Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite
Abstract
1. Introduction
2. Results and Discussion
2.1. Swelling Degree
2.2. Bioactivity Assessment
2.3. Hemolytic Test
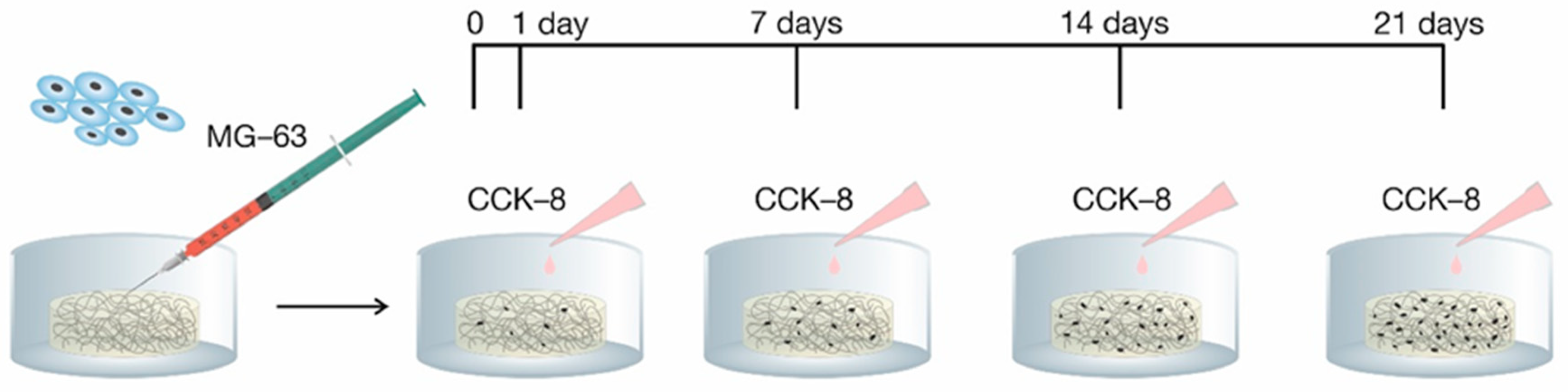
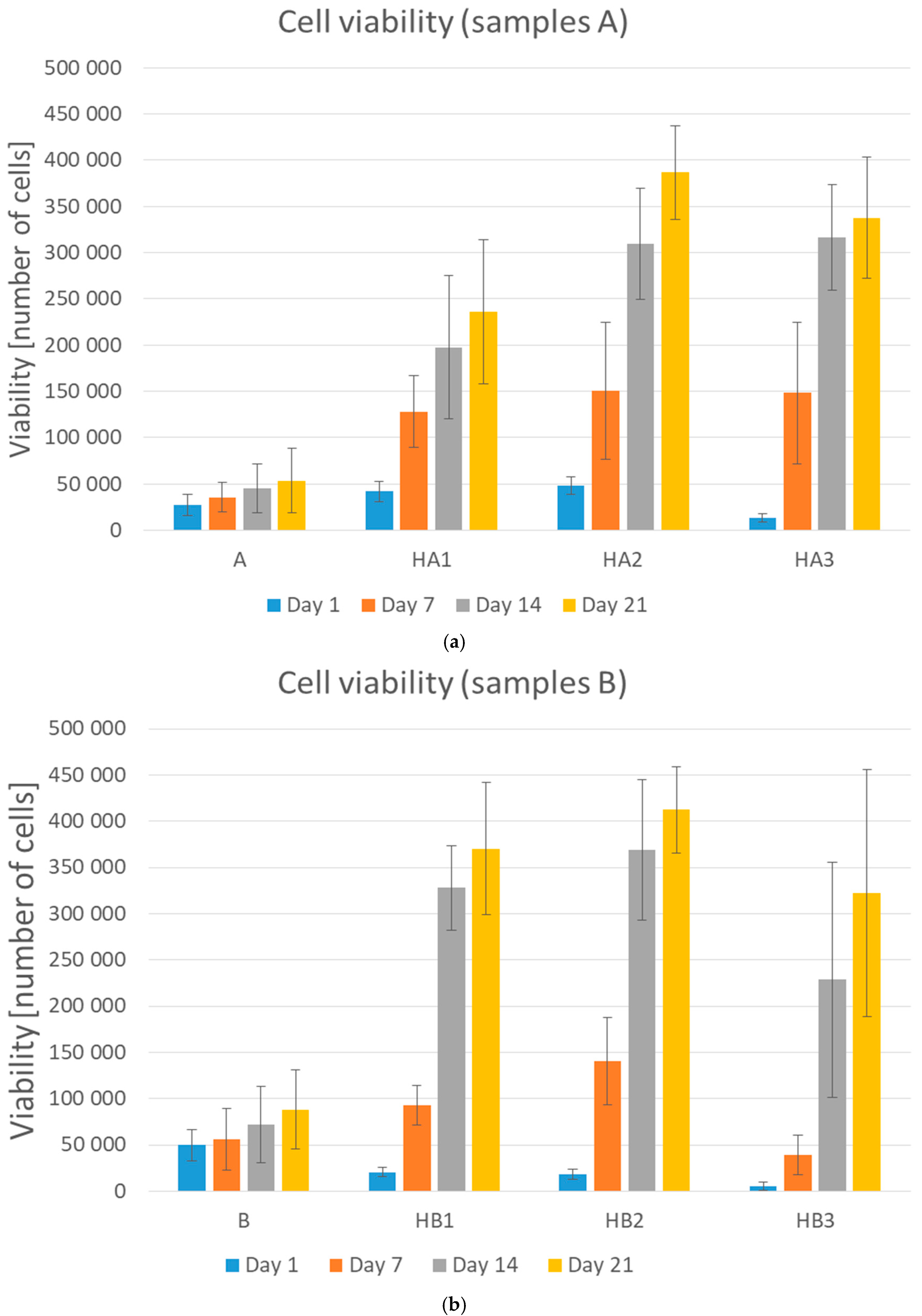
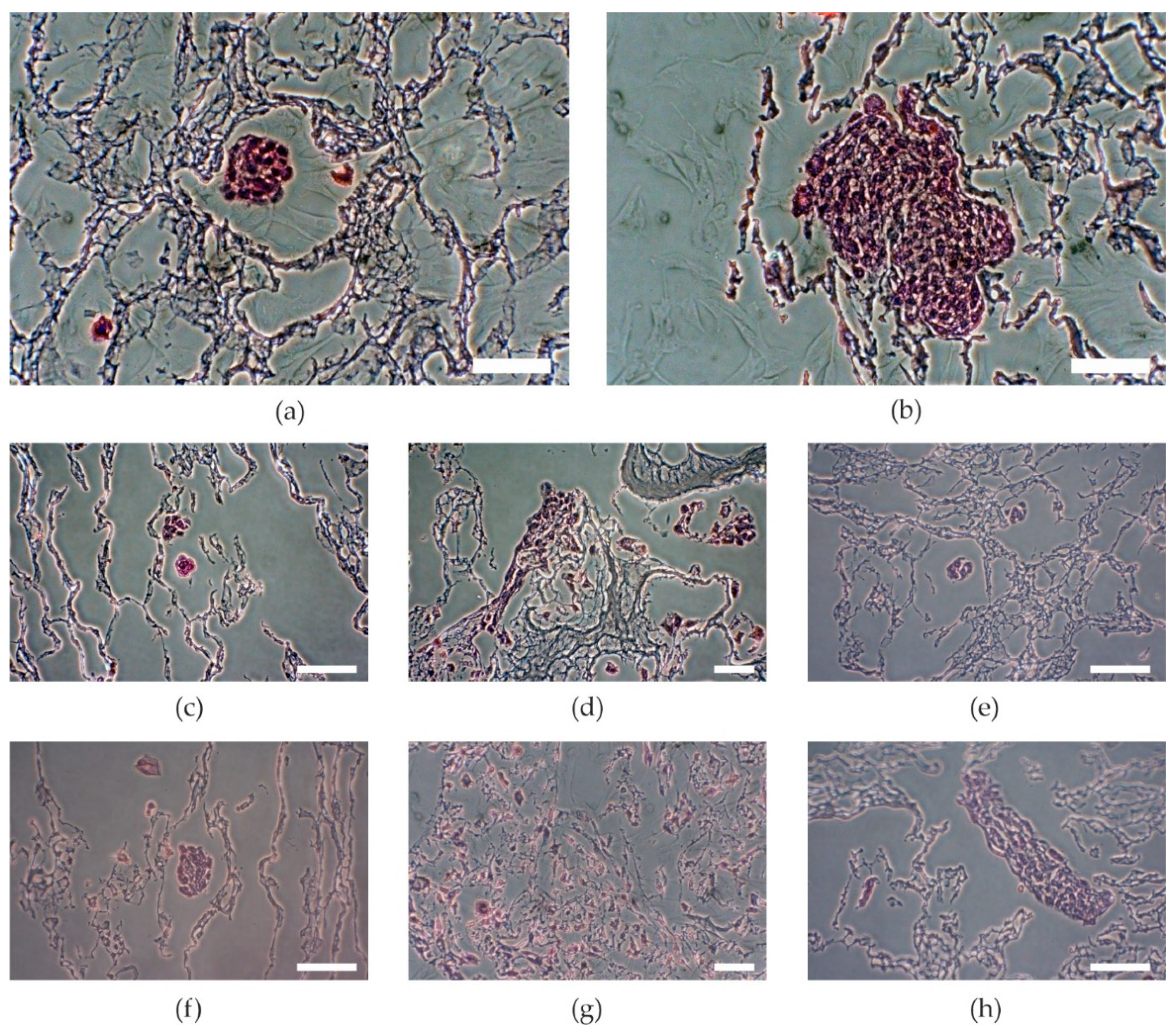
2.4. Cell Viability
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hydroxyapatite
3.3. Preparation of Scaffolds
3.4. In Vitro Biological Evaluation of Scaffíolds
3.4.1. Swelling Degree
3.4.2. Bioactivity Assessment
3.4.3. Hemolytic Test
3.5. Cell Viability Tests
3.5.1. Cell Cultures
3.5.2. Tests of Cell Adhesion and Proliferation
3.6. Histological Preparation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Order | Reagent | Amount |
|---|---|---|
| 1 | NaCl | 4.018 g |
| 2 | NaHCO3 | 0.178 g |
| 3 | KCl | 0.114 g |
| 4 | K2HPO4 3H2O | 0.116 g |
| 5 | MgCl2 6H2O | 0.159 g |
| 6 | 1M HCl | 19.5 mL |
| 7 | CaCl2 | 0.146 g |
| 8 | Na2SO4 | 0.036 g |
| 9 | Tris | 3.059 g |
| 10 | 1M HCl | 1.84 mL |
References
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, C.I.A.; Cardoso, D.A.; Van Oirschot, B.A.J.A.; Ulrich, D.J.O.; Jansen, J.A.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P. Porous titanium scaffolds with injectable hyaluronic acid-DBM gel for bone substitution in a rat critical-sized calvarial defect model. J. Tissue Eng. Regen. Med. 2016, 11, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.A.; Singh, S.R.; Pillai, S. