Single Dose of N-Acetylcysteine in Local Anesthesia Increases Expression of HIF1α, MAPK1, TGFβ1 and Growth Factors in Rat Wound Healing
Abstract
:1. Introduction
2. Results
2.1. General Considerations
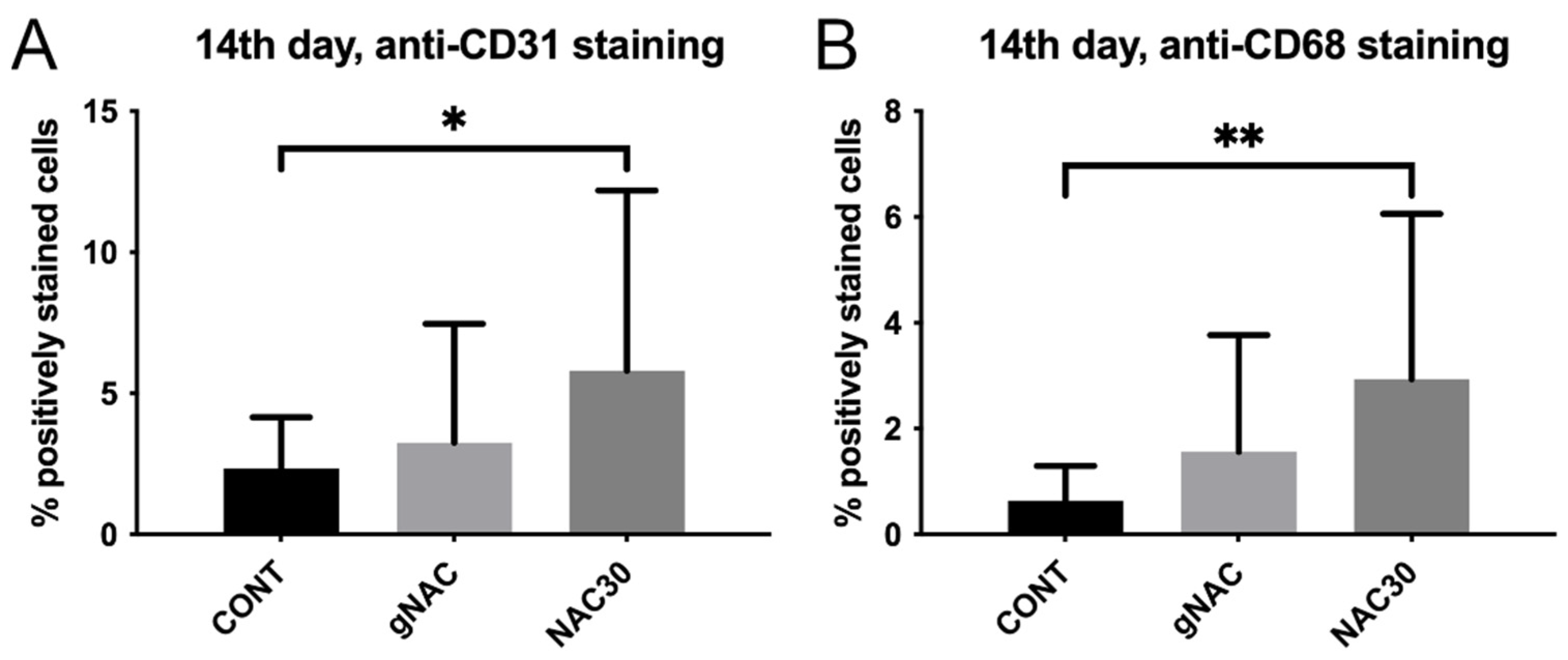
2.2. Assessment of the Immunohistochemical Staining
2.3. Genes’ Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Surgical Procedure
4.2. Scar Tissue Collection and Immunohistochemical Analysis
4.3. Gene Expression Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Wilgus, T.A. Inflammation as an orchestrator of cutaneous scar formation: A review of the literature. Plast. Aesthetic Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Papanas, N.; Maltezos, E. Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf. 2010, 33, 455–461. [Google Scholar] [CrossRef]
- Koria, P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs 2012, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lau, S.S.; Monks, T.J. The cytoprotective effect of N-acetyl-L-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol. Sci. 2011, 120, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Huang, H.P.; Hsu, J.D.; Lai, Y.R.; Hsiao, Y.P.; Lu, F.J.; Chang, H.R. Topical N-acetylcysteine accelerates wound healing in vitro and in vivo via the PKC/Stat3 pathway. Int. J. Mol. Sci. 2014, 15, 7563–7578. [Google Scholar] [CrossRef] [Green Version]
- Aktunc, E.; Ozacmak, V.H.; Ozacmak, H.S.; Barut, F.; Buyukates, M.; Kandemir, O.; Demircan, N. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin. Exp. Dermatol. 2010, 35, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, H.; Omma, T.; Bag, Y.M.; Uzunoglu, K.; Isildak, M.; Duymus, M.E.; Kismet, K.; Senes, M.; Fidanci, V.; Celepli, P.; et al. Topical and Systemic Effects of N-acetyl Cysteine on Wound Healing in a Diabetic Rat Model. Wounds 2019, 31, 91–96. [Google Scholar]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Paskal, W.; Paskal, A.M.; Pietruski, P.; Stachura, A.; Pełka, K.; Woessner, A.E.; Quinn, K.P.; Kopka, M.; Galus, R.; Wejman, J.; et al. N-Acetylcysteine Added to Local Anesthesia Reduces Scar Area and Width in Early Wound Healing—An Animal Model Study. Int. J. Mol. Sci. 2021, 22, 7549. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.P.; Golberg, A.; Broelsch, G.F.; Khan, S.; Villiger, M.; Bouma, B.; Austen, W.G.; Sheridan, R.L.; Mihm, M.C.; Yarmush, M.L.; et al. An automated image processing method to quantify collagen fiber organization within cutaneous scar tissue. Exp. Dermatol. 2015, 24, 78–80. [Google Scholar] [CrossRef] [Green Version]
- Betz, P.; Nerlich, A.; Wilske, J.; Tubel, J.; Wiest, I.; Penning, R.; Eisenmenger, W. The time-dependent rearrangement of the epithelial basement membrane in human skin wounds--immunohistochemical localization of collagen IV and VII. Int. J. Leg. Med. 1992, 105, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Abreu-Velez, A.; Howard, M. Collagen IV in normal skin and in pathological processes. North. Am. J. Med Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Solingen, C.; Araldi, E.; Chamorro-Jorganes, A.; Fernandez-Hernando, C.; Suarez, Y. Improved repair of dermal wounds in mice lacking microRNA-155. J. Cell. Mol. Med. 2014, 18, 1104–1112. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Aggarwal, R.; Singh, V.P.; Ramachandran, S.; Datta, M. Downregulation of miRNAs during Delayed Wound Healing in Diabetes: Role of Dicer. Mol. Med. 2016, 21, 847–860. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Abd-Elwahed, M.M.; Hawary, K.; Arafah, M.M.; Shier, M.K. Myofibroblast expression in skin wounds is enhanced by collagen III suppression. Biomed. Res. Int. 2015, 2015, 958695. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Wang, Y.; Mauldin, E.A.; Liechty, K.W.; Adams, S.L. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 2011, 194, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Gimona, M.; Kaverina, I.; Resch, G.P.; Vignal, E.; Burgstaller, G. Calponin repeats regulate actin filament stability and formation of podosomes in smooth muscle cells. Mol. Biol. Cell 2003, 14, 2482–2491. [Google Scholar] [CrossRef] [Green Version]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Rappolee, D.A.; Mark, D.; Banda, M.J.; Werb, Z. Wound macrophages express TGF-alpha and other growth factors in vivo: Analysis by mRNA phenotyping. Science 1988, 241, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kataoka, H.; Morimoto, M.; Nozaki, K.; Hashimoto, N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke 2007, 38, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, P.A.; Greaves, N.S.; Baguneid, M.; Bayat, A. Chemokines in Wound Healing and as Potential Therapeutic Targets for Reducing Cutaneous Scarring. Adv. Wound Care 2015, 4, 687–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Masaki, A.; Maeda, A.; Kilts, T.M.; Hara, E.S.; Komori, T.; Pham, H.; Kuboki, T.; Young, M.F. