3-dihydro-1,3,4-oxadiazoles, which showed lower antimicrobial activity in comparison with acylhydrazones. Especially, this dependence was observed for the compound with the t-butyl substituent where the hydrazone against S. aureus ATCC 29213 was active (MIC = 4–8 µM) and the corresponding 1,3,4-oxadiazole showed no activity [41], In two other studies, the cyclization of acylhydrazones into corresponding 3-acetyl-1,3,4-oxadiazolines resulted in lower values of bioactivity against S. aureus strain [12,16]
The bactericidal effect against Gram-positive bacteria for acylhydrazone with a 5-nitrofuroyl moiety was in the range of MIC from 3.91 to 62.5 µg/mL [32], whereas for the corresponding 1,3,4-oxadiazole, it was 3.91–250 µg/mL. The synthesized group of 1,3,4-oxadiazole derivatives showed higher activity only against fungi. This fact can be observed in case of compounds 8 and 23. Compound 23 showed the MIC values in the range of 125–500 µg/mL, and the corresponding acylhydrazone did not have this activity at all. The most active in both groups were compounds with a 5-nitrofuroyl moiety, which can be found in well-known medicines such as nitrofurantoin or furazolidone.
These results confirm the relationship presented in our previous two articles [34,42]. The fact that the 5-nitrofuran-2-yl moiety in the second position of the 1,3,4-oxadiazoline ring determines the activity of these derivatives and the cyclization of the N-heteroarylidene analogues to their 3-acetyl-1,3-4-oxadiazoline analogues resulted in a dramatic decrease of antibacterial activity [34,42]. On the basis of the conducted research, it can therefore be concluded that acylhydrazones are more active in terms of antimicrobial activity than the corresponding 1,3,4-oxadiazole derivatives.
Nevertheless, it seems justified to carry out additional tests, due to which it will be possible to establish the relationship between the activity and chemical structure of 3-acetyl-1,3,4-oxadiazolines.
5. Conclusions
In summary, the simple cyclization reaction of the corresponding acylhydrazones, described earlier by our research group [32], in the acetic anhydride, allowed to obtain a series of new 3-acetyl-2,5-disubstituted-1,3,4-oxadiazoline derivatives. This reaction was carried out with the efficiency of about 60%, which can be considered as satisfactory result. Among this group, one compound, namely 29 with 5-nitrofuran-2-yl moiety, deserves special attention. It showed the highest activity, especially against strains of Staphylococcus spp., including multi-drug resistant microorganisms belonging to methicillin-resistant S. aureus. Moreover, cytotoxicity tests showed that this compound showed low cytotoxicity. Conversely, the conducted lipophilicity studies showed that the presence of a halogen atom in the structure significantly influences the lipophilicity of the compound. We believe that the combination of this information provides a good foundation for the synthesis of new groups of compounds with potential antimicrobial activity.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms222413669/s1.
Author Contributions
Conceptualization, K.P.; Formal analysis, A.B. and Ł.P.; Methodology, K.P., A.B., A.B.-R., A.H. and Ł.P.; Writing—original draft, K.P. and Ł.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Son, N.T.; Huong, V.T.T.; Lien, V.T.K.; Nga, D.T.Q.; Hai Au, T.T.; Nga, T.T.; Minh Hoa, L.N.; Binh, T.Q. First report on multidrug-resistant methicillin-resistant staphylococcus aureus isolates in children admitted to tertiary hospitals in Vietnam S. J. Microbiol. Biotechnol. 2019, 29, 1460–1469. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Vollaro, A.; Catania, M.R.; Lesce, M.R.; Sferruzza, R.; D’Abrosca, B.; Donnarumma, G.; de Filippis, A.; Cermola, F.; DellaGreca, M.; Buommino, E. Antimicrobial and anti-biofilm properties of novel synthetic lignan-like compounds. New Microbiol. 2019, 42, 21–28. [Google Scholar]
- Fesatidou, M.; Petrou, A.; Athina, G. Heterocycle Compounds with Antimicrobial Activity. Curr. Pharm. Des. 2020, 26, 867–904. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotics Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar] [CrossRef]