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Nikbakht, M.; Karbasi, S.; Sorkhabadi, S.M.R. Biological evaluation of the effects of Hyaluronic acid on Poly (3-hydroxybutyrate) based Electrospun Nanocomposite scaffolds for cartilage tissue engineering application. Mater. Technol. 2019, 35, 141–151. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. 2016, 66, 159–182. [Google Scholar] [CrossRef]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Kanca, Y.; Milner, P.; Dini, D.; Amis, A.A. Tribological properties of PVA/PVP blend hydrogels against articular cartilage. J. Mech. Behav. Biomed. Mater. 2018, 78, 36–45. [Google Scholar] [CrossRef]
- Kim, T.H.; An, D.B.; Oh, S.H.; Kang, M.K.; Song, H.H.; Lee, J.H. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing–thawing method to investigate stem cell differentiation behaviors. Biomaterials 2015, 40, 51–60. [Google Scholar] [CrossRef]
- Liu, Y.; Geever, L.; Kennedy, J.E.; Higginbotham, C.L.; Cahill, P.A.; McGuinness, G. Thermal behavior and mechanical properties of physically crosslinked PVA/Gelatin hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 203–209. [Google Scholar] [CrossRef]
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; Liu, Y.; McGuinness, G.; Cahill, P.A. Characterization of Poly(vinyl alcohol)/Chitosan Hydrogels as Vascular Tissue Engineering Scaffolds. Macromol. Symp. 2008, 269, 106–110. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, 9. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef]
