New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy
Abstract
:1. Introduction
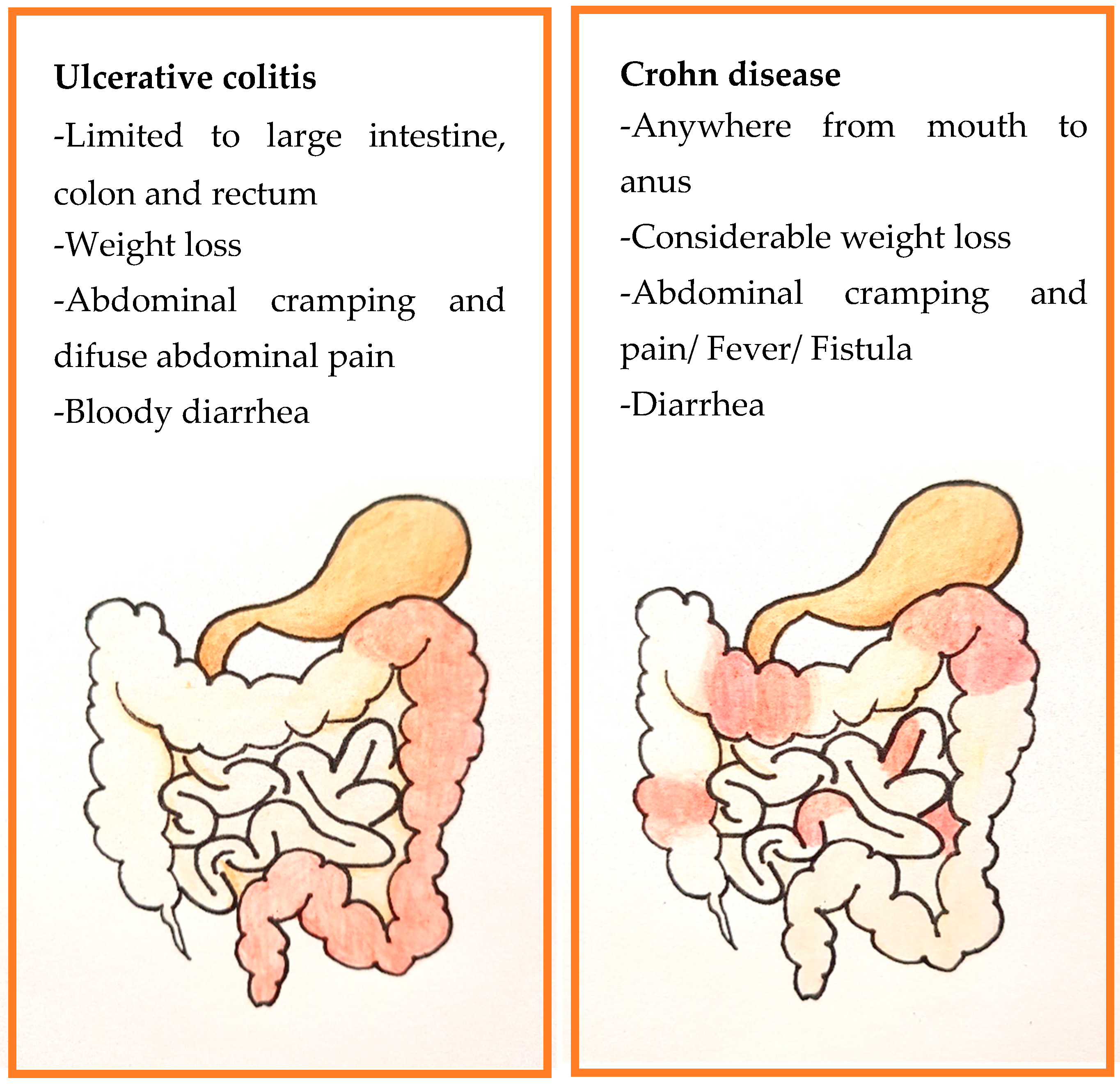
2. Inflammatory Bowel Disease: Ulcerative Colitis and Crohn’s Disease
3. IBD Treatments
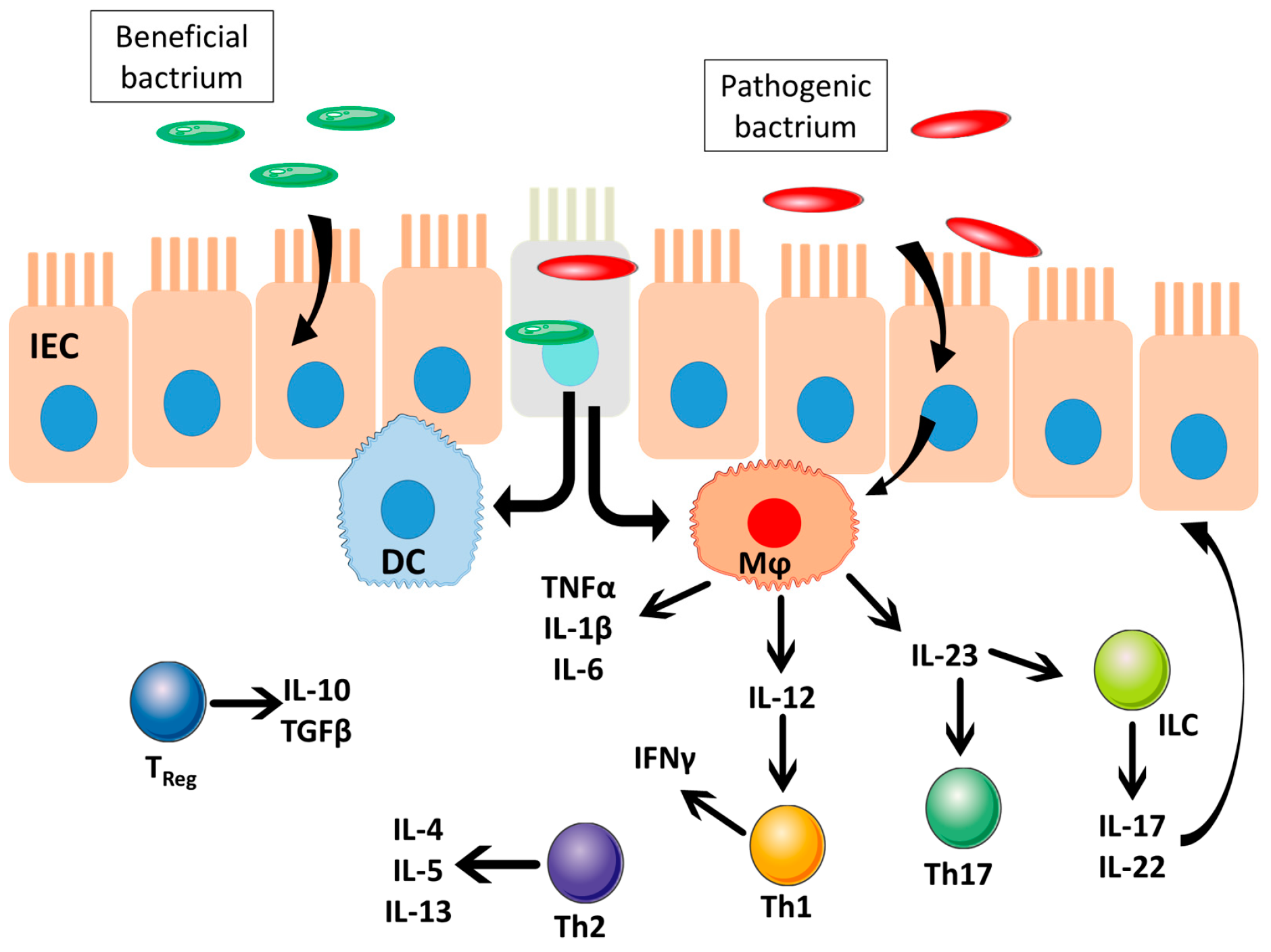
4. IBD Physiology and Microbiota Population
5. Oral CDDS for IBD Management
5.1. Oral CDDS Following the pH-Sensitive Approach
5.2. Oral CDDS Following Enzyme-Sensitive Approach
5.3. Oral CDDS Following Inflammation-Targeting and ROS-Responsive Approach
5.4. Oral CDDS Following a Dual or Combined Approach
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shah, N.K.; Rane, B.R.; Gujarathi, N.A. Developments in colon specific drug delivery systems—A review. Pharma Sci. Monit. 2014, 5, 95–110. [Google Scholar]
- Singh, G.; Kumar, D.; Singh, M.; Sharma, D.; Kaur, S. Emerging techniques and challenges in colon drug delivery systems. J. Appl. Pharm. Sci. 2012, 2, 139–147. [Google Scholar]
- Malik, K.; Goswami, L.; Kothiyal, P.; Mukhopadhyay, S. A Review on Colon Targeting Drug Delivery System: Novel Approaches, Anatomy and Evaluation. Pharma Innov. 2012, 1, 1–12. [Google Scholar]
- Lautenschläger, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Nakai, D.; Miyake, M.; Hashimoto, A. Comparison of the Intestinal Drug Permeation and Accumulation between Normal Human Intestinal Tissues and Human Intestinal Tissues with Ulcerative Colitis. J. Pharm. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory Bowel Disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.M.; Zhang, H.J. MiRNAs as new molecular insights into inflammatory bowel disease: Crucial regulators in autoimmunity and inflammation. World J. Gastroenterol. 2016, 22, 2206–2218. [Google Scholar] [CrossRef]
- Kim, D.H.; Cheon, J.H. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Inflammatory Bowel Disease | British Society for Immunology. Available online: https://www.immunology.org/es/public-information/bitesized-immunology/immune-dysfunction/enfermedad-inflamatoria-intestinal (accessed on 25 August 2020).
- Kwame, G.; Yeboah, G.S.W. Site-Specific Drug Delivery to the Gastrointestinal Tract. J. Mol. Pharm. Org. Process Res. 2013, 01, 240. [Google Scholar]
- Jay, M.; Beihn, R.M.; Digenis, G.A.; Deland, F.H.; Caldwell, L.; Mlodozeniec, A.R. Disposition of radiolabelled suppositories in humans. J. Pharm. Pharmacol. 1985, 37, 266–268. [Google Scholar] [CrossRef]
- Newton, A.M.J.; Lakshmanan, P. Effect of HPMC—E15 LV premium Polymer on Release Profile and Compression Characteristics of Chitosan/Pectin Colon Targeted Mesalamine Matrix Tablets and in vitro Study on Effect of pH Impact on the Drug Release Profile. Recent Pat. Drug Deliv. Formul. 2014, 8, 46–62. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Longman, R.; Harbus, M.; Dannenberg, K.; Scherl, E.J. Crohn’s Disease: Evolution, Epigenetics, and the Emerging Role of Microbiome-Targeted Therapies. Curr. Gastroenterol. Rep. 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Neurath, M.F.; Travis, S.P.L. Mucosal healing in inflammatory bowel diseases: A systematic review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Mawatari, S.; Shibata, N.; Takada, K.; Yoshikawa, H.; Arakawa, A.; Yosida, Y. Application of a biomagnetic measurement system (BMS) to the evaluation of gastrointestinal transit of intestinal pressure-controlled colon delivery capsules (PCDCs) in human subjects. Pharm. Res. 2000, 17, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.; Sharma, S.; Malik, A.; Kaur, J.; Prasad, K.K.; Sinha, S.K.; Singh, K. Small intestinal bacterial overgrowth and orocecal transit time in patients of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 2594–2598. [Google Scholar] [CrossRef]
- Philip, A.K.; Philip, B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med. J. 2010, 25, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Coupe, A.J.; Davis, S.S.; Wilding, I.R. Variation in Gastrointestinal Transit of Pharmaceutical Dosage Forms in Healthy Subjects. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1991, 8, 360–364. [Google Scholar]
- Rao, K.A.; Yazaki, E.; Evans, D.F.; Carbon, R. Objective evaluation of small bowel and colonic transit time using pH telemetry in athletes with gastrointestinal symptoms. Br. J. Sports Med. 2004, 38, 482–487. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Hebden, J.M.; Blackshaw, P.E.; Perkins, A.C.; Wilson, C.G.; Spiller, R.C. Limited exposure of the healthy distal colon to orally-dosed formulation is further exaggerated in active left-sided ulcerative colitis. Aliment. Pharmacol. Ther. 2000, 14, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J.; Christensen, L.A.; Jacobsen, B.A.; Rasmussen, S.N. Very low intraluminal colonic pH in patients with active ulcerative colitis. Dig. Dis. Sci. 1993, 38, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Bratten, J.; Jones, M.P. New directions in the assessment of gastric function: Clinical applications of physiologic measurements. Dig. Dis. 2006, 24, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Hada, R.; Nakajima, H.; Fukuda, S.; Munakata, A. Improved localizing method of radiopill in measurement of entire gastrointestinal pH profiles: Colonic luminal pH in normal subjects and patients with Crohn’s disease. Am. J. Gastroenterol. 1997, 92, 114–118. [Google Scholar]