- Morehead, M.S.; Scarbrough, C. Emergence of Global Antibiotic Resistance. Prim. Care Clin. Off. Pract. 2018, 45, 467–484. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO fact sheet. Global WHO summary report 2018. In Global Tuberculosis Reports; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Paruch, K.; Popiołek, Ł.; Wujec, M. Antimicrobial and antiprotozoal activity of 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines: A review. Med. Chem. Res. 2020, 29, 1–16. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; de Athayde-Filho, P.F. Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: A review of the literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Liu, Q.; Kim, W.; Tharmalingam, N.; Fuchs, B.B.; Mylonakis, E. Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Future Med. Chem. 2018, 10, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.D.; More, U.A.; Pansuriya, K.; Aminabhavi, T.M.; Gadad, A.K. Synthesis and molecular modeling studies of novel pyrrole analogs as antimycobacterial agents. J. Saudi Chem. Soc. 2017, 21, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Rollas, S.; Gulerman, N.; Erdeniz, H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 2002, 57, 171–174. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis and antimicrobial evaluation of some new oxadiazoles derived from phenylpropionohydrazides. Molecules 2009, 14, 1898–1903. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Arora, A.; Parameswaran, M.K.; Chan, P.; Sharma, D.; Michael, S.; Ravi, T.K. Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents. Acta Pol. Pharm. Drug Res. 2010, 67, 247–253. [Google Scholar]
- Dewangan, D.; Pandey, A.; Sivakumar, T.; Rajavel, R.; Dubey, R.D. Synthesis of some novel 2,5-disubstituted anti-tubercular activity. Int. J. ChemTech Res. 2010, 2, 1397–1412. [Google Scholar]
- El-Emam, A.A.; Alrashood, K.A.; Al-Omar, M.A.; Al-Tamimi, A.M.S. Synthesis and antimicrobial activity of N’-heteroarylidene-1- adamantylcarbohydrazides and (±)-2-(1-adamantyl)-4-acetyl-5-[5-(4- substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules 2012, 17, 3475–3483. [Google Scholar] [CrossRef] [Green Version]
- Koçyiğit-Kaymakçıoğlu, B.; Oruç-Emre, E.E.; Ünsalan, S.; Tabanca, N.; Khan, S.I.; Wedge, D.E.; İşcan, G.; Demirci, F.; Rolla, S. Synthesis and biological activity of hydrazide-hydrazones and their corresponding 3-Acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles. Med. Chem. Res. 2012, 21, 3499–3508. [Google Scholar] [CrossRef]
- Ke, S.; Liu, F.; Wang, N.; Yang, Q.; Qian, X. 1,3,4-Oxadiazoline derivatives as novel potential inhibitors targeting chitin biosynthesis: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2009, 19, 332–335. [Google Scholar] [CrossRef]
- Jadhav, G.R.; Deshmukh, D.G.; Medhane, V.J.; Gaikwad, V.B.; Bholay, A.D. 2,5-Disubstituted 1,3,4-oxadiazole derivatives of chromeno[4,3-b]pyridine: Synthesis and study of antimicrobial potency. Heterocycl. Commun. 2016, 22, 123–130. [Google Scholar] [CrossRef]
- Shyma, P.C.; Kalluraya, B.; Peethambar, S.K.; Telkar, S.; Arulmoli, T. Synthesis, characterization and molecular docking studies of some new 1,3,4-oxadiazolines bearing 6-methylpyridine moiety for antimicrobial property. Eur. J. Med. Chem. 2013, 68, 394–404. [Google Scholar] [CrossRef]
- Chaaban, I.; El Khawass, E.S.M.; Abd El Razik, H.A.; El Salamouni, N.S.; Redondo-Horcajo, M.; Barasoain, I.; Díaz, J.F.; Yli-Kauhaluoma, J.; Moreira, V.M. Synthesis and biological evaluation of new oxadiazoline-substituted naphthalenyl acetates as anticancer agents. Eur. J. Med. Chem. 2014, 87, 805–813. [Google Scholar] [CrossRef]
- Jin, L.; Chen, J.; Song, B.; Chen, Z.; Yang, S.; Li, Q.; Hu, D.; Xu, R. Synthesis, structure, and bioactivity of N′-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 5036–5040. [Google Scholar] [CrossRef] [PubMed]
- Salum, L.B.; Mascarello, A.; Canevarolo, R.R.; Altei, W.F.; Laranjeira, A.B.A.; Neuenfeldt, P.D.; Stumpf, T.R.; Chiaradia-Delatorre, L.D.; Vollmer, L.L.; Daghestani, H.N. N-(1’-naphthyl)-3,4,5-trimethoxybenzohydrazide as microtubule destabilizer: Synthesis, cytotoxicity, inhibition of cell migration and in vivo activity against acute lymphoblastic leukemia. Eur. J. Med. Chem. 2015, 96, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Han, D.; Xu, F.F.; Meng, X.B.; Li, Z.J. Microwave-assisted efficient synthesis of glucose-based 3-acetyl-5-alkyl-2,3-dihydro-1,3,4-oxadiazole derivatives catalyzed by sodium acetate. Carbohydr. Res. 2009, 344, 2113–2119. [Google Scholar] [CrossRef]
