Abstract
To overcome the limitations of the Limulus amebocyte lysate (LAL) assay method for the diagnosis of invasive fungal infection, we applied a reaction system combining recombinant β-glucan binding proteins and a scanning single-molecule counting (SSMC) method. A novel (1→3)-β-D-glucan recognition protein (S-BGRP) and a (1→6)-β-glucanase mutant protein were prepared and tested for the binding of (1→6)-branched (1→3)-β-D-glucan from fungi. S-BGRP and (1→6)-β-glucanase mutant proteins reacted with β-glucan from Candida and Aspergillus spp. Although LAL cross-reacted with plant-derived β-glucans, the new detection system using the SSMC method showed low sensitivity to plant (1→3)-β-D-glucan, which significantly improved the appearance of false positives, a recognized problem with the LAL method. Measurement of β-glucan levels by the SSMC method using recombinant β-glucan-binding proteins may be useful for the diagnosis of fungal infections. This study shows that this detection system could be a new alternative diagnostic method to the LAL method.
1. Introduction
As the number of immunocompromised patients increases, the number of opportunistic infections is also increasing annually [1]. The β-D-glucan (BG) test is a valuable diagnostic standard that covers a wide range of invasive fungal infections that have a poor prognosis [2]. Estimating the number for the year based on the data for June of the relevant year, the number of BG tests in Japan has rapidly increased from approximately 590,000 in 2010 to approximately 990,000 in 2018 [3]. Therefore, the medical value of the BG test is increasing. However, the current BG test method, the factor-G-based Limulus amebocyte lysate (LAL) assay, can produce false-positive reactions to 1,3-and 1,4-type BGs derived from cellulosic materials in the environment (e.g., gauze) [4,5,6].
Since the LAL assay reagent in BG testing detects both fungal and plant BGs [6], it is necessary to prevent contamination with plant BGs [7]. (1→3)-β-D-glucans are polysaccharides produced by plants, bacteria, and fungi. The chain length, degree of branching, and number and location of other glycosidic linkages, such as (1→4)-β- and/or (1→6)-β-bonds, vary widely. (1→3)-linked (1→4)-β-glucan structures are typically found in plant materials [8], but (1→3)- and (1→6)-β-D-glucosyl chains are more common in fungi [9]. (1→3)-β-D-glucans activate cells of the innate immune system by binding to glucan-specific receptors, such as dectin-1 and other cell membrane receptors [9]. Unlike mammals, insects have a unique pattern recognition receptor for (1→3)-β-D-glucan [9,10,11,12], called the BG recognition protein (BGRP). The interaction of BGRP with BG activates serine proteases, which in turn react with prophenoloxidase and phenol oxidase [10]. This reaction system can be applied to the detection of BGs using body fluids obtained from silkworm larvae [11,12]. We created a BG-binding protein, S-BGRP, by genetic modification of the insect BGRP [13]. In the design of the mutant, we modified 23 amino acids of the lepidopteran BGRP, while preserving the amino acid residues essential for BG binding [11]. Then, we could obtain the recombinant S-BGRP in high yield [13]. Furthermore, S-BGRP is a very stable protein that does not lose BG-binding ability even when heated to 90 °C [7]. Furthermore, S-BGRP maintains BG-binding ability over a wide pH range and exhibits more stable binding than antibodies [7,14].
Furthermore, we created a (1→6)-β-glucanase mutant protein (16BGM) with high specificity for fungal 1,6-β-glucan by replacing catalytic glutamic acid residue with glutamine to eliminate its hydrolase activity [15]. We have reported that 16BGM exhibits reactivity to various Candida fungi [16]. Since most of the fungal BGs have (1→3)-β-D-glucan and (1→6)-β-D-glucan, the combination of S-BGRP and 16BGM will enable the detection of fungal BGs with high specificity [17]. In this study, we developed a prototype BG reactive probe using S-BGRP and 16BGM and employed the scanning single-molecule counting (SSMC) method for detection.
2. Results
2.1. SSMC Method for BG Detection Using 16BGM and S-BGRP
To investigate the BG reactivity of 16BGM and S-BGRP, we prepared biotin-labeled 16BGM and Alexa Fluor 647 (AF647)-labeled S-BGRP and examined them. Streptavidin-conjugated magnetic beads were mixed with both proteins and samples to dissociate the AF647-labeled S-BGRP from the samples captured by the beads, and the number of AF647 molecules in the elution was counted (Figure 1).