- Collnot, E.M.; Ali, H.; Lehr, C.M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Colonic drug delivery: Prodrug approach. Pharm. Res. 2001, 18, 557–564. [Google Scholar] [CrossRef]
- Gorbach, S.L. Intestinal Microflora. Gastroenterology 1971, 60, 1110–1129. [Google Scholar] [CrossRef]
- Simon, G.L.; Gorbach, S.L. The human intestinal microflora. Dig. Dis. Sci. 1986, 31, 147–162. [Google Scholar] [CrossRef]
- Moore, W.E.C.; Holdeman, L.V. Discussion of Current Bacteriological Investigations of the Relationships between Instestinal Flora, Diet, and Colon Cancer. Cancer Res. 1975, 35. [Google Scholar]
- Rubinstein, A. Microbially controlled drug delivery to the colon. Biopharm. Drug Dispos. 1990, 11, 465–475. [Google Scholar] [CrossRef]
- Scheline, R.R. Metabolism of foreign compounds by gastrointestinal microorganisms. Pharmacol. Rev. 1973, 25, 451–532. [Google Scholar] [PubMed]
- Prasanth, V.; Jayaprakash, R.; Mathew, S. Colon Specific Drug Delivery Systems: A Review on Various Pharmaceutical Approaches. J. Appl. Pharm. Sci. 2012, 2, 163–169. [Google Scholar]
- Sartor, R.B. Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc. Natl. Acad. Sci. USA 2008, 105, 16413–16414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.-C.; Stappenbeck, T.S. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 127–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.B.; Dhake, A.S. Multiparticulate approach: An emerging trend in colon specific drug delivery for chronotherapy. J. Appl. Pharm. Sci. 2011, 1, 59–63. [Google Scholar]
- Xiao, B.; Merlin, D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin. Drug Deliv. 2012, 9, 1393–1407. [Google Scholar] [CrossRef]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J. Pharmacol. Exp. Ther. 2005, 315, 196–202. [Google Scholar] [CrossRef]
- Beloqui, A.; Cococ, R.; Alhouayek, M.; Solińis, M.Á.; Rodríguez-Gáscon, A.; Muccioli, G.G.; Préat, V. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int. J. Pharm. 2013, 454, 775–783. [Google Scholar] [CrossRef]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef]
- Naeem, M.; Bae, J.; Oshi, M.A.; Kim, M.S.; Moon, H.R.; Lee, B.L.; Im, E.; Jung, Y.; Yoo, J.W. Colon-targeted delivery of cyclosporine a using dual-functional eudragit® FS30D/PLGA nanoparticles ameliorates murine experimental colitis. Int. J. Nanomed. 2018, 13, 1225–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshi, M.A.; Naeem, M.; Bae, J.; Kim, J.; Lee, J.; Hasan, N.; Kim, W.; Im, E.; Jung, Y.; Yoo, J.W. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr. Polym. 2018, 198, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vass, P.; Démuth, B.; Hirsch, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; Nagy, Z.K.; Marosi, G. Drying technology strategies for colon-targeted oral delivery of biopharmaceuticals. J. Control. Release 2019, 296, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, Y.D.; Bahramsoltani, R.; Marques, A.M.; Naseri, R.; Rahimi, R.; Haratipour, P.; Panah, A.I.; Farzaei, M.H.; Abdollahi, M. A systematic review of nano formulation of natural products for the treatment of inflammatory bowel disease: Drug delivery and pharmacological targets. DARU J. Pharm. Sci. 2018, 26, 229–239. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef]
- Varum, F.; Freire, A.C.; Bravo, R.; Basit, A.W. OPTICORETM, an innovative and accurate colonic targeting technology. Int. J. Pharm. 2020, 583. [Google Scholar] [CrossRef]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.W.; Park, B.J.; Han, H.K. Strategic approaches for colon targeted drug delivery: An overview of recent advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: A brief review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar]
- Yoon, S.W.; Shin, D.H.; Kim, J.S. Liposomal itraconazole formulation for the treatment of glioblastoma using inclusion complex with HP-β-CD. J. Pharm. Investig. 2019, 49, 477–483. [Google Scholar] [CrossRef]