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via alpha5beta1 and TNFalpha. Matrix Biol. 2018, 68, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J.; Feldman, M.; Durum, S.K.; Hirano, T.; Vilcek, J.; Nicola, N.A. Cytokine Reference: A Compendium of Cytokines and Other Mediators of Host Defense; Academic Press: Boston, MA, USA, 2001. [Google Scholar]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Miles, R.H.; Paxton, T.P.; Zacheis, D.; Dries, D.J.; Gamelli, R.L. Systemic Administration of Interferon-γ Impairs Wound Healing. J. Surg. Res. 1994, 56, 288–294. [Google Scholar] [CrossRef]
- Kameyama, H.; Udagawa, O.; Hoshi, T.; Toukairin, Y.; Arai, T.; Nogami, M. The mRNA expressions and immunohistochemistry of factors involved in angiogenesis and lymphangiogenesis in the early stage of rat skin incision wounds. Leg. Med. 2015, 17, 255–260. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Qi, S.; Xu, Y. Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system. PLoS ONE 2014, 9, e92691. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Burdick, M.; Low, Q.E.; Kunkel, S.L.; Strieter, R.M. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J. Clin. Investig. 1998, 101, 1693–1698. [Google Scholar] [CrossRef]
- Kobayashi, Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008, 13, 2400–2407. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Balaji, S.; Le, L.D.; Crombleholme, T.M.; Keswani, S.G. Regenerative Wound Healing: The Role of Interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laato, M.; Heino, J.; Gerdin, B.; Kahari, V.M.; Niinikoski, J. Interferon-gamma-induced inhibition of wound healing in vivo and in vitro. Ann. Chir. Gynaecol. 2001, 90, 19–23. [Google Scholar]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef]
- Ishida, Y.; Kondo, T.; Takayasu, T.; Iwakura, Y.; Mukaida, N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J. Immunol. 2004, 172, 1848–1855. [Google Scholar] [CrossRef] [Green Version]
- Michel, N.A.; Zirlik, A.; Wolf, D. CD40L and Its Receptors in Atherothrombosis-An Update. Front. Cardiovasc. Med. 2017, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liang, D.; Li, X.; Liu, H.-H.; Zhang, X.; Zheng, M.; Dill, D.; Shi, X.; Qiao, Y.; Yeomans, D.; et al. The Role of Interleukin-1 in Wound Biology. Part II: In Vivo and Human Translational Studies. Anesth. Analg. 2010, 111, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Uslukaya, O.; Alabalik, U.; Turkoglu, A.; Kapan, M.; Bozdag, Z. Topical N-acetylcysteine improves wound healing comparable to dexpanthenol: An experimental study. Int. Surg. 2015, 100, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Ferreira, A.M.; Oberyszyn, T.M.; Bergdall, V.K.; Dipietro, L.A. Regulation of scar formation by vascular endothelial growth factor. Lab. Investig. A J. Tech. Methods Pathol. 2008, 88, 579–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Mace, K.A.; Yu, D.H.; Paydar, K.Z.; Boudreau, N.; Young, D.M. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007, 15, 636–645. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Taheri, S.; Liu, K.J.; Shi, H. Hypoxia-inducible factor 1 contributes to N-acetylcysteine’s protection in stroke. Free. Radic. Biol. Med. 2014, 68, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Lund, L.R.; Green, K.A.; Stoop, A.A.; Ploug, M.; Almholt, K.; Lilla, J.; Nielsen, B.S.; Christensen, I.J.; Craik, C.S.; Werb, Z.; et al. Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J. 2006, 25, 2686–2697. [Google Scholar] [CrossRef] [Green Version]
- Gemmati, D.; Vigliano, M.; Burini, F.; Mari, R.; El Mohsein, H.H.; Parmeggiani, F.; Serino, M.L. Coagulation Factor XIIIA (F13A1): Novel Perspectives in Treatment and Pharmacogenetics. Curr. Pharm. Des. 2016, 22, 1449–1459. [Google Scholar] [CrossRef]