- Kumar, S.G.V.; Rajendraprasad, Y.; Mallikarjuna, B.P.; Chandrashekar, S.M.; Kistayya, C. Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2010, 45, 2063–2074. [Google Scholar] [CrossRef]
- Baquero, E.; Quiñones, W.; Ribon, W.; Caldas, M.L.; Sarmiento, L.; Echeverri, F. Effect of an Oxadiazoline and a Lignan on Mycolic Acid Biosynthesis and Ultrastructural Changes of Mycobacterium tuberculosis. Tuberc. Res. Treat. 2011, 2011, 986409. [Google Scholar] [CrossRef]
- Pasqualoto, K.F.M.; Ferreira, E.I.; Santos-Filho, O.A.; Hopfinger, A.J. Rational design of new antituberculosis agents: Receptor-independent four-dimensional quantitative structure-activity relationship analysis of a set of isoniazid derivatives. J. Med. Chem. 2004, 47, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Jorge, S.D.; de Oliveira, A.A.; Palace-Berl, F.; Sonehara, I.Y.; Pasqualoto, K.F.M.; Tavares, L.C. Synthesis, molecular modeling and preliminary biological evaluation of a set of 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazole as potential antibacterial, anti-Trypanosoma cruzi and antifungal agents. Bioorg. Med. Chem. 2011, 19, 6292–6301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palace-Berl, F.; Jorge, S.D.; Pasqualoto, K.F.M.; Ferreira, A.K.; Maria, D.A.; Zorzi, R.R.; de Sá Bortolozzo, L.; Lindoso, J.Â.L.; Tavares, L.C. 5-Nitro-2-furfuriliden derivatives as potential anti-Trypanosoma cruzi agents: Design, synthesis, bioactivity evaluation, cytotoxicity and exploratory data analysis. Bioorg. Med. Chem. 2013, 21, 5395–5406. [Google Scholar] [CrossRef] [Green Version]
- Paneth, A.; Hawryl, A.; Plech, T.; Hawryl, M.; Swieboda, R.; Janowska, D.; Wujec, M.; Paneth, P. Lipophilicity studies on thiosemicarbazide derivatives. Molecules 2017, 22, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Hawrył, A.; Hawrył, M.; Dzitko, K. Discovery of potent and selective halogen-substituted imidazole-thiosemicarbazides for inhibition of toxoplasma gondii growth in vitro via structure-based design. Molecules 2019, 24, 1618. [Google Scholar] [CrossRef] [Green Version]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Berecka-Rycerz, A.; Malm, A.; Gumieniczek, A.; Wujec, M. Novel derivatives of 4-methyl-1,2,3-thiadiazole-5-carboxylic acid hydrazide: Synthesis, lipophilicity, and in vitro antimicrobial activity screening. Appl. Sci. 2021, 11, 1180. [Google Scholar] [CrossRef]
- Komsta, Ł.; Skibiński, R.; Berecka, A.; Gumieniczek, A.; Radkiewicz, B.; Radoń, M. Revisiting thin-layer chromatography as a lipophilicity determination tool-A comparative study on several techniques with a model solute set. J. Pharm. Biomed. Anal. 2010, 53, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł.; Biernasiuk, A.; Paruch, K.; Malm, A.; Wujec, M. Synthesis and in vitro antimicrobial activity screening of new 3-acetyl-2,5-disubstituted-1,3,4-oxadiazoline derivatives. Chem. Biodivers. 2019, 16, e1900082. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). EUCAST discussion document E. Dis 5. 1 March 2003 Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
- Clinical and Laboratory Standards Institute. Document M27-A4. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Hordyjewska, A.; Malm, M.; Wujec, M. Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and biological activity. Molecules 2020, 25, 5844. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Khanal, A.; Sucholan, G.C.; Gaire, A.; Khanal, A.; Estrada, R.; Ghimire, R.; Panthee, S. Methicillin-resistant Staphylococcus aureus in Nepal: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 103, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.D.; Jenabi, A.; Montazeri, E.A. Distribution of genes encoding resistance to aminoglycoside modifying enzymes in methicillin-resistant Staphylococcus aureus (MRSA) strains. Kaohsiung J. Med. Sci. 2017, 33, 587–593. [Google Scholar] [CrossRef]
- Zorzi, R.R.; Jorge, S.D.; Palace-Berl, F.; Pasqualoto, K.F.M.; Bortolozzo, L.D.S.; de Castro Siqueira, A.M.; Tavares, L.C. Exploring 5-nitrofuran derivatives against nosocomial pathogens: Synthesis, antimicrobial activity and chemometric analysis. Bioorg. Med. Chem. 2014, 22, 2844–2854. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A.; Berecka, A.; Gumieniczek, A.; Malm, A.; Wujec, M. New hydrazide–hydrazones of isonicotinic acid: Synthesis, lipophilicity and in vitro antimicrobial screening. Chem. Biol. Drug Des. 2018, 91, 915–923. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).