- Bazan, L.; Bendas, E.R.; El Gazayerly, O.N.; Badawy, S.S. Comparative pharmaceutical study on colon targeted micro-particles of celecoxib: In-vitro–in-vivo evaluation. Drug Deliv. 2016, 23, 3339–3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Hatton, G.B.; Merchant, H.A.; Basit, A.W. Gastrointestinal release behaviour of modified-release drug products: Dynamic dissolution testing of mesalazine formulations. Int. J. Pharm. 2015, 484, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Battat, R.; Dulai, P.S.; Parker, C.E.; Sandborn, W.J.; Feagan, B.G.; Jairath, V. Innovations in Oral Therapies for Inflammatory Bowel Disease. Drugs 2019, 79, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Ashford, M.; Brayden, D.J. Local delivery of macromolecules to treat diseases associated with the colon. Adv. Drug Deliv. Rev. 2018, 136–137, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Baker, J.R.; Fioritto, A.F.; Wang, Y.; Luo, R.; Li, S.; Wen, B.; Bly, M.; Tsume, Y.; Koenigsknecht, M.J.; et al. Measurement of in vivo gastrointestinal release and dissolution of three locally acting mesalamine formulations in regions of the human gastrointestinal tract. Mol. Pharm. 2017, 14, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, V.C.; Fadda, H.M.; McConnell, E.L.; Khela, M.K.; Evans, D.F.; Basit, A.W. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm. Res. 2008, 25, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef]
- Duan, H.; Lü, S.; Gao, C.; Bai, X.; Qin, H.; Wei, Y.; Wu, X.; Liu, M. Mucoadhesive microparticulates based on polysaccharide for target dual drug delivery of 5-aminosalicylic acid and curcumin to inflamed colon. Colloids Surf. B Biointerfaces 2016, 145, 510–519. [Google Scholar] [CrossRef]
- Cong, Z.; Shi, Y.; Wang, Y.Y.; Wang, Y.Y.; Niu, J.; Chen, N.; Xue, H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018, 107, 855–864. [Google Scholar] [CrossRef]
- Gareb, B.; Dijkstra, G.; Kosterink, J.G.W.; Frijlink, H.W. Development of novel zero-order release budesonide tablets for the treatment of ileo-colonic inflammatory bowel disease and comparison with formulations currently used in clinical practice. Int. J. Pharm. 2019, 554, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gareb, B.; Posthumus, S.; Beugeling, M.; Koopmans, P.; Touw, D.J.; Dijkstra, G.; Kosterink, J.G.W.; Frijlink, H.W. Towards the oral treatment of ileo-colonic inflammatory bowel disease with infliximab tablets: Development and validation of the production process. Pharmaceutics 2019, 11, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Alvarez, M.; Coll, C.; Gonzalez-Alvarez, I.; Giménez, C.; Aznar, E.; Martínez-Bisbal, M.C.; Lozoya-Agulló, I.; Bermejo, M.; Martínez-Máñez, R.; Sancenón, F. Gated Mesoporous Silica Nanocarriers for a “Two-Step” Targeted System to Colonic Tissue. Mol. Pharm. 2017, 14, 4442–4453. [Google Scholar] [CrossRef]
- Deng, X.Q.; Zhang, H.B.; Wang, G.F.; Xu, D.; Zhang, W.Y.; Wang, Q.S.; Cui, Y.L. Colon-specific microspheres loaded with puerarin reduce tumorigenesis and metastasis in colitis-associated colorectal cancer. Int. J. Pharm. 2019, 570, 118644. [Google Scholar] [CrossRef]
- Shi, X.; Yan, Y.; Wang, P.; Sun, Y.; Zhang, D.; Zou, Y.; Hu, S.; Zhang, L.; Xing, J.; Dong, Y. In vitro and in vivo study of pH-sensitive and colon-targeting P(LE-IA-MEG) hydrogel microspheres used for ulcerative colitis therapy. Eur. J. Pharm. Biopharm. 2018, 122, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Malviya, T.; Joshi, S.; Dwivedi, L.M.; Baranwal, K.; Pandey, A.K.; Singh, V. Synthesis of Aloevera/Acrylonitrile based Nanoparticles for targeted drug delivery of 5-Aminosalicylic acid. Int. J. Biol. Macromol. 2018, 106, 930–939. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Chen, L.; Xie, F. Starch film-coated microparticles for oral colon-specific drug delivery. Carbohydr. Polym. 2018, 191, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Günter, E.A.; Popeyko, O.V. Calcium pectinate gel beads obtained from callus cultures pectins as promising systems for colon-targeted drug delivery. Carbohydr. Polym. 2016, 147, 490–499. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Kim, W.; Nam, J.; Jeong, S.; Lee, S.; Yoo, J.W.; Kim, M.S.; Jung, Y. Celecoxib coupled to dextran via a glutamic acid linker yields a polymeric prodrug suitable for colonic delivery. Drug Des. Devel. Ther. 2015, 9, 4105–4113. [Google Scholar]
- Qiao, H.; Fang, D.; Chen, J.; Sun, Y.; Kang, C.; Di, L.; Li, J.; Chen, Z.; Chen, J.; Gao, Y. Orally delivered polycurcumin responsive to bacterial reduction for targeted therapy of inflammatory bowel disease. Drug Deliv. 2017, 24, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Ray, S. Advanced colon-specific delivery systems for treating local disorders. In Polysaccharide Carriers for Drug Delivery; Woodhead Publishing: Sawston, UK, 2019; ISBN 9780081025536. [Google Scholar]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ (accessed on 4 June 2020).