- Obata, H.; Yamazaki, Y.; Matsunaga, T.; Sato, T. Synthesis and evaluation of hyaluronic acid hydrogels modified with various crosslinkers as biodegradable polymers. J. Appl. Polym. Sci. 2017, 134, 45453. [Google Scholar] [CrossRef]
- Haroun, A.A.; Gamal-Eldeen, A.M.; Harding, D.R.K. Preparation, characterization and in vitro biological study of biomimetic three-dimensional gelatin–montmorillonite/cellulose scaffold for tissue engineering. J. Mater. Sci. Mater. Electron. 2009, 20, 2527–2540. [Google Scholar] [CrossRef]
- Kaur, T.; Thirugnanam, A.; Pramanik, K. Tailoring the in vitro characteristics of poly (vinyl alcohol)-nanohydroxyapatite composite scaffolds for bone tissue engineering. J. Polym. Eng. 2016, 36, 771–784. [Google Scholar] [CrossRef]
- Pal, K.; Pal, S. Development of Porous Hydroxyapatite Scaffolds. Mater. Manuf. Process. 2006, 21, 325–328. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Batra, U.; Kadam, A.; Mulik, P. Development of Hydroxyapatite Bio-Scaffold. IOSR J. Mech. C Eng. 2009, 6, 33–37. [Google Scholar]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2018, 43, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Babuska, V.; Dobrá, J.K.; Dluhos, L.; Dvorakova, J.; Moztarzadeh, J.; Hrušák, D.; Kulda, V. Repeated Exposure of Nanostructured Titanium to Osteoblasts with Respect to Peri-Implantitis. Materials 2020, 13, 697. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, Y.; Lu, W.W.; Leong, J.C.; Zhang, W.; Zhang, J.; Zhang, M.; Yao, K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials 2002, 23, 3227–3234. [Google Scholar] [CrossRef]