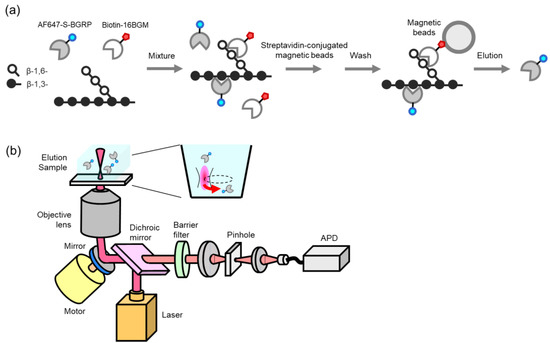
Figure 1.
Schematic diagrams of molecular counting interacted with β-D-glucans. AF647-labeled molecules bound to BG complex were eluted (a), the elution sample was scanned, and a molecule in the confocal site was counted (b).
2.2. Comparison of BG Detection by LAL and SSMC
Japanese cedar pollen is suggested to contain BG, which can be a false-positive for the diagnosis of invasive fungal infections [7]. Therefore, we also examined the SSMC method in combination with 16BGM and S-BGRP. First, we calculated the Pachyman equivalents of CSBG, ASBG, cedar pollen, and algae BG (laminarin) in 10 ng/mL of BG using the LAL method. As a result, 490 and 819 pg/mL of ASBG and CSBG, 51.6 pg/mL of pollen BG (Pollen BG) of Japanese cedar and 338 pg/mL of laminarin were found, as shown in Table 1. The fluorescence counts of CSBG, ASBG, Pollen BG, and laminarin at each concentration were determined using the SSMC method (Figure 2). The results showed that CSBG and ASBG had similar counts of approximately 5 × 104 at 100 pg/mL, whereas the Pollen BG and laminarin count was lower, at approximately 3 × 104 at 10 µg/mL and 1.7 × 104 at 50 µg/mL, respectively, indicating that it is difficult to detect Japanese cedar pollen BG and algae BG using the SSMC method.

Table 1.
BG content of CSBG, ASBG, Pollen BG, and laminarin in the LAL test.
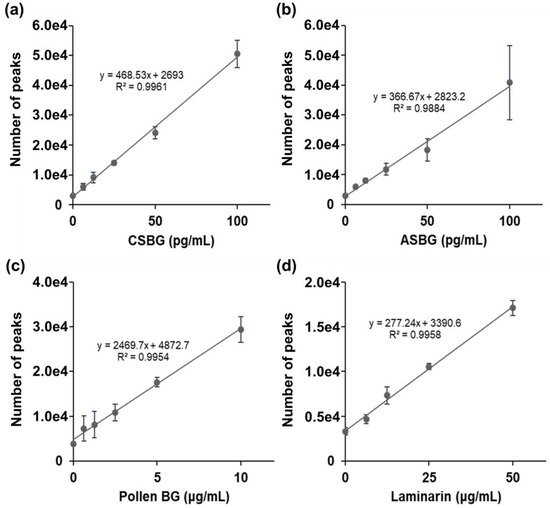
Figure 2.
Detection of various BGs using S-BGRP and 16BGM. CSBG (a), ASBG (b), Pollen BG (c), and laminarin (d) are detected using 0.5 μg/mL AF647-labeled S-BGRP and 0.1 μg/mL biotin-labeled 16BGM. Each value represents a mean ± standard deviation (SD). n = 6 for 0 g/mL; n = 3 for all other concentrations.
2.3. Correlation between LAL Method and SSMC Method
As shown in Figure 3, there was a positive correlation between the Pachyman equivalent value of CSBG and the fluorescence count value of the SSMC method (Pearson’s R2 = 0.9928). These results indicate that the SSMC method has comparable reactivity to the LAL method for BG from candida.