- Morales-Burgos, A.M.; Carvajal-Millan, E.; Rascón-Chu, A.; Martínez-López, A.L.; Lizardi-Mendoza, J.; López-Franco, Y.L.; Brown-Bojorquez, F. Tailoring reversible insulin aggregates loaded in electrosprayed arabinoxylan microspheres intended for colon-targeted delivery. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Miramontes-Corona, C.; Escalante, A.; Delgado, E.; Corona-González, R.I.; Vázquez-Torres, H.; Toriz, G. Hydrophobic agave fructans for sustained drug delivery to the human colon. React. Funct. Polym. 2020, 146, 104396. [Google Scholar] [CrossRef]
- Zhu, A.Z.; Ho MC, D.; Gemski, C.K.; Chuang, B.C.; Liao, M.; Xia, C.Q. Utilizing In Vitro Dissolution-Permeation Chamber for the Quantitative Prediction of pH-Dependent Drug-Drug Interactions with Acid-Reducing Agents: A Comparison with Physiologically Based Pharmacokinetic Modeling. AAPS J. 2016, 18, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Kim, W.; Cao, J.; Jung, Y.; Yoo, J.W. Enzyme/pH dual sensitive polymeric nanoparticles for targeted drug delivery to the inflamed colon. Colloids Surf. B Biointerfaces 2014, 123, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Teruel, A.H.; Coll, C.; Costero, A.M.; Ferri, D.; Parra, M.; Gaviña, P.; González-Álvarez, M.; Merino, V.; Marcos, M.D.; Martínez-Máñez, R.; et al. Functional magnetic mesoporous silica microparticles capped with an azo-derivative: A promising colon drug delivery device. Molecules 2018, 23, 375. [Google Scholar] [CrossRef] [Green Version]
- Teruel, A.H.; Pérez-Esteve, É.; González-Álvarez, I.; González-Álvarez, M.; Costero, A.M.; Ferri, D.; Gaviña, P.; Merino, V.; Martínez-Máñez, R.; Sancenón, F. Double Drug Delivery Using Capped Mesoporous Silica Microparticles for the Effective Treatment of Inflammatory Bowel Disease. Mol. Pharm. 2019, 16, 2418–2429. [Google Scholar] [CrossRef]
- Rafii, F.; Franklin, W.; Cerniglia, C.E. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl. Environ. Microbiol. 1990, 56, 2146–2151. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Gulati, M.; Singh, S.K. Role of synbiotics in polysaccharide assisted colon targeted microspheres of mesalamine for the treatment of ulcerative colitis. Int. J. Biol. Macromol. 2017, 95, 438–450. [Google Scholar] [CrossRef]
- Ferri, D.; Gaviña, P.; Parra, M.; Costero, A.M.; El Haskouri, J.; Amorós, P.; Merino, V.; Teruel, A.H.; Sancenón, F.; Martínez-Máñez, R. Mesoporous silica microparticles gated with a bulky azo derivative for the controlled release of dyes/drugs in colon. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.G.; Ma, R.; Xiao, X.L.; Zhang, Y.H.; Zhang, X.Z.; Hu, N.; Gao, J.L.; Zheng, Y.F.; Dong, D.L.; Sun, Z.J. Azo polymeric micelles designed for colon-targeted dimethyl fumarate delivery for colon cancer therapy. Acta Biomater. 2016, 44, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Karrout, Y.; Dubuquoy, L.; Piveteau, C.; Siepmann, F.; Moussa, E.; Wils, D.; Beghyn, T.; Neut, C.; Flament, M.P.; Guerin-Deremaux, L.; et al. In vivo efficacy of microbiota-sensitive coatings for colon targeting: A promising tool for IBD therapy. J. Control. Release 2015, 197, 121–130. [Google Scholar] [CrossRef]
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B Biointerfaces 2017, 150, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Iwao, Y.; Bani-Jaber, A.; Noguchi, S.; Itai, S. Preparation and evaluation of newly developed chitosan salt coating dispersions for colon delivery without requiring overcoating. Chem. Pharm. Bull. 2015, 63, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Dagli, Ü.; Balk, M.; Yücel, D.; Ülker, A.; Över, H.; Saydam, G.; Şahin, B. The Role of Reactive Oxygen Metabolites in Ulcerative Colitis. Inflamm. Bowel Dis. 1997, 3, 260–264. [Google Scholar] [CrossRef]
- Simmonds, N.J.; Rampton, D.S. Inflammatory, bowel, disease—A, radical, view. Gut 1993, 34, 865–868. [Google Scholar] [CrossRef] [Green Version]
- Grisham, M.B. Oxidants and free radicals in inflammatory bowel disease. Lancet (London, England) 1994, 344, 859–861. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, H.; Lin, Y.; Hu, Y.; An, H.; Zhang, D.; Feng, S.; Hu, H.; Wang, R.; Li, X.; et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials 2016, 105, 206–221. [Google Scholar] [CrossRef]
- Sedghi, S.; Fields, J.Z.; Klamut, M.; Urban, G.; Durkin, M.; Winship, D.; Fretland, D.; Olyaee, M.; Keshavarzian, A. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut 1993, 34, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, N.J.; Allen, R.E.; Stevens, T.R.; Van Someren, R.N.; Blake, D.R.; Rampton, D.S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 1992, 103, 186–196. [Google Scholar] [CrossRef]
- Vong, L.B.; Mo, J.; Abrahamsson, B.; Nagasaki, Y. Specific accumulation of orally administered redox nanotherapeutics in the inflamed colon reducing inflammation with dose-response efficacy. J. Control. Release 2015, 210, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.B.; Nagasaki, Y. Combination Treatment of Murine Colon Cancer with Doxorubicin and Redox Nanoparticles. Mol. Pharm. 2016, 13, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Babbs, C.F. Oxygen radicals in ulcerative colitis. Free Radic. Biol. Med. 1992, 13, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Kotakadi, V.S.; Ying, L.; Hofseth, A.B.; Cui, X.; Wood, P.A.; Windust, A.; Matesic, L.E.; Pena, E.A.; Chiuzan, C.; et al. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis 2008, 29, 2351–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguí, J.; Gironella, M.; Sans, M.; Granell, S.; Gil, F.; Gimeno, M.; Coronel, P.; Piqué, J.M.; Panés, J. Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J. Leukoc. Biol. 2004, 76, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Laroui, H.; Viennois, E.; Ayyadurai, S.; Charania, M.A.; Zhang, Y.; Zhang, Z.; Baker, M.T.; Zhang, B.; Gewirtz, A.T.; et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology 2014, 146, 1289–1300.e1–19. [Google Scholar] [CrossRef] [Green Version]
- Fromont Hankard, G.; Cezard, J.P.; Aigrain, Y.; Navarro, J.; Peuchmaur, M. CD44 variant expression in inflammatory colonic mucosa is not disease specific but associated with increased crypt cell proliferation. Histopathology 1998, 32, 317–321. [Google Scholar] [CrossRef]