- Simone, T.M.; Higgins, C.E.; Czekay, R.P.; Law, B.K.; Higgins, S.P.; Archambeault, J.; Kutz, S.M.; Higgins, P.J. SERPINE1: A Molecular Switch in the Proliferation-Migration Dichotomy in Wound-"Activated" Keratinocytes. Adv. Wound Care 2014, 3, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Stavrou, E.X.; Fang, C.; Bane, K.L.; Long, A.T.; Naudin, C.; Kucukal, E.; Gandhi, A.; Brett-Morris, A.; Mumaw, M.M.; Izadmehr, S.; et al. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Investig. 2018, 128, 944–959. [Google Scholar] [CrossRef] [Green Version]
- Drew, A.F.; Liu, H.; Davidson, J.M.; Daugherty, C.C.; Degen, J.L. Wound-healing defects in mice lacking fibrinogen. Blood 2001, 97, 3691–3698. [Google Scholar] [CrossRef] [Green Version]
- Achen, M.G.; Williams, R.A.; Minekus, M.P.; Thornton, G.E.; Stenvers, K.; Rogers, P.A.; Lederman, F.; Roufail, S.; Stacker, S.A. Localization of vascular endothelial growth factor-D in malignant melanoma suggests a role in tumour angiogenesis. J. Pathol. 2001, 193, 147–154. [Google Scholar] [CrossRef]
- Paquet-Fifield, S.; Levy, S.M.; Sato, T.; Shayan, R.; Karnezis, T.; Davydova, N.; Nowell, C.J.; Roufail, S.; Ma, G.Z.; Zhang, Y.F.; et al. Vascular endothelial growth factor-d modulates caliber and function of initial lymphatics in the dermis. J. Investig. Dermatol. 2013, 133, 2074–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plichta, J.K.; Radek, K.A. Sugar-coating wound repair: A review of FGF-10 and dermatan sulfate in wound healing and their potential application in burn wounds. J. Burn. Care Res. 2012, 33, 299–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, S. A novel enhancer of the wound healing process: The fibroblast growth factor-binding protein. Am. J. Pathol. 2011, 179, 2144–2147. [Google Scholar] [CrossRef] [PubMed]
- Kashpur, O.; LaPointe, D.; Ambady, S.; Ryder, E.F.; Dominko, T. FGF2-induced effects on transcriptome associated with regeneration competence in adult human fibroblasts. BMC Genom. 2013, 14, 656. [Google Scholar] [CrossRef] [Green Version]
- Li, J.F.; Duan, H.F.; Wu, C.T.; Zhang, D.J.; Deng, Y.; Yin, H.L.; Han, B.; Gong, H.C.; Wang, H.W.; Wang, Y.L. HGF accelerates wound healing by promoting the dedifferentiation of epidermal cells through beta1-integrin/ILK pathway. Biomed. Res. Int. 2013, 2013, 470418. [Google Scholar] [CrossRef] [Green Version]
- Bevan, D. Hepatocyte growth factor/scatter factor (HGF/SF) increases cutaneous wound healing in the diabetes mouse. Int. J. Exp. Pathol. 2000, 81, A4–A5. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell. Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.H.; Park, J.W.; Kim, D.Y. Enhanced regenerative healing efficacy of a highly skin-permeable growth factor nanocomplex in a full-thickness excisional mouse wound model. Int. J. Nanomed. 2014, 9, 4551–4567. [Google Scholar]
- Gilbertson, D.G.; Duff, M.E.; West, J.W.; Kelly, J.D.; Sheppard, P.O.; Hofstrand, P.D.; Gao, Z.; Shoemaker, K.; Bukowski, T.R.; Moore, M.; et al. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J. Biol. Chem. 2001, 276, 27406–27414. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.H.; Mace, K.A.; Hansen, S.L.; Boudreau, N.; Young, D.M. Effects of decreased insulin-like growth factor-1 stimulation on hypoxia inducible factor 1-alpha protein synthesis and function during cutaneous repair in diabetic mice. Wound Repair Regen. 2007, 15, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Kratz, G.; Lake, M.; Ljungstrom, K.; Forsberg, G.; Haegerstrand, A.; Gidlund, M. Effect of recombinant IGF binding protein-1 on primary cultures of human keratinocytes and fibroblasts: Selective enhancement of IGF-1 but not IGF-2-induced cell proliferation. Exp. Cell Res. 1992, 202, 381–385. [Google Scholar] [CrossRef]
- Hassmann-Poznanska, E.; Taranta, A.; Bialuk, I.; Poznanska, M.; Zajaczkiewicz, H.; Winnicka, M.M. Analysis of gene expression profiles in tympanic membrane following perforation using PCR Array in rats--preliminary investigation. Int. J. Pediatric Otorhinolaryngol. 2013, 77, 1753–1759. [Google Scholar] [CrossRef]
- Alfaro, M.P.; Deskins, D.L.; Wallus, M.; DasGupta, J.; Davidson, J.M.; Nanney, L.B.; Guney, M.A.; Gannon, M.; Young, P.P. A physiological role for connective tissue growth factor in early wound healing. Lab. Investig. A J. Tech. Methods Pathol. 2013, 93, 81–95. [Google Scholar] [CrossRef]