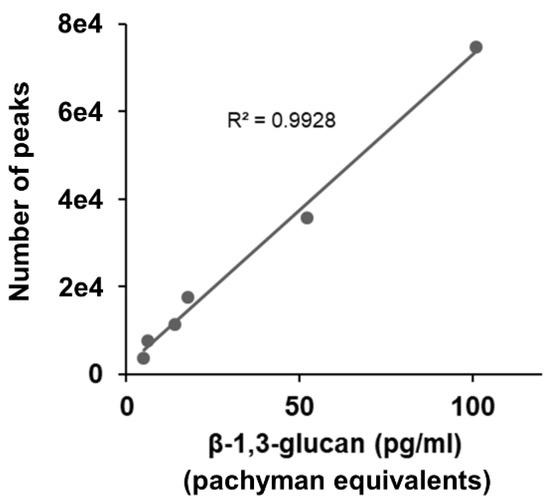
Figure 3.
Comparison of LAL test and SSMC. 0–1000 pg/mL CSBG (two-fold serial dilution) are detected using LAL test and SSMC. A total of 0.5 μg/mL AF647-labeled S-BGRP and 0.1 μg/mL biotin-labeled 16BGM were used in SSMC.
2.4. Effect of Human Sera on the Reaction with CSBG in the SSMC Method
We then investigated the effect of human serum components on the detection of BG using the SSMC method with S-BGRP and 16BGM. We compared the detection of CSBG (50 pg/mL) in the presence of 10% human serum from eight different donors with that in the absence of serum (Figure 4). When CSBG measured in the absence of serum was set at 100%, the BG detection rates for all the normal human sera samples were between 73% and 95%, indicating that the presence of 10% serum did not significantly interfere with the detection of BGRP or the SSMC method using 16BGM.
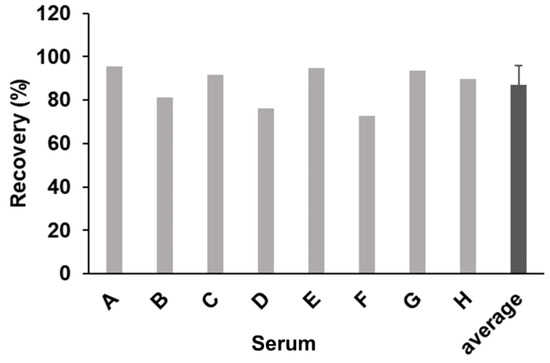
Figure 4.
Spiking of CSBG into human sera and recovery test. A total of 50 pg/mL CSBG spiked into human sera were detected using 0.5 μg/mL AF647-labeled S-BGRP and 0.1 μg/mL biotin-labeled 16BGM. An error bar of average represents SD.
2.5. Comparison of Reactivity to Immunoglobulin Preparations
Since it has been reported that some immunoglobulin preparations for intravenous injection may contain LAL-detectable BGs, we investigated the reactivity of the SSMC method to human immunoglobulin preparations [17]. Six immunoglobulin preparations were assayed by LAL and SSMC methods; the BG concentrations are shown as Pachyman and CSBG equivalents (Table 2). Venilon-I was low in both the LAL and SSMC methods, but Venoglobulin IH 5%, Gammagard, and Glovenin-I were above the detection limit in the LAL method. Polyglobin-N was detected as 60.6 and 41.1 pg/mL in the LAL and SSMC methods, respectively, whereas Sanglopor was detected as a high value of 1.5 ng/mL in the LAL method but was below the detection limit (32.4 pg/mL) in the SSMC method. These results showed that the SSMC method showed almost no positive results in the detection of BG in immunoglobulin preparations, whereas the LAL method registered high BG detection depending on the product.

Table 2.
Comparison of LAL and SSMC methods for determination of BG content in intravenous immunoglobulin.
3. Discussion
The mainstream serodiagnostic method for invasive fungal infection is the horseshoe crab Limulus assay [2,18], which is highly sensitive in detecting BGs but is also highly reactive to BGs other than fungi, resulting in problems in diagnostic accuracy.