- Farkas, S.; Hornung, M.; Sattler, C.; Anthuber, M.; Gunthert, U.; Herfarth, H.; Schlitt, H.J.; Geissler, E.K.; Wittig, B.M. Short-term treatment with anti-CD44v7 antibody, but not CD44v4, restores the gut mucosa in established chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. 2005, 142, 260–267. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Z.; Viennois, E.; Kang, Y.; Zhang, M.; Han, M.K.; Chen, J.; Merlin, D. Combination therapy for ulcerative colitis: Orally targeted nanoparticles prevent mucosal damage and relieve inflammation. Theranostics 2016, 6, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohns. Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.X.; Zhou, J.T.; Wang, T.T.; Huang, Y.F.; Chen, V.P.; Xie, Y.L.; Lin, Z.X.; Gao, J.S.; Su, Z.R.; Zeng, H.F. Self-nanoemulsifying drug delivery system of bruceine D: A new approach for anti-ulcerative colitis. Int. J. Nanomed. 2018, 13, 5887–5907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higa, L.H.; Jerez, H.E.; De Farias, M.A.; Portugal, R.V.; Romero, E.L.; Morilla, M.J. Ultra-small solid archaeolipid nanoparticles for active targeting to macrophages of the inflamed mucosa. Nanomedicine 2017, 12, 1165–1175. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [Green Version]
- Melero, A.; Draheim, C.; Hansen, S.; Giner, E.; Carreras, J.J.; Talens-Visconti, R.; Garrigues, T.M.; Peris, J.E.; Recio, M.C.; Giner, R.; et al. Targeted delivery of Cyclosporine A by polymeric nanocarriers improves the therapy of inflammatory bowel disease in a relevant mouse model. Eur. J. Pharm. Biopharm. 2017, 119, 361–371. [Google Scholar] [CrossRef]
- Wang, J.L.; Gan, Y.J.; Iqbal, S.; Jiang, W.; Yuan, Y.Y.; Wang, J. Delivery of tacrolimus with cationic lipid-assisted nanoparticles for ulcerative colitis therapy. Biomater. Sci. 2018, 6, 1916–1922. [Google Scholar] [CrossRef]
- Sun, Q.; Luan, L.; Arif, M.; Li, J.; Dong, Q.J.; Gao, Y.; Chi, Z.; Liu, C.G. Redox-sensitive nanoparticles based on 4-aminothiophenol-carboxymethyl inulin conjugate for budesonide delivery in inflammatory bowel diseases. Carbohydr. Polym. 2018, 189, 352–359. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Hatton, G.B.; Yadav, V.; Basit, A.W.; Merchant, H.A. Animal Farm: Considerations in Animal Gastrointestinal Physiology and Relevance to Drug Delivery in Humans. J. Pharm. Sci. 2015, 104, 2747–2776. [Google Scholar] [CrossRef]
- Hatton, G.B.; Madla, C.M.; Rabbie, S.C.; Basit, A.W. All disease begins in the gut: Influence of gastrointestinal disorders and surgery on oral drug performance. Int. J. Pharm. 2018, 548, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Choi, M.; Cao, J.; Lee, Y.; Ikram, M.; Yoon, S.; Lee, J.; Moon, H.R.; Kim, M.S.; Jung, Y.; et al. Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy. Drug Des. Devel. Ther. 2015, 9, 3789–3799. [Google Scholar] [PubMed] [Green Version]
- Kim, M.S.; Yeom, D.W.; Kim, S.R.; Yoon, H.Y.; Kim, C.H.; Son, H.Y.; Kim, J.H.; Lee, S.; Choi, Y.W. Development of a chitosan based double layer-coated tablet as a platform for colon-specific drug delivery. Drug Des. Devel. Ther. 2017, 11, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibekwe, V.C.; Khela, M.K.; Evans, D.F.; Basit, A.W. A new concept in colonic drug targeting: A combined pH-responsive and bacterially-triggered drug delivery technology. Aliment. Pharmacol. Ther. 2008, 28, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Fischer, M.; Sagi, S.V.; Bohm, M.E.; Fadda, H.M.; Ranmal, S.R.; Budree, S.; Basit, A.W.; Glettig, D.L.; de la Serna, E.L.; et al. Fecal Microbiota Transplantation Capsules with Targeted Colonic Versus Gastric Delivery in Recurrent Clostridium difficile Infection: A Comparative Cohort Analysis of High and Low Dose. Dig. Dis. Sci. 2019, 64, 1672–1678. [Google Scholar] [CrossRef]
- Varum, F.; Freire, A.C.; Fadda, H.M.; Bravo, R.; Basit, A.W. A dual pH and microbiota-triggered coating (PhloralTM) for fail-safe colonic drug release. Int. J. Pharm. 2020, 583, 119379. [Google Scholar] [CrossRef]
- Nguyen, M.N.U.; Tran, P.H.L.; Tran, T.T.D. A single-layer film coating for colon-targeted oral delivery. Int. J. Pharm. 2019, 559, 402–409. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, J.; Jia, L.; Guo, G.; Wang, C.; Zang, Y.; Ding, Z.; Chen, J.; Zhang, J.; Dong, L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials 2015, 48, 26–36. [Google Scholar] [CrossRef]
- Hou, L.; Shi, Y.; Jiang, G.; Liu, W.; Han, H.; Feng, Q.; Ren, J.; Yuan, Y.; Wang, Y.; Shi, J.; et al. Smart nanocomposite hydrogels based on azo crosslinked graphene oxide for oral colon-specific drug delivery. Nanotechnology 2016, 27, 315105. [Google Scholar] [CrossRef]
- Teruel, A.H.; Pérez-Esteve, É.; González-Álvarez, I.; González-Álvarez, M.; Costero, A.M.; Ferri, D.; Parra, M.; Gaviña, P.; Merino, V.; Martínez-Mañez, R.; et al. Smart gated magnetic silica mesoporous particles for targeted colon drug delivery: New approaches for inflammatory bowel diseases treatment. J. Control. Release 2018, 281, 58–69. [Google Scholar] [CrossRef]
- Maurer, J.M.; Hofman, S.; Schellekens, R.C.A.; Tonnis, W.F.; Dubois, A.O.T.; Woerdenbag, H.J.; Hinrichs, W.L.J.; Kosterink, J.G.W.; Frijlink, H.W. Development and potential application of an oral ColoPulse infliximab tablet with colon specific release: A feasibility study. Int. J. Pharm. 2016, 505, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Alange, V.V.; Birajdar, R.P.; Kulkarni, R.V. Functionally modified polyacrylamide-graft-gum karaya pH-sensitive spray dried microspheres for colon targeting of an anti-cancer drug. Int. J. Biol. Macromol. 2017, 102, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Mol. Ther. 2017, 25, 1628–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Approach/Mechanism | Dosage Form | Description | Drug Loaded | In Vitro Model and Release | In Vivo Model | Year/Ref |
|---|---|---|---|---|---|---|
| pH-responsive | Nanofibers: Polyvinylpyrrolidone (PVP). | Diclofenac encapsulated in a hydrophilic nanocomposite coated with a thin shellac layer. The nanosystem was obtained using a modified coaxial electrospinning method and showed specificity and pulsatile colon delivery. | Diclofenac sodium | Drug release studies in buffered solutions at different pH and ex vivo permeation studies. | - | 2018 [15] |
| pH-responsive | NPs: Eudragit FS30D/PLGA nanoparticles | Nanoparticles of cyclosporine A encapsulated with Eudragit FS30D nanoparticles (ENPs), PLGA nanoparticles (PNPs), and Eudragit FS30D/PLGA nanoparticles (E/PNPs) were synthetized. In vivo assays using a mouse model of colitis, showed a significant improvement of the symptoms of the pathology | Cyclosporine A | Drug release studies in buffered solutions at different pH. | Mice Dextran Sulfate Sodium (DSS) induced colitis | 2018 [42] |
| pH-responsive | Microcrystals: Chitosan/alginate/Eudragit S multilayers | Dexamethasone microcrystals coated with different layers: chitosan, alginate, and Eudragit S 100 (ES) allows a pH-dependent dexamethasone release, providing an important drug release in colon segment. The formulation shows therapeutic activity in an in vivo mouse model of ulcerative colitis. | Dexamethasone | Drug release studies in buffered solutions at different pH. | Mice DSS induced colitis | 2018 [43] |