- de Winter, P.; Leoni, P.; Abraham, D. Connective tissue growth factor: Structure-function relationships of a mosaic, multifunctional protein. Growth Factors 2008, 26, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Lin, B.B.; Hu, H.W.; Lin, C.; Jin, W.Y.; Zhang, F.B.; Zhu, Y.A.; Lu, C.J.; Wei, X.J.; Chen, R.J. NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways. Biomed. Res. Int. 2014, 2014, 547187. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Yager, D.R.; Zhang, L.Y.; Liang, H.X.; Diegelmann, R.F.; Cohen, I.K. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J. Investig. Dermatol. 1996, 107, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.-H.; Huang, P.-H.; Hsu, Y.-J.; Peng, Y.-J.; Lee, C.-H.; Wang, J.-C.; Chen, J.-W.; Lin, S.-J. Inhibition of hypoxia inducible factor-1α attenuates abdominal aortic aneurysm progression through the down-regulation of matrix metalloproteinases. Sci. Rep. 2016, 6, 28612. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [Green Version]
- Meigs, T.E.; Fedor-Chaiken, M.; Kaplan, D.D.; Brackenbury, R.; Casey, P.J. Galpha12 and Galpha13 negatively regulate the adhesive functions of cadherin. J. Biol. Chem. 2002, 277, 24594–24600. [Google Scholar] [CrossRef] [Green Version]
- Hunter, M.V.; Lee, D.M.; Harris, T.J.; Fernandez-Gonzalez, R. Polarized E-cadherin endocytosis directs actomyosin remodeling during embryonic wound repair. J. Cell Biol. 2015, 210, 801–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarrigle, D.; Shan, D.; Yang, S.; Huang, X.Y. Role of tyrosine kinase Csk in G protein-coupled receptor- and receptor tyrosine kinase-induced fibroblast cell migration. J. Biol. Chem. 2006, 281, 10583–10588. [Google Scholar] [CrossRef] [Green Version]
- Cole, A.M.; Shi, J.; Ceccarelli, A.; Kim, Y.H.; Park, A.; Ganz, T. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood 2001, 97, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Zhao, N.; Long, S.; Ge, L.; Wang, A.; Sun, H.; Ran, X.; Zou, Z.; Wang, J.; Su, Y. Downregulation of miR-205 in migrating epithelial tongue facilitates skin wound re-epithelialization by derepressing ITGA5. Biochim. Biophys. Acta 2016, 1862, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Griffiths, M.; Wu, J.; Farese, R.V., Jr.; Sheppard, D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol. Cell. Biol. 2000, 20, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Breuss, J.M.; Gallo, J.; DeLisser, H.M.; Klimanskaya, I.V.; Folkesson, H.G.; Pittet, J.F.; Nishimura, S.L.; Aldape, K.; Landers, D.V.; Carpenter, W.; et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 1995, 108, 2241–2251. [Google Scholar] [CrossRef]
- Gilbert, R.W.D.; Vickaryous, M.K.; Viloria-Petit, A.M. Signalling by Transforming Growth Factor Beta Isoforms in Wound Healing and Tissue Regeneration. J. Dev. Biol. 2016, 4, 21. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-beta family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma 2012, 2, 18–28. [Google Scholar]
- El Gazaerly, H.; Elbardisey, D.M.; Eltokhy, H.M.; Teaama, D. Effect of transforming growth factor Beta 1 on wound healing in induced diabetic rats. Int. J. Health Sci. 2013, 7, 160–172. [Google Scholar] [CrossRef]
- Yamano, S.; Kuo, W.P.; Sukotjo, C. Downregulated gene expression of TGF-betas in diabetic oral wound healing. J. Cranio Maxillofac. Surg. 2013, 41, e42–e48. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, B.; Fan, J.; Guan, S.; Li, Y.; Chen, M.; Woodley, D.T.; Li, W. A “traffic control” role for TGFbeta3: Orchestrating dermal and epidermal cell motility during wound healing. J. Cell Biol. 2006, 172, 1093–1105. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tomann, P.; Andl, T.; Gallant, N.M.; Huelsken, J.; Jerchow, B.; Birchmeier, W.; Paus, R.; Piccolo, S.; Mikkola, M.L.; et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev. Cell 2009, 17, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, M.; Chen, W.L.; Takatori, A.; Peng, Z.; Zhang, L.; Mongan, M.; Parthasarathy, R.; Sartor, M.; Miller, M.; Yang, J.; et al. A role for the mitogen-activated protein kinase kinase kinase 1 in epithelial wound healing. Mol. Biol. Cell 2006, 17, 3446–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escuin-Ordinas, H.; Li, S.; Xie, M.W.; Sun, L.; Hugo, W.; Huang, R.R.; Jiao, J.; de-Faria, F.M.; Realegeno, S.; Krystofinski, P.; et al. Cutaneous wound healing through paradoxical MAPK activation by BRAF inhibitors. Nat. Commun. 2016, 7, 12348. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M. PTEN: A promising pharmacological target to enhance epithelial wound healing. Br. J. Pharmacol. 2007, 152, 1141–1144. [Google Scholar] [CrossRef] [Green Version]