Moro et al. reported the detection of BG in immunoglobulin preparations [19]. This report stated that several immunoglobulin products react positively in the LAL method and that these immunoglobulin products may be a source of false-positive substances [6,20]. In the present study, we also found that several immunoglobulin products contained LAL-reactive BGs. This outcome seems to be reproducible, as similar results have been obtained in other studies [20,21]. In contrast, the newly developed SSMC method for BG detection using a combination of 16BGM and S-BGRP did not detect BG in immunoglobulin products (Table 2) but showed high reactivity to candida-derived BG and CSBG. The reactivity against fungal BG was highly correlated with that of the LAL method, and the reaction remained effective even in the presence of serum components, indicating that the method could be applied to the detection of candida BGs in clinical specimens from infected patients. No clear answer has been found as to why Sanglopor showed LAL reactivity or what the substance was that reacted with the LAL method. Since our SSMC method suggests that (1→6)-branched (1→3)-β-D-glucan is specifically detected, the LAL-reactive substance in Sanglopor may contain only linear (1→3)-β-D-glucan. At least, it is unlikely that Sanglopor contains (1→6)-branched fungal-derived BGs.
Although certain cutoff values have been set for BG detection in the diagnosis of invasive fungal infection [22], the criteria for diagnosis in all cases are to detect BG at a concentration of several tens of pg/mL or higher [23]. Since (1→3)-β-D-glucan levels in the blood diagnostics of patients with fungal infections can be very low, at the pg/mL level, high sensitivity is required for diagnosis [2]. However, (1→3)-β-D-glucan is present not only in fungi but also in algae and higher plants [24,25,26]. Most fungal (1→3)-β-D-glucans can be structurally characterized by their branched (1→6)-β-glucan structure, and (1→6)-branched (1→3)-β-D-glucan is abundant in the cell walls of yeast and fungi [9]. In contrast, plant β-glucans contain 1,4-β-glucosyl-linked (1→3)-β-D-glucans, which are different from fungal (1→3)-β-D-glucans. Focusing on these structural features, we measured (1→6)-β-glucan and (1→3)-β-D-glucan to distinguish them from plant cellulose. Another approach for the simultaneous measurement of (1→6)-β-glucan and (1→3)-β-D-glucan can be performed using 16BGM and S-BGRP conjugated with fragment-luciferase, where both proteins accumulate on β-glucan and luciferase activity is expressed [17]. However, the BG concentration that can be detected by the fragment-luciferase-based assay is in the µg/mL range, and the assay lacks sufficient sensitivity for BG concentrations in the pg/mL range [17].
Several papers have been published on the detection of BGs by enzyme-linked immunosorbent assay. Using antibodies specific for (1→3)-β-D-glucan, it is possible to detect fungal BGs in the ng/mL range with a monoclonal antibody against laminarin and bovine serum albumin conjugate [27] and in the pg/mL range with a monoclonal antibody against laminariheptaose-human transferrin-conjugate antigen [28]. All of these are specific to the linear (1→3)-β-D-glucan structure, and the possibility of cross-reactivity with plant BGs other than fungi cannot be completely excluded [29].
The SSMC method is an ultra-sensitive analytical method with high sensitivity and a good signal-to-noise ratio, which can detect fluorescent molecules even at concentrations of several tens of aM [30]. To improve the measurement efficiency, we labeled the binding protein with the fluorescent dye Alexa Fluor 647, which is resistant to photobleaching [31], and adopted a method to rapidly count fluorescent molecules in a limited volume of the confocal site. The SSMC method has also been applied to the sensitive detection of oligonucleotide interactions hybridized to specific sequences [30]. The SSMC method has the potential to become an analytical method with excellent specificity and sensitivity for the detection of interactions between macromolecules and small molecules.
In this study, we successfully detected the interaction between polysaccharides and proteins with high sensitivity using the SSMC method. BG detection by the SSMC method uses recombinant proteins expressed in E. coli and is, therefore, inexpensive due to the availability of large quantities of reagents. This method has the potential to become a new diagnostic method for invasive fungal infections that can compensate for defects in the LAL method. Our next aim is to evaluate the practicality of this method in clinical specimens from patients with invasive fungal infections. In addition, since this measurement device is based on a confocal microscope system, one of the future tasks is to make the measurement device more compact. If a space-saving and compact measurement device can be realized, it may be used as a bedside measurement device.