| pH-responsive | NPs: Chitosan based polymeric nanomicelles | pH-sensitive nanomicelles of curcumin coated with N-naphthyl-N,O-succinyl chitosan (NSCS) and N-octyl-N,O- succinyl chitosan (OSCS) allow colon-targeted drug delivery. | Curcumin | Drug release studies with simulated GIT fluids. Cytotoxicity: Caco-2 and HT-29 cells. Anti-cancer activity: HT-29 cells. | - | 2018 [23] |
| pH-responsive | Nanostructured lipid carrier (NLCs) systems coated with Eudragit S100 | Budesonide loaded nanostructured lipid carriers obtained by high pressure homogenization technique and coated with Eudragit® S100 prevent release of drug at acidic pHs. | Budesonide (BDS) | Drug release studies in buffered solutions at different pH. | - | 2018 [27] |
| pH-responsive | NPs: Mesoporous nanoparticles capped with (1) a molecule containing a disulfide bond, (2) a starch derivative or (3) a lipid bilayer. | Gated mesoporous nanoparticles able to deliver their cargo triggered by different stimuli such as redox ambient, enzymatic hydrolysis, and presence of surfactant or contact with cell membrane) allow an increase of drug release and drug concentration in intestine and colon reducing plasma drug levels. | Safranin O (dye for preliminary studies) | Drug release studies with simulated GIT fluids. Cytotoxicity & permeability: Caco-2 cell line. | Wistar rats Biodistribution studies (S2 coated with Eudragit FS30D) | 2017 [64] |
| pH-responsive | MPs: Alginate hydrogel/chitosan micelle composites | pH-sensitive drug delivery systems based on the cross-linked unimolecular micelles dispersed in a hydrogel matrix at different ratios. The hydrogel/micelle (3:1) showed a colon-specific release. The release of drug from these formulations is a complex process that involves several mechanisms simultaneously. | Emodin | Drug release studies with simulated GIT fluids. | - | 2018 [61] |
| pH-responsive | Alginate microparticles | pH-sensitive drug delivery microparticles of puerarin in order to reduce the complications associated to ulcerative colitis | Puerarin | Drug release studies with simulated GIT fluids | Mice DSS-induced colitis-associated colorectal cancer | 2019 [65] |
| pH-responsive | MPs: P(LE-IA- MEG) hydrogel microspheres obtained using emulsion crosslinking method with itaconic acid, poly (ethylene glycol) methyl ether methacrylate and PLE-AC (a new polymer obtained from poly (ethylene glycol) methyl ether methacrylate and acryloyl choride) | pH-sensitive hydrogel microspheres as a drug carrier of hydrocortisone have been obtained. The in vitro assays showed that the microspheres are pH-sensitivity. In vivo assays carried out on a mice colitis model indicate that mice treated with microspheres exhibited more therapeutic effects than those treated with free hydrocortisone. | Hydrocortisone sodium succinate (HSS) | Drug release studies in buffered solutions at different pH. | Mice 2,4,6-Trinitrobenzenesulfonic acid (TNBS) induced colitis | 2018 [66] |
| pH-responsive | Tablet of aloe vera polysaccharide/Acrylonitrile nanoparticles + guar gum + drug | Self-assembled nanoparticles with aloe vera polysaccharide and acrylonitrile loaded with 5-ASA were synthetized. The resulting allows the cargo delivery at colonic pH. | 5-ASA | Drug release studies in buffered solutions at different pH. | - | 2018 [67] |
| pH-responsive | Eudragit microparticles | Celecoxib microparticles formulated using Eudragit S100, Eudragit L100-55 or Eudragit L100 | Celecoxib | In vitro and in vivo assays | Rat model | 2016 [52] |
| pH-responsive | ColoPulse coated tablets | Sustained-release budesonide tablets coated with ColoPulse | Budesonide | In vitro and in vivo assays | Human | 2019 [62] |
| pH-responsive | ColoPulse coated tablets | Sustained-release infliximab tablets coated with ColoPulse | Infliximab | In vitro assays | 2019 [63] |
| Approach/Mechanism | Dosage Form | Description | Drug Loaded | In Vitro Model & Release | In Vivo Model | Year/Ref |
|---|---|---|---|---|---|---|
| Enzyme-responsive | Microparticles: Starch film-coated | Microparticles loaded with 5-ASA coated with a resistant starch films prepared with different techniques showed an important enzymatic resistance. In vivo studies indicated that formulation orally administered resist acidic medium and delivered drug in colon | 5-ASA | - | Healthy mice | 2018 [68] |
| Enzyme-responsive | Microspheres: guar gum and xanthan gum | 5-ASA encapsulated in microspheres with guar gum and xanthan gum was combined with probiotics such as Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium longum, and Saccharomyces boulardi. The coadministration of probiotics and drugs show therapeutic benefits in the in vivo assays carried out in rats with ulcerative colitis. | 5-ASA | - | Wistar rats. Acetic acid- induced ulcerogenic colitis. | 2017 [82] |
| Enzyme-responsive | MPs: Mesoporous silica microparticles capped with a bulky azo derivative | Gated silica microparticles loaded with the dye safranin O were prepared and characterized. Microparticles release takes place under reducing conditions typical of the colonic mucosa. Preliminary in vivo experiments using healthy mice indicate that solid release the dye in the last part of GIT mucosa. | Safranin O (dye for preliminary studies) | Drug release studies in buffered solutions at different pH (with/without enzyme stimuli). | Healthy mice | 2018 [83] |
| Enzyme-responsive | MPs: Magnetic mesoporous silica microparticles capped with a bulky azo derivative | Magnetic micro-sized mesoporous silica particles loaded with safranin O and functionalized with an azo derivative allow colon-targeted delivery. Controlled release assays were carried out using simulated digestion process. | Safranin O (dye for preliminary studies) | Drug release studies with simulated GIT fluids. | - | 2018 [79] |
| Enzyme-responsive | NPs: Star-shape amphiphilic polymer of polycaprolactone (PCL), olsalazine, and methoxypolyethylene glycols (mPEG). | Azo four-arm polymeric micelles for colon-targeted delivery of dimethyl fumarate can be use in colon cancer therapy. In vitro drug release assays indicated that the cumulative drug release from the polymeric micelles was lower than 20% in the gastric fluid of rats within 10 h. However release in colonic fluids of drugs reached 100% in the same period of time | Dimethyl fumarate | LIVE/DEAD® Viability/Cytotoxicity Assay Kit—Colon cancer cell lines CT26, HT29, and HCT116 cells. | - | 2016 [84] |
| Enzyme-responsive | MPs: Calcium pectinate gel beads | Low methyl-esterified pectins microparticles were used as the carriers for colon delivery of prednisolone. Release aspects of prednisolone in the simulated gastric (pH 1.25), intestinal (pH 7.0) and colonic (pH 7.0 + pectinase) media were investigated. Prednisolone release occurred in a larger extent in colonic medium due to the enzymatic erosion of the beads. | Prednisolone | Drug release studies with simulated GIT fluids. | - | 2016 [69] |