- Gillis, J.; Gebremeskel, S.; Phipps, K.D.; MacNeil, L.A.; Sinal, C.J.; Johnston, B.; Hong, P.; Bezuhly, M. Effect of N-Acetylcysteine on Adipose-Derived Stem Cell and Autologous Fat Graft Survival in a Mouse Model. Plast. Reconstr. Surg. 2015, 136, 179e–188e. [Google Scholar] [CrossRef]
- Pietruski, P.; Paskal, W.; Paluch, Ł.; Paskal, A.M.; Nitek, Ż.; Włodarski, P.; Walecki, J.; Noszczyk, B. The Impact of N-Acetylcysteine on Autologous Fat Graft: First-in-Human Pilot Study. Aesthetic. Plast. Surg. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponugoti, B.; Xu, F.; Zhang, C.; Tian, C.; Pacios, S.; Graves, D.T. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J. Cell Biol. 2013, 203, 327–343. [Google Scholar] [CrossRef] [Green Version]
- Demir, E.O.; Cakmak, G.K.; Bakkal, H.; Turkcu, U.O.; Kandemir, N.; Demir, A.S.; Tascilar, O. N-acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J. Investig. Surg. 2011, 24, 151–158. [Google Scholar] [CrossRef]
- Tascilar, O.; Cakmak, G.; Emre, A.; Bakkal, H.; Kandemir, N.; Turkcu, U.; Demir, E. N-acetylcycsteine attenuates the deleterious effects of radiation therapy on inci-sional wound healing in rats. Hippokratia 2014, 18, 17–23. [Google Scholar] [PubMed]
- McMahon, S.; Charbonneau, M.; Grandmont, S.; Richard, D.E.; Dubois, C.M. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J. Biol. Chem. 2006, 281, 24171–24181. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Elsner, T.; Botella, L.M.; Velasco, B.; Corbí, A.; Attisano, L.; Bernabéu, C. Synergistic Cooperation between Hypoxia and Transforming Growth Factor-β Pathways on Human Vascular Endothelial Growth Factor Gene Expression. J. Biol. Chem. 2001, 276, 38527–38535. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Bragina, O.; Xu, Y.; Cao, Z.; Chen, H.; Zhou, B.; Morgan, M.; Lin, Y.; Jiang, B.-H.; Liu, K.J.; et al. Glucose up-regulates HIF-1α expression in primary cortical neurons in response to hypoxia through maintaining cellular redox status. J. Neurochem. 2008, 105, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Botusan, I.R.; Gupta Sunkari, V.; Savu, O.; Catrina, A.I.; Grünler, J.; Lindberg, S.; Pereira, T.; Ylä-Herttuala, S.; Poellinger, L.; Brismar, K.; et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef] [Green Version]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Aparicio, L.S.; Bernaldez-Sarabia, J.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H.; Diaz-Martinez, N.E.; Padilla-Camberos, E.; Licea-Navarro, A.; Castro-Cesena, A.B. Improvement of the wound healing properties of hydrogels with N-acetylcysteine through their modification with methacrylate-containing polymers. Biomater. Sci. 2021, 9, 726–744. [Google Scholar] [CrossRef]
- Yagiela, J.A. Vasoconstrictor agents for local anesthesia. Anesth. Prog. 1995, 42, 116–120. [Google Scholar] [PubMed]
- Wejman, J.; Pyzlak, M.; Szukiewicz, D.; Jarosz, D.; Tarnowski, W.; Szewczyk, G. Thrombospondin and VEGF-R: Is There a Correlation in Inflammatory Bowel Disease? Mediat. Inflamm. 2013, 2013, 908259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [Green Version]
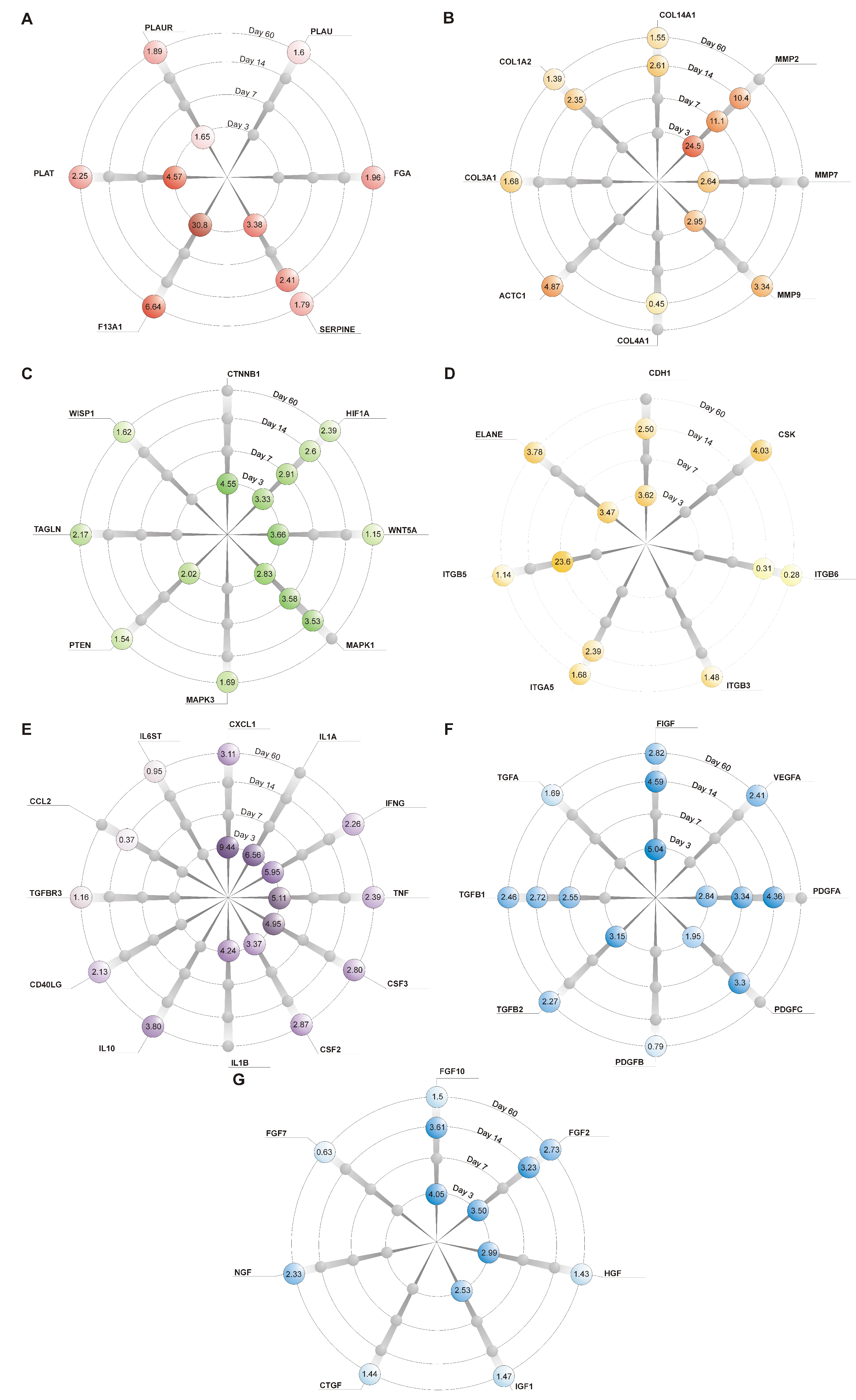
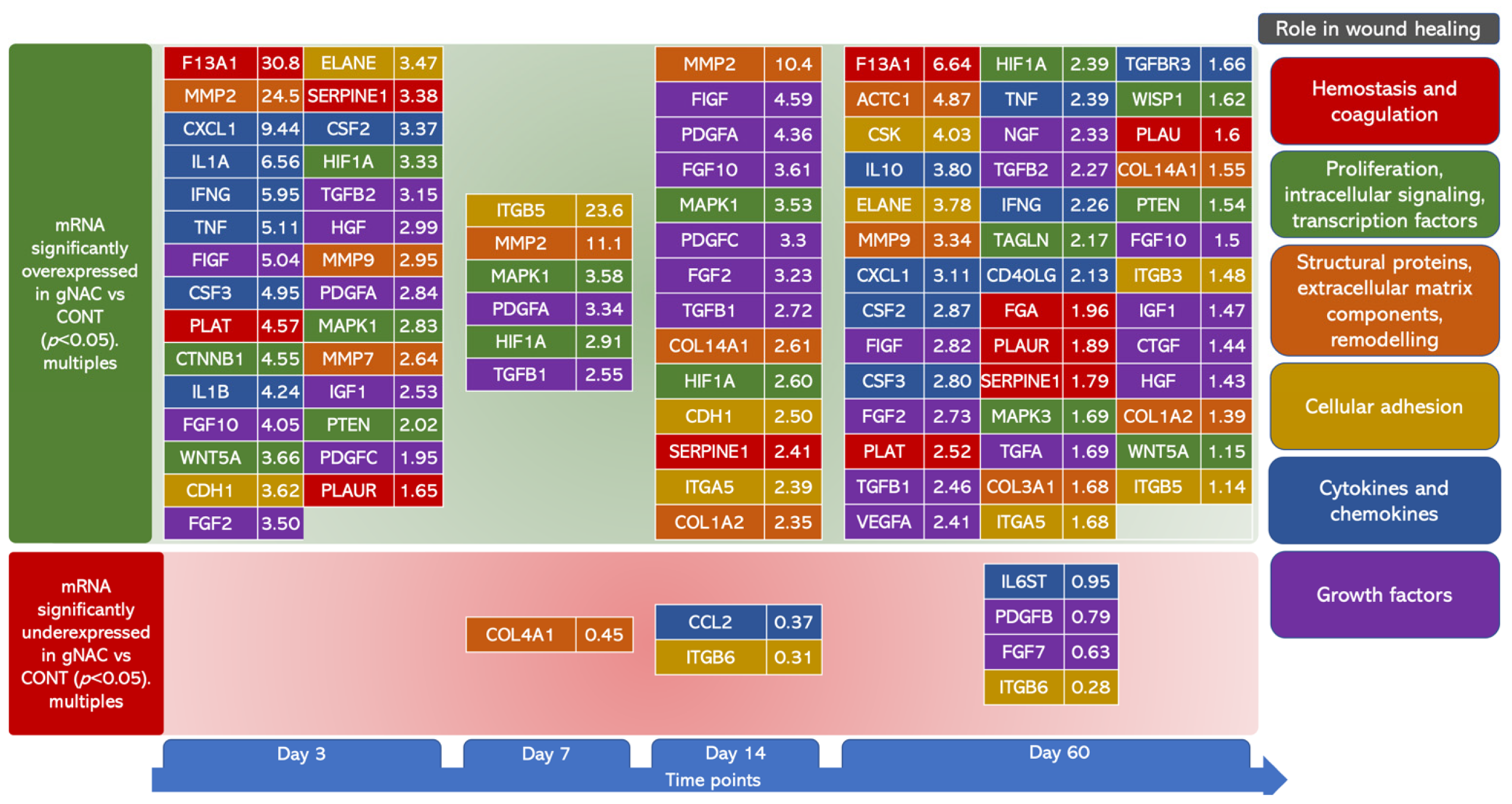
| Day3 | Day 7 | Day 14 | Day 60 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CONT | gNAC | p | CONT | gNAC | p | CONT | gNAC | p | NAC30 | p | CONT | gNAC | p | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||||
| CD31% of positive cells | 8.92 ± 4.49 | 10.5 ± 7.23 | 0.42 | 6.24 ± 3.92 | 5.22 ± 3.01 | 0.38 | 2.34 ± 1.81 | 3.25 ± 4.22 | 0.40 | 5.81 ± 6.37 | 0.04 | 3.08 ± 2.99 | 2.36 ± 1.27 | 0.88 |
| CD31 n of positive cells/mm2 | 179.58 ± 103.56 | 207.89 ± 129.16 | 0.47 | 135.09 ± 99.88 | 112.67 ± 76.35 | 0.45 | 41.182 ± 38.698 | 58.362 ± 79.914 | 0.41 | 105.51 ± 120.66 | 0.05 | 62.277 ± 70.29 | 46.962 ± 29.53 | 0.76 |
| CD68% of positive cells | 9.14 ± 5.06 | 9.51 ± 4.44 | 0.81 | 4.30 ± 3.29 | 3.52 ± 2.54 | 0.43 | 0.64 ± 0.65 | 1.56 ± 2.21 | 0.42 | 2.94 ± 3.12 | 0.006 | 1.39 ± 1.27 | 1.50 ± 1.75 | 0.82 |
| CD68 n of positive cells/mm2 | 334.75 ± 182.45 | 353.34 ± 175.26 | 0.75 | 164.72 ± 128.46 | 131.10 ± 90.12 | 0.37 | 23.003 ± 26.45 | 55.603 ± 80.79 | 0.46 | 106.04 ± 115.75 | 0.008 | 45.854 ± 43.33 | 47.069 ± 53.14 | 0.94 |