4. Materials and Methods
4.1. Fungal Culture
Aspergillus fumigatus NBRC 30870 and Candida albicans NBRC 1385 were purchased from the National Institute of Technology and Evaluation (Tokyo, Japan). Fungi were maintained on Sabouraud agar (Difco, Franklin Lakes, NJ, USA) at 25 °C and transferred once every three months. To obtain yeast cells of Candida albicans, a C-limiting medium originally described by Shepherd and Sullivan [32] was used unless stated otherwise. The medium contained (per liter): sucrose, 10 g; (NH4)2SO4, 2 g; KH2PO4, 2 g; CaCl2·2H2O, 0.05 g; MgSO4·7H2O, 0.05 g; ZnSO4·7H2O, 1 mg; CuSO4·5H2O, 1 mg; FeSO4·7H2O, 0.01 g; biotin, 25 µg; final pH, 5.2. Five liters of medium were placed in the glass jar of a microfermenter (Sakura Seiki, Tokyo, Japan) and cultured at 27 °C with 5 L/min of aeration and stirring at 400 rpm. The cells were precipitated by adding ethanol and then dried with acetone.
4.2. Reagents
Sodium hypochlorite solution, sodium hydroxide (NaOH), and Dulbecco’s phosphate-buffered saline (PBS) were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Distilled water (DIW) was purchased from Otsuka Co., Ltd. (Tokyo, Japan). Laminarin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Human sera were purchased from BioIVT (Hicksville, NY, USA). Intravenous immunoglobulin products, Venilon-I, Venoglobulin IH 5%, Gammagard, Glovenin-I, Polyglobin-N, and Sanglopor were obtained from KM Biologics (Kumamoto, Japan), Japan Blood Products Organization (Tokyo, Japan), Shire Japan (Tokyo, Japan), Nihon Pharmaceutical (Tokyo, Japan), Japanese Red Cross Society (Tokyo, Japan), and CSL Behring (King of Prussia, PA, USA).
4.3. Preparation of Candida Albicans-Derived Soluble Beta-Glucan
Preparation of the NaClO-oxidized yeast was followed by the procedure used in a previous paper [33]. Briefly, Candida albicans NBRC 1385 yeast cells (2 g) were suspended in 200 mL of 0.1 M NaOH and oxidized with an appropriate volume of NaClO solution for 1 d at 4 °C. After the reaction was completed, the reaction mixture was dialyzed extensively with distilled water to collect the non-dialyzable and insoluble fraction, or the reaction product was directly centrifuged to collect the insoluble fraction. The insoluble fractions were dried by washing with ethanol and acetone. Each dried fraction was suspended in Me2SO and extracted by occasional sonication and boiling. After centrifugation to remove any insoluble fraction, the solubilized part was again precipitated with ethanol and acetone. The resulting Me2SO-soluble material was designated as CSBG.
4.4. Preparation of Aspergillus Cell Wall Glucans
Acetone-dried mycelium of Aspergillus fumigatus (2 g) was suspended in 200 mL of 0.1 M NaOH with NaClO at various available chlorine concentrations for 1 d at 4 °C. After the reaction was complete, the reaction mixture was centrifuged to collect the insoluble fraction. The insoluble fractions were dried by washing with ethanol and acetone (NaClO-treated Aspergillus, OX-Asp). OX-Asp suspended in 8 M urea was autoclaved at 120 °C for 20 min, and the resulting solutions were centrifuged (12,000 rpm, 20 min) and divided into supernatant and precipitant. Each fraction was dried in ethanol and acetone. The supernatant fraction was designated as ASBG.
4.5. Purification of Pollen BG Using BGRP-Assisted Affinity Chromatography
Preparation of the Pollen BG was followed by the procedure used in a previous paper [7]. To prepare the BGRP column, a Hitrap NHS-activated column (Cytiva, Marlborough, MA, USA) was conjugated with S-BGRP according to the manufacturer’s protocol. Before use for BG purification, the BGRP column was washed with 10 mL of PBS that was flowed through a peristaltic pump set at 2 mL/min. The pollen extract was prepared as follows. Five grams of Japanese cedar pollen (Fujifilm Wako, Osaka, Japan) were suspended in 1 L of 0.1M NaHCO3 aqueous solution and stirred for 30 min at room temperature. Then the supernatant was collected by 2 step centrifugations with 6000 × g for 10 min and 10,000 × g for 10 min. Finally, the supernatant was filtered with a 0.20 µm aPES bottle top filter (Thermofisher Scientific, Waltham, MA, USA). Then, 900 mL of the pollen extract was passed through the column at a rate of 2 mL/min. After washing the column with 10 mL of PBS (wash fluid), the BG was eluted as five fractions (900 µL/fraction) using 0.03 M NaOH. The eluates containing Pollen BG were immediately neutralized with 300 µL of 0.1 M phosphate citrate buffer (pH 3.0), dialyzed against deionized water, and lyophilized.