| Enzyme-responsive | Pellets (0.7–1 mm) +microbiota sensitive film coating | 5-ASA pellets coated with Nutriose: ethylcellulose 1:4 or peas starch:ethylcellulose 1:2 blends were synthetized. In vivo assays in a rat model revealed the efficacy of these colon targeting pellets. | 5-ASA | - | Wistar rats TNBS induced colitis | 2015 [85] |
| Enzyme-responsive | NPs: MSNs guar gum capping (GG-MSN) | In vitro release studies of 5-Fluorouracil loaded mesoporous nanoparticles capped with guar gum allow drug release specifically triggered by colonic enzymes. The released drug produced cytostatic effect in cells cultured with simulated colonic microenvironment | 5FU | Drug release studies with simulated GIT fluids Cell proliferation study human colon cancer model (HT-29). | - | 2017 [86] |
| Enzyme-responsive | NPs: Amphiphilic curcumin polymer (PCur) | An amphiphilic curcumin polymer conjugate containing a hydrophilic poly(ethylene glycol) and hydrophobic curcumin linked by disulfide bond was designed to release curcumin in the intestinal reduction environment. The results obtained from in vitro and in vivo assays confirmed higher therapeutic level of curcumin in intestinal tissue damaged. | Curcumin | Drug release studies in buffered solutions at different pH and reductive environment. Cytotoxicity and permeability: Caco-2 cells. | Sprague–Dawley (SD) rats and C57BL/6 mice DSS-induced IBD model | 2017 [71] |
| Enzyme-responsive | Tablets: Chitosan-laurate coating | Chitosan-laurate dispersions were used as coating films of acetaminophen tablets. Results indicate that formulation was stable in acidic environment and allow drug delivery in the colon tissues. | Acetaminophen | Drug release studies in buffered solutions at different pH (with/without enzyme stimuli). | - | 2015 [87] |
| Approach/Mechanism | Dosage Form | Description | Drug Loaded | In Vitro Model & Release | In Vivo Model | Year/Ref |
|---|---|---|---|---|---|---|
| Inflammation targeting (ROS-responsive) | NPs: An oxidation-responsive β-cyclodextrine nanoparticles loaded with Templol (Tpl/OxbCD) | An oxidation-responsive β-cyclodextrine (OxbCD) nanoparticles was obtained and loaded with tempol (Tpl). The drug release from formulation is allowed by hydrolysis of OxbCD NPs by means of hydrogen peroxide. Oral administration of nanoparticles allows to obtain increased amounts of drug in colon and less biodistribution in other organs. The efficacy was better than free drug or other tested formulations used as control | Tempol (Tpl): ROS scavenger anti-inflammation | ROS-sensitivity, hydrolysis and drug release evaluation in buffered solutions at different pH. | Mice DSS and TNBS induced colitis | 2016 [92] |
| Inflammation targeting | NPs: PLGA | PLGA NPs loaded with siCD98 and curcumin demonstrated that codelivery of both drugs increase the efficacy of colitis treatment. This structurally simple platform is suitable for orally administered delivery of drugs to target colon for ulcerative colitis or other pathologies | CD98 siRNA and curcumin | - | Mice DSS induced colitis | 2016 [101] |
| Inflammation targeting | NPs: Ultra-small solid archeolipid nanoparticles | Archeolipid nanoparticles loaded with dexamethasone are synthetized and characterized | Dexamethasone (Dex) | Anti-inflammatory activity: J774A1 cells. | - | 2017 [107] |
| Inflammation targeting | NPs: PLGA and amphiphilic copolymer Polylactic acid-Polyethylene glycol-Folate (PLGA/PLA- PEG-FA) nanoparticles | PLGA/PLA-PEG-FA nanoparticles containing 6-shogaol are a promising formulation because its effectivity in targeting colitis tissue, improving symptoms and accelerating wound repair. | 6-shogaol | - | Mice DSS induced colitis | 2018 [105] |
| Inflammation targeting | NPs: ginseng-derived nanoparticles (GDNPs 2) | GDNPs 2 nanoparticles ability for controlled and it is an optimal option for ulcerative colitis prevention and treatment due to its effectivity in colon-targeted, low toxicity and easy production. | Lipids, proteins, microRNAs and ginger bioactives (6-gingerol and 6-shogaol) | Internalization and citotoxicity: RAW 264.7 microphage, Caco-2BBE and Colon-26 cells. | Mice DSS induced colitis | 2016 [108] |
| Inflammation targeting | NPs: PLGA nanocarriers | PLGA nanocarriers loaded with cyclosporine A have demonstrated to be useful as drug delivery system, targeting inflamed issues, providing high drug concentrations at inflamed tissues, demonstrating superior efficacy and safety in a relevant preclinical mouse model in vivo. | Cyclosporine A | Drug release studies with simulated gastric fluid (pH = 3.0). | Balb/C mice DSS-induced acute colitis | 2017 [109] |
| Inflammation targeting | NPs: Cationic lipid-assisted nanoparticles (CLAN) | Cationic lipid-assisted nanoparticles loaded with Tacrolimus (FK506) are tested as drug delivery system for ulcerative colitis treatment Results indicate that the formulation accumulate at the inflamed issues and improve the therapeutic effects of the treatment. | Tacrolimus (FK506) | Drug release studies in buffered solutions at different pH. | C57BL/6 mice DSS-induced acute colitis | 2018 [110] |
| Inflammation targeting (ROS-responsive) | NPs: Self-assembling copolymer nanoparticles | Redox nanoparticles (RNPO) administered by oral route specifically accumulated in inflamed tissues and scavenged reactive oxygen species (ROS) demonstrating the potential therapeutic of this approach. | Tempol (Tpl): ROS scavenger anti-inflammation | Cellular uptake: Caco-2 cells. | Mice DSS-induced acute colitis | 2015 [95] |
| Inflammation targeting (ROS-responsive) | NPs: Superoxide dismutase (SOD)/catalase mimetic nanosystem | An oxidation-responsive ß-cyclodextrin material (OxbCD) was synthesized, and loaded with the ROS scavenger Tempol. Hydrogen peroxide presence promote the on-demand release of loaded drug. In vivo assays revealed that nanoparticles accumulate in the inflamed tissues after oral delivery. | Tempol (Tpl): ROS scavenger anti-inflammation | Drug release studies in buffered solutions at different pH + hydrogen peroxide. | DSS induced acute and chronic colitis. & TNBS induced acute colitis | 2016 [92] |
| Inflammation targeting (redox-responsive) | NPs based on 4-aminothiophenol-carboxymethyl inulin conjugate | Budesonide loaded nanoparticles based on an amphiphilic inulin derivative (ATP-CMI) were obtained. In vitro release and in vivo assays indicate that this formulation can be a promising option for colitis treatment. | Budesonide | Drug release studies with simulated GIT fluids. Cytotoxicity: Caco-2 cell line. | Mice DSS-induced acute colitis | 2018 [111] |