| MPO% of positive cells | 8.87 ± 4.83 | 8.84 ± 4.76 | 0.98 | 2.76 ± 1.61 | 2.88 ± 1.63 | 0.82 | 0.69 ± 0.40 | 0.88 ± 0.78 | 0.96 | 1.01 ± 1.02 | 0.27 | 1.81 ± 1.52 | 1.52 ± 1.11 | 0.48 |
| MPO n of positive cells/mm2 | 303.38 ± 192.10 | 322.24 ± 195.06 | 0.77 | 102.35 ± 63.24 | 100.76 ± 60.13 | 0.93 | 21.342 ± 13.62 | 28.009 ± 26.41 | 0.98 | 34.230 ± 37.23 | 0.21 | 51.286 ± 39.32 | 43.775 ± 33.28 | 0.54 |
| Folds of Expression Versus CONT (2ΔΔcT) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | Day 60 | |||||||||
| CONT | gNAC | CONT | gNAC | CONT | gNAC | CONT | gNAC | |||||
| Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | Mean ± SD | Mean ± SD | p | |
| ACTC1 | 1 ± 1.00 | 1.35 ± 1.50 | 0.44 | 1 ± 2.11 | 4.15 ± 2.29 | 0.09 | 1 ± 1.14 | 1.78 ± 1.53 | 0.21 | 1 ± 0.65 | 4.87 ± 2.27 | 0.00 |
| ACTA2 | 1 ± 2.79 | 1.74 ± 1.35 | 0.67 | 1 ± 0.66 | 0.98 ± 0.99 | 0.96 | 1 ± 0.67 | 0.69 ± 1.65 | 0.30 | 1 ± 0.38 | 0.51 ± 2.82 | 0.89 |
| ANGPT1 | 1 ± 3.80 | 2.29 ± 1.94 | 0.48 | 1 ± 2.53 | 1.11 ± 2.41 | 0.91 | 1 ± 2.85 | 0.82 ± 3.09 | 0.86 | 1 ± 1.35 | 0.31 ± 1.99 | 0.86 |
| COL14A1 | 1 ± 0.36 | 1.07 ± 1.44 | 0.78 | 1 ± 0.91 | 1.84 ± 1.80 | 0.15 | 1 ± 0.92 | 2.61 ± 2.06 | 0.04 | 1 ± 0.48 | 1.55 ± 1.55 | 0.02 |
| COL1A2 | 1 ± 0.80 | 1.37 ± 0.98 | 0.28 | 1 ± 1.04 | 1.68 ± 1.69 | 0.25 | 1 ± 0.41 | 2.35 ± 2.04 | 0.02 | 1 ± 0.45 | 1.39 ± 2.05 | 0.02 |
| COL3A1 | 1 ± 0.67 | 1.49 ± 1.09 | 0.16 | 1 ± 0.84 | 1.87 ± 2.55 | 0.22 | 1 ± 0.98 | 2.40 ± 2.29 | 0.43 | 1 ± 0.70 | 1.68 ± 1.97 | 0.01 |
| COL4A1 | 1 ± 0.46 | 1.32 ± 0.83 | 0.15 | 1 ± 0.64 | 0.45 ± 1.52 | 0.02 | 1 ± 0.51 | 0.86 ± 1.12 | 0.56 | 1 ± 0.30 | 0.65 ± 1.98 | 0.33 |
| COL4A3 | - | - | - | - | - | - | - | - | - | - | - | - |
| COL5A1 | 1 ± 0.68 | 1.13 ± 1.41 | 0.69 | 1 ± 0.61 | 0.76 ± 2.40 | 0.56 | 1 ± 0.67 | 1.57 ± 1.70 | 0.77 | 1 ± 0.49 | 1.12 ± 1.05 | 0.71 |
| COL5A3 | 1 ± 3.34 | 3.77 ± 1.05 | 0.34 | 1 ± 1.16 | 1.18 ± 1.13 | 0.69 | 1 ± 0.68 | 0.90 ± 1.38 | 0.75 | 1 ± 0.61 | 0.78 ± 1.61 | 0.28 |
| CCL2 | 1 ± 0.77 | 1.45 ± 1.96 | 0.35 | 1 ± 1.21 | 0.36 ± 1.57 | 0.05 | 1 ± 0.79 | 0.37 ± 1.77 | 0.02 | 1 ± 0.80 | 0.82 ± 1.72 | 0.06 |
| CD40LG | 1 ± 1.16 | 1.50 ± 1.28 | 0.32 | 1 ± 1.28 | 1.72 ± 2.38 | 0.34 | 1 ± 1.02 | 1.24 ± 2.28 | 0.66 | 1 ± 1.14 | 2.13 ± 1.77 | 0.04 |
| CDC42 | 1 ± 6.02 | 0.09 ± 1.77 | 0.25 | 1 ± 4.61 | 1.92 ± 2.87 | 0.68 | 1 ± 4.07 | 0.10 ± 2.19 | 0.11 | 1 ± 1.21 | 2.41 ± 2.27 | 0.23 |
| CDH1 | 1 ± 1.02 | 3.62 ± 1.04 | 0.00 | 1 ± 0.48 | 2.23 ± 1.64 | 0.15 | 1 ± 0.49 | 2.50 ± 2.11 | 0.02 | 1 ± 0.16 | 2.49 ± 2.04 | 2.01 |
| CSF2 | 1 ± 1.02 | 3.37 ± 2.09 | 0.01 | 1 ± 1.34 | 1.80 ± 2.78 | 0.35 | 1 ± 1.47 | 1.10 ± 1.83 | 0.85 | 1 ± 1.24 | 2.87 ± 1.96 | 0.04 |
| CSF3 | 1 ± 0.86 | 4.95 ± 2.40 | 0.02 | 1 ± 1.37 | 2.65 ± 2.94 | 0.15 | 1 ± 1.26 | 1.31 ± 2.85 | 0.66 | 1 ± 0.60 | 2.80 ± 1.91 | 0.01 |
| CSK | 1 ± 1.07 | 1.32 ± 1.04 | 0.44 | 1 ± 2.95 | 1.29 ± 3.16 | 0.81 | 1 ± 0.32 | 2.23 ± 2.71 | 0.58 | 1 ± 1.12 | 4.03 ± 1.42 | 0.01 |
| CTGF | 1 ± 1.89 | 2.22 ± 1.41 | 0.21 | 1 ± 0.80 | 1.16 ± 1.40 | 0.65 | 1 ± 0.91 | 2.14 ± 1.68 | 0.07 | 1 ± 0.33 | 1.44 ± 1.59 | 0.01 |
| CTNNB1 | 1 ± 2.69 | 4.55 ± 1.12 | 0.00 | 1 ± 0.83 | 0.59 ± 1.62 | 0.18 | 1 ± 0.94 | 0.73 ± 2.72 | 0.59 | 1 ± 0.46 | 1.21 ± 1.63 | 0.30 |
| CTSG | - | - | - | 1 ± 1.15 | 0.36 ± 1.59 | 0.30 | - | - | - | 1 ± 0.01 | 10.3 ± 0.65 | 0.67 |
| CTSK | 1 ± 2.16 | 2.55 ± 1.40 | 0.19 | 1 ± 0.92 | 1.20 ± 1.02 | 0.59 | 1 ± 0.63 | 1.60 ± 1.51 | 0.15 | 1 ± 0.46 | 1.29 ± 1.63 | 0.15 |
| CTSV | - | - | - | 1 ± 0.01 | 0.32 ± 9.92 | - | - | - | - | - | - | - |
| CXCL1 | 1 ± 2.79 | 9.44 ± 2.38 | 0.03 | 1 ± 2.41 | 1.45 ± 2.42 | 0.67 | 1 ± 2.02 | 1.25 ± 2.53 | 0.77 | 1 ± 0.64 | 3.11 ± 1.82 | 0.00 |
| CXCL2 | 1 ± 1.54 | 2.88 ± 3.26 | 0.14 | 1 ± 3.80 | 2.44 ± 3.50 | 0.51 | 1 ± 2.23 | 0.26 ± 4.26 | 0.19 | 1 ± 1.12 | 0.83 ± 3.74 | 0.84 |
| CXCL8 | ||||||||||||
| EGF | 1 ± 2.23 | 1.31 ± 1.25 | 0.72 | 1 ± 0.57 | 0.97 ± 1.69 | 0.94 | 1 ± 1.05 | 0.54 ± 1.50 | 0.21 | 1 ± 1.83 | 0.86 ± 1.19 | 0.74 |
| EGFR | 1 ± 1.14 | 1.10 ± 0.94 | 0.79 | 1 ± 0.48 | 1.04 ± 1.83 | 0.89 | 1 ± 0.82 | 1.22 ± 1.82 | 0.61 | 1 ± 0.10 | 0.75 ± 1.30 | 0.21 |
| ELANE | 1 ± 1.41 | 3.47 ± 2.13 | 0.03 | 1 ± 1.75 | 2.24 ± 2.55 | 0.26 | 1 ± 1.38 | 2.35 ± 2.30 | 0.16 | 1 ± 0.73 | 3.78 ± 2.07 | 0.00 |