4.6. Preparation of S-BGRP and 16BGM
S-BGRP and 16BGM were prepared as recombinant proteins using the E. coli BL21 and pCold-I expression systems [15,16]. The proteins were purified using a TALON affinity resin column (Takara Bio, Shiga, Japan) as reported previously. The purified proteins were fluorescently labeled using the Alexa Fluor 647 Antibody Labeling Kit (Invitrogen, Carlsbad, CA, USA), and biotin-labeled using the Biotin Labeling Kit-NH2 (DOJINDO Laboratories, Kumamoto, Japan).
4.7. Basic Assay Method for SSMC
Ten microliters of β-glucan sample, 10 µL of human serum, and 60 µL of PBS containing 1% BSA were pretreated at 95 °C for 1 min and then mixed with AF647-labeled S-BGRP (5 µg/mL, 10 µL) and biotin-labeled 16BGM (1 µg/mL, 10 µL), and incubated at 37 °C for 30 min with agitation and light shielding. Then, 10 µg of streptavidin-conjugated magnetic beads (Invitrogen, Carlsbad, CA, USA) were mixed with the mixture and incubated at 37 °C for 1 min with agitation. The beads were washed five times with 100 µL of PBS containing 0.1% Triton X-100 and once with 10 mM glycine-HCl buffer (pH 2.5) containing 0.1% Triton X-100, and then 20 µL of 10 mM Tris-HCl (pH 8.0) containing 0.1% SDS was added to the magnetic beads. After incubation at 95 °C for 30 s, the filtrate containing AF647-labeled S-BGRP was eluted by filtering the supernatant with a 0.22 μm filter plate (Millipore, MA, USA) and measured by SSMC.
4.8. SSMC
SSMC used a laser light source (Showa Optronics, Tokyo, Japan) with a wavelength of 642 nm, barrier filter transmitting 660–710 nm (Chroma Technology, Bellows Falls, VT, USA), 40× water immersion objective lens (UAPON40XW340, NA = 1.15; Olympus, Tokyo, Japan), and avalanche photodiode (APD) (Perkin Elmer, Waltham, MA, USA). Optical scanning was performed at a scanning speed of 69 mm sec-1 and with an excitation light of 1 mW. Fluorescence time series data were acquired for 600 s and analyzed as previously described [30].
Author Contributions
Conceptualization, H.N., T.T. and Y.A.; methodology, H.N. and T.T.; data analysis, H.N. and T.T.; investigation, H.N., T.T., D.Y. and Y.A.; project administration, H.N., T.T. and Y.A.; resources, Y.A., D.Y., T.K., K.-i.I., N.O.; writing—original draft preparation, H.N. and Y.A.; writing—review and editing, H.N., T.T., D.Y. and Y.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (C), JSPS KAKENHI, grant number JP18K06636 to N.O., JP18K06723 to Y.A., JP 20K07487 to D.Y., a Grant-in-Aid for Research Activity Start-up JSPS KAKENHI, grant number JP20K22791 to T.K. This work was also supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (N.O.) and KAKENHI (Y.A., 22590407).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank J. Tetsui and T. Watanabe for their technical assistance.
Conflicts of Interest
Two of the authors (H.N. and T.T.) were salaried employees of Olympus Corporation. The authors (Y.A., D.Y., T.K., K.-i.I. and N.O.) declare no conflicts of interest.
References
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar]
- Obayashi, T.; Yoshida, M.; Mori, T.; Goto, H.; Yasuoka, A.; Iwasaki, H.; Teshima, H.; Kohno, S.; Horiuchi, A.; Ito, A.; et al. Plasma (1-->3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 1995, 345, 17–20. [Google Scholar] [CrossRef]
- Japan Ministry of Health, Labor and Welfare, Statistics by Social Medical Practices. Available online: https://www.mhlw.go.jp/toukei/list/26-19c.html (accessed on 27 June 2019).