| Inflammation targeting (NPs intrinsic properties) | Nanoemulsion: Self-nanoemulsifying drug delivery system (SNEDDS) | Self-nanoemulsifying formulation that contains medium-chain triglycerides oil (MCT oil), Solutol HS-15 (surfactant), propylene glycol (co-surfactant) and Bruceine D is able to show high therapeutic effectivity in a colitis animal model. | Bruceine D | Drug release studies in buffered solutions at different pH. | Sprague Dawley rats TNBS-induced colitis | 2018 [106] |
| Inflammation targeting | NPs: Broccoli-Derived Nanoparticles | Nanoparticles based on broccoli extracts have demonstrated to protect mice against colitis Assays have been carried out using three mouse colitis models and preliminary studies indicate that activation of adenosine monophosphate- activated protein kinase (AMPK) in dendritic cells (DCs) play an important role y in prevention. | BDN | - | 1. Adoptive T cell transfer chronic colitis. 2. DSS-induced colitis. 3. Agonistic αCD40 colitis. | 2017 [112] |
| Approach/Mechanism | Dosage Form | Description | Drug Loaded | In Vitro Model & Release | In Vivo Model | Year/Ref |
|---|---|---|---|---|---|---|
| Dual pH-/time-responsive | NPs: Eudragit FS30D, Eudragit RS100 | Loaded budesonide nanoparticles using Eudragit® FS30D as a pH-responsive polymer, and Eudragit® RS100 as a time-dependent controlled release polymer were obtained in order to reach release at a colonic pH. In vivo assays confirmed that the dual approach pH/time-dependent is useful to obtain colon specific delivery and to enhance the efficacy of budesonide treatment. | Budesonide | Drug release studies in buffered solutions at different pH. | Mice DSS induced colitis | 2015 [115] |
| Swelling properties & enzyme-responsive | Microspheric vehicle: Cationic konjac glucomannan (cKGM) phytagel | Microspheric particles obtained with cationic konjac glucomannan phytagel are able to target colonic macrophages and suppress the local expression of TNF-α by specific delivery of antisense oligonucleotide anti-TNFα, providing excellent results in the in vivo assays using a colitis mice model. | Antisense oligonucleotide anti-TNFα | Drug release studies in buffered solutions at different pH. Cytotoxicity: Raw 264.7 and CT-26 cell lines. | Mice DSS induced colitis | 2015 [121] |
| Dual pH-/time-responsive | ColoPulse coated tablet (croscarmellose sodium enhance disintegration) | Infliximab incorporated in a sugar glass matrix showed activity compared to a fresh infliximab solution and demonstrated advantages such as high stability and targeted colon delivery | Infliximab | Drug release studies in buffered solutions at different pH. | - | 2016 [124] |
| Dual pH-/enzyme-responsive | Microspheres: polyacrylamide-graft-gum karaya pH-sensitive spray dried microspheres (PAAm-g-GK) | Microspheres based on pH-sensitive PAAm-g-GK copolymer having cross-linked with glutaraldehyde and loaded with capecitabine are used as drug carriers to target colon tissue. In vitro results indicate that, after 5h later to star the assay, it is observed an important drug release because of colonic bacteria’s action on PAAm-g-GK copolymer contained in fecal contents medium accelerated drug delivery. | Capecitabine | Drug release studies with simulated GIT fluids and rat cecal contents. | - | 2017 [125] |
| Dual pH-/enzyme-responsive | Nanocomposite hydrogel based on graphene oxide pH-sensitive and biocompatible graphene oxide (GO) containing azoaromatic crosslinks and poly (vinyl alcohol) (PVA) (GO–N = N–GO/PVA) | Nanocomposite hydrogel based on graphene oxide, azoaromatic crosslinks, and polyvinyl alcohol, and loaded with curcumin designed for colon cancer drug delivery. The results demonstrated that the nanocomposite hydrogels are able to protect curcumin from acidic pHs and enhance drug concentration and residence time in the colon tissue. | Curcumin | Drug release studies in buffered solutions at different pH. | Healthy Sprague-Dawley rats Gastrointestinal distribution, imaging analysis and PK studies | 2016 [122] |
| Dual pH-/enzyme responsive | PhloralTM | Unique patented coating that include Eudragit® and a resistant starch | 5-ASA | Drug release at different pH and in pH = 6.8 human fecal slurry | 2020 [119] | |
| Dual pH-/enzyme responsive | OPTICORETM | Coating technology consisting on an inner layer of Duocoat® to accelerate the release and an outer layer of Phloral (pH and enzyme responsive coating) | 5ASA | Drug release at different pH and in pH = 6.8 human fecal slurry | 2020 [48] | |
| pH-responsive & target of CD44 receptors (HA-CD44) | NPs: Hyaluronic Acid-Functionalized nanoparticles encapsulated in a hydrogel of alginate and chitosan (7:3). | Tripeptide lysine-proline-valine was loaded into polymeric nanoparticles obtained from functionalized hyaluronic acid. These nanoparticles are nontoxic and biocompatible with intestinal cells an oral administration of the formulation allow the alleviation of colitis symptoms combining both accelerating mucosal healing and reducing inflammation. | Tripeptide KPV (Lysine-proline-valine) | Cytotoxicity and cellular uptake: Raw 264.7 and Colon-26 cells. | Mice DSS induced colitis | 2017 [126] |
| Magnetically-driven & pH-responsive microparticles | Magnetic mesoporous microparticles with azo-derivative molecular gate | Hydrocortisone magnetic mesoporous microparticles decorated with bulky azo-derivatives allows a colon specific delivery and high in vivo efficacy in a rat model | Hydrocortisone | In vitro and in vivo assays | Sprague Dawley rats TNBS-induced colitis | 2018 [123] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teruel, A.H.; Gonzalez-Alvarez, I.; Bermejo, M.; Merino, V.; Marcos, M.D.; Sancenon, F.; Gonzalez-Alvarez, M.; Martinez-Mañez, R. New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy. Int. J. Mol. Sci. 2020, 21, 6502. https://doi.org/10.3390/ijms21186502
Teruel AH, Gonzalez-Alvarez I, Bermejo M, Merino V, Marcos MD, Sancenon F, Gonzalez-Alvarez M, Martinez-Mañez R. New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy. International Journal of Molecular Sciences. 2020; 21(18):6502. https://doi.org/10.3390/ijms21186502
Chicago/Turabian StyleTeruel, Adrian H., Isabel Gonzalez-Alvarez, Marival Bermejo, Virginia Merino, Maria Dolores Marcos, Felix Sancenon, Marta Gonzalez-Alvarez, and Ramon Martinez-Mañez. 2020. "New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy" International Journal of Molecular Sciences 21, no. 18: 6502. https://doi.org/10.3390/ijms21186502
APA StyleTeruel, A. H., Gonzalez-Alvarez, I., Bermejo, M., Merino, V., Marcos, M. D., Sancenon, F., Gonzalez-Alvarez, M., & Martinez-Mañez, R. (2020). New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy. International Journal of Molecular Sciences, 21(18), 6502. https://doi.org/10.3390/ijms21186502