| F13A1 | 1 ± 1.18 | 30.8 ± 2.68 | 0.01 | 1 ± 0.80 | 3.49 ± 2.95 | 0.23 | 1 ± 1.60 | 3.24 ± 1.87 | 0.12 | 1 ± 1.11 | 6.64 ± 2.25 | 0.01 |
| F3 | 1 ± 3.27 | 3.26 ± 1.29 | 0.26 | 1 ± 0.46 | 1.69 ± 1.29 | 0.06 | 1 ± 0.93 | 1.54 ± 1.53 | 0.28 | 1 ± 0.21 | 0.76 ± 1.83 | 0.08 |
| FGA | 1 ± 0.57 | 1.66 ± 1.27 | 0.07 | 1 ± 0.26 | 1.77 ± 1.44 | 0.17 | 1 ± 1.23 | 1.87 ± 1.90 | 0.23 | 1 ± 0.70 | 1.96 ± 1.39 | 0.00 |
| FGF10 | 1 ± 1.22 | 4.05 ± 2.43 | 0.04 | 1 ± 1.05 | 3.39 ± 1.95 | 0.06 | 1 ± 0.78 | 3.61 ± 2.81 | 0.03 | 1 ± 1.14 | 1.50 ± 2.24 | 0.00 |
| FGF2 | 1 ± 1.32 | 3.50 ± 1.85 | 0.02 | 1 ± 1.30 | 4.25 ± 2.87 | 0.06 | 1 ± 1.07 | 3.23 ± 2.37 | 0.03 | 1 ± 0.73 | 2.73 ± 2.08 | 0.00 |
| FGF7 | 1 ± 2.63 | 0.21 ± 2.11 | 0.10 | 1 ± 1.49 | 0.52 ± 2.18 | 0.30 | 1 ± 1.80 | 0.62 ± 1.93 | 0.54 | 1 ± 1.09 | 0.63 ± 1.60 | 0.02 |
| FIGF | 1 ± 0.93 | 5.04 ± 2.20 | 0.02 | 1 ± 1.41 | 2.66 ± 2.66 | 0.25 | 1 ± 1.50 | 4.59 ± 2.73 | 0.03 | 1 ± 0.21 | 2.82 ± 1.53 | 0.00 |
| IL1A | 1 ± 2.26 | 6.56 ± 2.12 | 0.03 | 1 ± 1.27 | 1.54 ± 2.45 | 0.46 | 1 ± 0.43 | 1.05 ± 1.55 | 0.70 | 1 ± 0.41 | 0.83 ± 1.77 | 0.13 |
| IL1B | 1 ± 1.66 | 4.24 ± 2.67 | 0.04 | 1 ± 2.28 | 1.49 ± 2.40 | 0.63 | 1 ± 1.38 | 1.11 ± 2.78 | 0.87 | 1 ± 0.43 | 2.97 ± 2.17 | 0.06 |
| IL4 | 1 ± 1.76 | 27.7 ± 4.70 | 0.62 | 1 ± 1.07 | 1.23 ± 2.44 | 0.81 | 1 ± 0.01 | 1.37 ± 2.98 | - | - | - | - |
| IL6 | 1 ± 1.37 | 3.06 ± 3.15 | 0.10 | 1 ± 2.25 | 1.27 ± 2.39 | 0.76 | 1 ± 1.49 | 0.85 ± 2.68 | 0.80 | 1 ± 0.55 | 3.16 ± 3.61 | 0.16 |
| IL6ST | 1 ± 1.01 | 1.36 ± 3.80 | 0.25 | 1 ± 0.66 | 0.84 ± 1.64 | 0.63 | 1 ± 0.48 | 0.79 ± 1.57 | 0.72 | 1 ± 0.29 | 0.95 ± 3.58 | 0.01 |
| ITGA1 | 1 ± 0.87 | 2.04 ± 3.84 | 0.92 | 1 ± 0.28 | 0.86 ± 0.92 | 0.43 | 1 ± 0.34 | 0.92 ± 1.51 | 0.79 | 1 ± 0.58 | 1.26 ± 4.35 | 0.94 |
| ITGA5 | 1 ± 1.11 | 1.90 ± 1.34 | 0.12 | 1 ± 0.76 | 1.69 ± 1.57 | 0.15 | 1 ± 0.68 | 2.39 ± 1.20 | 0.00 | 1 ± 0.37 | 1.68 ± 1.37 | 0.00 |
| ITGB1 | 1 ± 4.83 | 3.16 ± 3.33 | 0.46 | 1 ± 3.15 | 1.08 ± 3.16 | 0.94 | 1 ± 4.06 | 0.97 ± 3.44 | 0.98 | 1 ± 1.38 | 0.09 ± 3.05 | 0.71 |
| ITGB3 | 1 ± 1.08 | 2.25 ± 1.34 | 0.05 | 1 ± 0.67 | 1.33 ± 1.39 | 0.37 | 1 ± 0.36 | 1.30 ± 1.41 | 0.34 | 1 ± 0.54 | 1.48 ± 1.44 | 0.02 |
| ITGB5 | 1 ± 1.07 | 1.76 ± 0.92 | 0.17 | 1 ± 4.58 | 23.6 ± 1.64 | 0.02 | 1 ± 0.66 | 1.27 ± 1.34 | 0.44 | 1 ± 1.29 | 1.14 ± 1.85 | 0.02 |
| ITGB6 | 1 ± 2.77 | 0.91 ± 1.57 | 0.92 | 1 ± 0.62 | 0.26 ± 2.28 | 0.20 | 1 ± 1.00 | 0.31 ± 1.97 | 0.02 | 1 ± 1.31 | 0.28 ± 1.91 | 0.00 |
| MAPK1 | 1 ± 0.79 | 2.83 ± 1.17 | 0.00 | 1 ± 0.47 | 3.58 ± 2.08 | 0.00 | 1 ± 0.60 | 3.53 ± 1.98 | 0.00 | 1 ± 0.44 | 3.12 ± 1.91 | 1.92 |
| MAPK3 | 1 ± 0.74 | 1.58 ± 1.40 | 0.19 | 1 ± 0.35 | 1.40 ± 1.19 | 0.15 | 1 ± 0.58 | 1.65 ± 1.82 | 0.17 | 1 ± 0.39 | 1.69 ± 1.26 | 0.00 |
| MMP1 | 1 ± 0.95 | 2.08 ± 1.38 | 0.08 | 1 ± 0.48 | 1.71 ± 2.91 | 0.92 | 1 ± 0.14 | 1.46 ± 2.47 | 0.64 | 1 ± 2.24 | 2.94 ± 1.20 | 0.05 |
| MMP2 | 1 ± 1.88 | 24.5 ± 2.55 | 0.00 | 1 ± 0.54 | 11.1 ± 2.40 | 0.00 | 1 ± 0.79 | 10.4 ± 2.71 | 0.00 | 1 ± 0.89 | 7.72 ± 3.21 | 3.06 |
| MMP7 | 1 ± 0.76 | 2.64 ± 1.53 | 0.00 | 1 ± 1.70 | 0.97 ± 1.82 | 0.97 | 1 ± 1.40 | 1.16 ± 2.55 | 0.80 | 1 ± 0.73 | 1.73 ± 1.67 | 0.06 |
| MMP9 | 1 ± 1.07 | 2.95 ± 1.40 | 0.01 | 1 ± 1.42 | 2.28 ± 2.95 | 0.23 | 1 ± 1.71 | 1.07 ± 2.89 | 0.92 | 1 ± 0.94 | 3.34 ± 2.67 | 0.01 |
| NGF | 1 ± 1.34 | 1.44 ± 1.34 | 0.42 | 1 ± 1.31 | 1.53 ± 1.85 | 0.42 | 1 ± 1.14 | 1.33 ± 2.85 | 0.62 | 1 ± 1.03 | 2.33 ± 1.36 | 0.03 |
| PDGFA | 1 ± 0.34 | 2.84 ± 1.37 | 0.00 | 1 ± 0.94 | 3.34 ± 2.46 | 0.03 | 1 ± 1.48 | 4.36 ± 1.96 | 0.02 | 1 ± 0.91 | 2.73 ± 1.33 | 1.86 |
| PDGFB | 1 ± 0.80 | 0.71 ± 1.10 | 0.26 | 1 ± 0.51 | 0.58 ± 2.10 | 0.18 | 1 ± 0.74 | 0.98 ± 1.54 | 0.96 | 1 ± 0.41 | 0.79 ± 1.32 | 0.01 |
| PDGFC | 1 ± 0.55 | 1.95 ± 1.40 | 0.02 | 1 ± 0.61 | 1.94 ± 2.23 | 0.12 | 1 ± 0.63 | 3.30 ± 1.81 | 0.00 | 1 ± 0.24 | 2.06 ± 1.72 | 9.33 |
| PDGFD | 1 ± 1.09 | 1.78 ± 1.88 | 0.20 | 1 ± 0.59 | 0.82 ± 1.76 | 0.58 | 1 ± 0.88 | 0.89 ± 1.56 | 0.78 | 1 ± 0.33 | 0.48 ± 2.74 | 0.80 |
| PDGFRA | - | - | - | 1 ± 6.35 | 0.01 ± 1.04 | 0.8 | - | - | - | - | - | - |
| PDFGRB | 1 ± 1.83 | 1.90 ± 1.31 | 0.39 | 1 ± 1.21 | 2.67 ± 1.12 | 0.08 | 1 ± 3.15 | 0.83 ± 1.41 | 0.86 | 1 ± 2.05 | 3.11 ± 2.67 | 0.09 |
| PLAT | 1 ± 0.58 | 4.57 ± 2.07 | 0.01 | 1 ± 1.41 | 2.01 ± 2.24 | 0.24 | 1 ± 1.02 | 1.67 ± 2.02 | 0.28 | 1 ± 0.37 | 2.52 ± 1.70 | 0.00 |
| PLAU | 1 ± 1.16 | 2.33 ± 1.34 | 0.06 | 1 ± 1.35 | 1.87 ± 1.91 | 0.26 | 1 ± 0.56 | 0.97 ± 2.34 | 0.95 | 1 ± 0.82 | 1.60 ± 1.63 | 0.01 |