- Nakao, A.; Yasui, M.; Kawagoe, T.; Tamura, H.; Tanaka, S.; Takagi, H. False-positive endotoxemia derives from gauze glucan after hepatectomy for hepatocellular carcinoma with cirrhosis. Hepatogastroenterology 1997, 44, 1413–1418. [Google Scholar]
- Kato, A.; Takita, T.; Furuhashi, M.; Takahashi, T.; Maruyama, Y.; Hishida, A. Elevation of blood (1-->3)-beta-D-glucan concentrations in hemodialysis patients. Nephron 2001, 89, 15–19. [Google Scholar] [CrossRef]
- Nagasawa, K.; Yano, T.; Kitabayashi, G.; Morimoto, H.; Yamada, Y.; Ohata, A.; Usami, M.; Horiuchi, T. Experimental proof of contamination of blood components by (1-->3)-beta-D-glucan caused by filtration with cellulose filters in the manufacturing process. J. Artif. Organs 2003, 6, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Kim, C.; Yamanaka, D.; Ishibashi, K.I.; Tanaka, H.; Ohno, N.; Adachi, Y. Possibility of Japanese cedar pollen causing false positives in the deep mycosis test. Int. J. Mol. Sci. 2021, 22, 2135. [Google Scholar] [CrossRef] [PubMed]
- Hrmova, M.; Farkas, V.; Lahnstein, J.; Fincher, G.B. A Barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-beta-D-glucans. J. Biol. Chem. 2007, 282, 12951–12962. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal β-Glucans and Mammalian Immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Ochiai, M.; Ashida, M. Purification of a beta-1,3-glucan recognition protein in the prophenoloxidase activating system from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1988, 263, 12056–12062. [Google Scholar] [CrossRef]
- Ochiai, M.; Ashida, M. A pattern-recognition protein for beta-1,3-glucan. The binding domain and the cDNA cloning of beta-1,3-glucan recognition protein from the silkworm, Bombyx mori. J. Biol. Chem. 2000, 275, 4995–5002. [Google Scholar] [CrossRef]
- Takahasi, K.; Ochiai, M.; Horiuchi, M.; Kumeta, H.; Ogura, K.; Ashida, M.; Inagaki, F. Solution structure of the silkworm betaGRP/GNBP3 N-terminal domain reveals the mechanism for beta-1,3-glucan-specific recognition. Proc. Natl. Acad. Sci. USA 2009, 106, 11679–11684. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ishii, M.; Kanno, T.; Tetsui, J.; Ishibashi, K.-I.; Yamanaka, D.; Miura, N.; Ohno, N. N-Terminal (1-->3)-beta-D-glucan recognition proteins from insects recognize the difference in ultra-structures of (1-->3)-beta-D-glucan. Int. J. Mol. Sci. 2019, 20, 3498. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Kurokawa, T. A sensitive sandwich ELISA to measure (1-->3)-beta-D-glucan levels in blood. J. Immunol. Methods 2011, 365, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Kanno, T.; Ishibashi, K.-i.; Yamanaka, D.; Motoi, A.; Motoi, M.; Ohno, N. Binding specificity of a new artificial beta-glucan recognition protein and its application to beta-glucan detection in mushroom extracts. Int. J. Med. Mushrooms 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, D.; Takatsu, K.; Kimura, M.; Swamydas, M.; Ohnishi, H.; Umeyama, T.; Oyama, F.; Lionakis, M.S.; Ohno, N. Development of a novel β-1,6-glucan-specific detection system using functionally-modified recombinant endo-β-1,6-glucanase. J. Biol. Chem. 2020, 295, 5362–5376. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, D.; Kurita, S.; Hanayama, Y.; Adachi, Y. Split enzyme-based biosensors for structural characterization of soluble and insoluble β-glucans. Int. J. Mol. Sci. 2021, 22, 1576. [Google Scholar] [CrossRef]