| Sample | Composition Ratio [PVA/HA/HAp] | Swelling Degree [%] | Bioactivity Assessment [%] | Hemolysis [%] 1 |
|---|---|---|---|---|
| A | 3:1:0 | 902.06 | 110.55 | 0.97 (+++) |
| B | 1:1:0 | 999.61 | 118.70 | 0.68 (+++) |
| HA1 | 3:1:1 | 917.23 | 102.50 | 0.39 (+++) |
| HA2 | 3:1:2 | 804.27 | 97.25 | 1.46 (+++) |
| HA3 | 3:1:3 | 770.82 | 113.30 | 1.55 (+++) |
| HB1 | 1:1:1 | 1020.45 | 124.80 | 0.10 (+++) |
| HB2 | 1:1:2 | 797.92 | 122.70 | 0.58 (+++) |
| HB3 | 1:1:3 | 404.79 | 102.70 | 1.46 (+++) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chocholata, P.; Kulda, V.; Dvorakova, J.; Kolaja Dobra, J.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. https://doi.org/10.3390/ijms21165719
Chocholata P, Kulda V, Dvorakova J, Kolaja Dobra J, Babuska V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. International Journal of Molecular Sciences. 2020; 21(16):5719. https://doi.org/10.3390/ijms21165719
Chicago/Turabian StyleChocholata, Petra, Vlastimil Kulda, Jana Dvorakova, Jana Kolaja Dobra, and Vaclav Babuska. 2020. "Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite" International Journal of Molecular Sciences 21, no. 16: 5719. https://doi.org/10.3390/ijms21165719
APA StyleChocholata, P., Kulda, V., Dvorakova, J., Kolaja Dobra, J., & Babuska, V. (2020). Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. International Journal of Molecular Sciences, 21(16), 5719. https://doi.org/10.3390/ijms21165719