| PLAUR | 1 ± 0.46 | 1.65 ± 1.20 | 0.04 | 1 ± 0.82 | 1.26 ± 0.89 | 0.45 | 1 ± 0.55 | 1.28 ± 2.16 | 0.54 | 1 ± 0.33 | 1.89 ± 1.53 | 0.00 |
| PLG | 1 ± 0.24 | 0.46 ± 1.50 | 0.58 | 1 ± 0.90 | 2.92 ± 1.27 | 0.14 | 1 ± 1.91 | 1.27 ± 1.18 | 0.82 | 1 ± 2.10 | 1.01 ± 1.34 | 0.98 |
| PRTN3 | - | - | - | 1 ± 1.58 | 0.86 ± 1.09 | 0.85 | - | - | - | 1 ± 0.73 | 1.44 ± 0.97 | 0.43 |
| PTEN | 1 ± 0.40 | 2.02 ± 1.16 | 0.00 | 1 ± 0.32 | 1.05 ± 1.31 | 0.84 | 1 ± 0.57 | 1.65 ± 1.74 | 0.15 | 1 ± 0.24 | 1.54 ± 1.36 | 0.00 |
| PTGS2 | 1 ± 0.95 | 4.69 ± 2.58 | 0.10 | 1 ± 2.41 | 1.88 ± 2.99 | 0.50 | 1 ± 1.56 | 0.94 ± 2.05 | 0.93 | 1 ± 1.00 | 1.41 ± 1.87 | 0.21 |
| RAC1 | 1 ± 0.83 | 1.45 ± 1.37 | 0.27 | 1 ± 0.37 | 0.80 ± 0.76 | 0.23 | 1 ± 0.53 | 1.31 ± 1.72 | 0.42 | 1 ± 0.23 | 0.61 ± 2.89 | 0.21 |
| RHOA | 1 ± 2.25 | 1.49 ± 1.45 | 0.57 | 1 ± 0.98 | 0.80 ± 3.40 | 0.76 | 1 ± 0.82 | 2.60 ± 3.88 | 0.23 | 1 ± 0.78 | 0.87 ± 1.81 | 0.91 |
| SERPINE1 | 1 ± 0.64 | 3.38 ± 1.38 | 0.00 | 1 ± 1.65 | 1.83 ± 1.88 | 0.33 | 1 ± 0.54 | 2.41 ± 1.85 | 0.02 | 1 ± 0.44 | 1.79 ± 1.63 | 0.00 |
| STAT3 | 1 ± 4.31 | 3.54 ± 1.92 | 0.41 | 1 ± 3.58 | 1.10 ± 2.67 | 0.93 | 1 ± 2.49 | 0.88 ± 1.54 | 0.89 | 1 ± 2.47 | 0.63 ± 2.01 | 0.92 |
| TAGLN | 1 ± 4.85 | 4.99 ± 1.14 | 0.45 | 1 ± 0.48 | 1.08 ± 0.90 | 0.70 | 1 ± 0.72 | 1.78 ± 0.88 | 0.05 | 1 ± 0.32 | 2.17 ± 1.13 | 0.00 |
| TGFA | 1 ± 1.24 | 2.31 ± 1.15 | 0.06 | 1 ± 0.54 | 1.52 ± 1.55 | 0.19 | 1 ± 0.63 | 1.59 ± 1.34 | 0.13 | 1 ± 0.41 | 1.69 ± 1.70 | 0.00 |
| TGFB1 | 1 ± 0.44 | 1.78 ± 1.60 | 0.06 | 1 ± 0.45 | 2.55 ± 1.44 | 0.00 | 1 ± 0.77 | 2.72 ± 1.57 | 0.01 | 1 ± 0.35 | 2.46 ± 1.53 | 0.0 |
| TGFB2 | 1 ± 0.76 | 3.15 ± 1.35 | 0.00 | 1 ± 0.71 | 1.83 ± 1.56 | 0.09 | 1 ± 0.79 | 1.98 ± 2.18 | 0.13 | 1 ± 0.49 | 2.27 ± 1.57 | 0.00 |
| TGFBR3 | 1 ± 0.94 | 1.11 ± 1.22 | 0.76 | 1 ± 0.51 | 1.78 ± 1.54 | 0.07 | 1 ± 0.69 | 1.90 ± 1.62 | 0.07 | 1 ± 0.32 | 1.66 ± 1.58 | 0.02 |
| TIMP1 | 1 ± 2.67 | 2.54 ± 1.39 | 0.28 | 1 ± 0.36 | 0.71 ± 0.94 | 0.11 | 1 ± 0.64 | 1.58 ± 2.89 | 0.40 | 1 ± 0.22 | 0.68 ± 2.28 | 0.83 |
| TNF | 1 ± 0.91 | 5.11 ± 2.12 | 0.01 | 1 ± 1.01 | 1.84 ± 2.19 | 0.23 | 1 ± 0.97 | 1.90 ± 1.92 | 0.16 | 1 ± 0.94 | 2.39 ± 2.09 | 0.00 |
| VEGFA | - | - | - | - | - | - | 1 ± 0.67 | 0.77 ± 2.74 | 0.68 | 1 ± 0.01 | 2.41 ± 1.04 | 0.03 |
| VEGFB | 1 ± 4.65 | 10.3 ± 1.30 | 0.25 | 1 ± 0.73 | 0.87 ± 1.82 | 0.72 | 1 ± 0.96 | 1.04 ± 2.03 | 0.93 | 1 ± 0.91 | 0.89 ± 1.69 | 0.15 |
| VEGFC | 1 ± 0.66 | 1.18 ± 1.42 | 0.58 | 1 ± 0.55 | 1.19 ± 1.03 | 0.47 | 1 ± 0.56 | 1.78 ± 1.72 | 0.10 | 1 ± 0.23 | 1.59 ± 1.28 | 0.07 |
| WISP1 | 1 ± 0.68 | 1.60 ± 1.39 | 0.13 | 1 ± 0.63 | 1.55 ± 1.11 | 0.12 | 1 ± 0.42 | 1.60 ± 1.77 | 0.53 | 1 ± 0.50 | 1.62 ± 1.50 | 0.02 |
| WNT5A | 1 ± 0.78 | 3.66 ± 1.35 | 0.00 | 1 ± 0.34 | 1.02 ± 1.99 | 0.94 | 1 ± 0.38 | 1.55 ± 1.99 | 0.07 | 1 ± 0.51 | 1.15 ± 2.10 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskal, W.; Kopka, M.; Stachura, A.; Paskal, A.M.; Pietruski, P.; Pełka, K.; Woessner, A.E.; Quinn, K.P.; Galus, R.; Wejman, J.; et al. Single Dose of N-Acetylcysteine in Local Anesthesia Increases Expression of HIF1α, MAPK1, TGFβ1 and Growth Factors in Rat Wound Healing. Int. J. Mol. Sci. 2021, 22, 8659. https://doi.org/10.3390/ijms22168659
Paskal W, Kopka M, Stachura A, Paskal AM, Pietruski P, Pełka K, Woessner AE, Quinn KP, Galus R, Wejman J, et al. Single Dose of N-Acetylcysteine in Local Anesthesia Increases Expression of HIF1α, MAPK1, TGFβ1 and Growth Factors in Rat Wound Healing. International Journal of Molecular Sciences. 2021; 22(16):8659. https://doi.org/10.3390/ijms22168659
Chicago/Turabian StylePaskal, Wiktor, Michał Kopka, Albert Stachura, Adriana M. Paskal, Piotr Pietruski, Kacper Pełka, Alan E. Woessner, Kyle P. Quinn, Ryszard Galus, Jarosław Wejman, and et al. 2021. "Single Dose of N-Acetylcysteine in Local Anesthesia Increases Expression of HIF1α, MAPK1, TGFβ1 and Growth Factors in Rat Wound Healing" International Journal of Molecular Sciences 22, no. 16: 8659. https://doi.org/10.3390/ijms22168659
APA StylePaskal, W., Kopka, M., Stachura, A., Paskal, A. M., Pietruski, P., Pełka, K., Woessner, A. E., Quinn, K. P., Galus, R., Wejman, J., & Włodarski, P. (2021). Single Dose of N-Acetylcysteine in Local Anesthesia Increases Expression of HIF1α, MAPK1, TGFβ1 and Growth Factors in Rat Wound Healing. International Journal of Molecular Sciences, 22(16), 8659. https://doi.org/10.3390/ijms22168659