- Obayashi, T. The plasma (1->3)-beta-D-glucan assay, a Japanese contribution to the diagnosis of invasive fungal infection. Med. Mycol. J. 2017, 58, J141–J147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moro, H.; Koshio, N.; Bamba, Y.; Koizumi, T.; Cho, H.; Aoki, N.; Hayashi, M.; Tsubata, C.; Sakagami, A.; Sato, M.; et al. The effect of intravenous gamma-globulin reagents on the measurement results of (1->3)-β-D-glucan. Kansenshogaku Zasshi 2017, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Buchacher, A.; Krause, D.; Wiry, G.; Weinberger, J. Elevated endotoxin levels in human intravenous immunoglobulin concentrates caused by (1->3)-beta-D-glucans. PDA J. Pharm. Sci. Technol. 2010, 64, 536–544. [Google Scholar]
- Ogawa, M.; Hori, H.; Niiguchi, S.; Azuma, E.; Komada, Y. False-positive plasma (1-->3)-beta-D-glucan test following immunoglobulin product replacement in an adult bone marrow recipient. Int. J. Hematol. 2004, 80, 97–98. [Google Scholar] [CrossRef]
- Yoshida, K.; Niki, Y.; Mitekura, H.; Nakajima, M.; Kawane, H.; Matsushima, T. A discrepancy in the values of serum (1-3)-beta-D-glucan measured by two kits using different methods. Nihon Ishinkin Gakkai Zasshi 2001, 42, 237–242. [Google Scholar] [CrossRef][Green Version]
- Kato, K.; Onoda, S.; Asano, J.; Fukaya, S.; Yoshida, S. Evaluation of the clinical cutoff level of serum (1 --> 3)-beta-D-glucan in patients with connective tissue diseases complicated by deep fungal infections. Mod. Rheumatol. 2010, 20, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Shibakami, M.; Tsubouchi, G.; Nakamura, M.; Hayashi, M. Polysaccharide nanofiber made from euglenoid alga. Carbohydr. Polym. 2013, 93, 499–505. [Google Scholar] [CrossRef]
- Rylander, R.; Fogelmark, B.; McWilliam, A.; Currie, A. (1-->3)-beta-D-glucan may contribute to pollen sensitivity. Clin. Exp. Immunol. 1999, 115, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Lemmon, B.E.; Stone, B.A.; Olsen, O.A. Cell wall (1-->3)- and (1-->3, 1-->4)-beta-glucans during early grain development in rice (Oryza sativa L.). Planta 1997, 202, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Sander, I.; Fleischer, C.; Borowitzki, G.; Brüning, T.; Raulf-Heimsoth, M. Development of a two-site enzyme immunoassay based on monoclonal antibodies to measure airborne exposure to (1-->3)-beta-D-glucan. J. Immunol. Methods 2008, 337, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sunamura, E.-i.; Iwasaki, M.; Shiina, S.; Kitahara, S.-i.; Yotani, T.; Manabe, M.; Miyazaki, O. A novel enzyme immunoassay for the measurement of plasma (1-->3)-beta-D-glucan levels. J. Immunol. Methods 2020, 487, 112872. [Google Scholar] [CrossRef]
- Meikle, P.J.; Bonig, I.; Hoogenraad, N.J.; Clarke, A.E.; Stone, B.A. The location of (1→3)-β-glucans in the walls of pollen tubes of Nicotiana alata using a (1→3)-β-glucan-specific monoclonal antibody. Planta 1991, 185, 1–8. [Google Scholar] [CrossRef]
- Hanami, T.; Tanabe, T.; Hanashi, T.; Yamaguchi, M.; Nakata, H.; Mitani, Y.; Kimura, Y.; Soma, T.; Usui, K.; Isobe, M.; et al. Scanning single-molecule counting system for Eprobe with highly simple and effective approach. PLoS ONE 2020, 15, e0243319. [Google Scholar] [CrossRef]
- Anderson, G.P.; Nerurkar, N.L. Improved fluoroimmunoassays using the dye Alexa Fluor 647 with the RAPTOR, a fiber optic biosensor. J. Immunol. Methods 2002, 271, 17–24. [Google Scholar] [CrossRef]
- Shepherd, M.G.; Sullivan, P.A. The production and growth characteristics of yeast and mycelial forms of Candida albicans in continuous culture. J. Gen. Microbiol. 1976, 93, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Uchiyama, M.; Tsuzuki, A.; Tokunaka, K.; Miura, N.N.; Adachi, Y.; Aizawa, M.W.; Tamura, H.; Tanaka, S.; Yadomae, T. Solubilization of yeast cell-wall beta-(1->3)-D-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr. Res. 1999, 316, 161–172. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).