Metabolic Syndrome, Clusterin and Elafin in Patients with Psoriasis Vulgaris
Abstract
1. Introduction
2. Methods
2.1. Study Groups
2.2. PASI Score
2.3. Blood Samples Collection
2.4. Metabolic Syndrome (MetS) and BMI
2.5. Analysis of Clusterin
2.6. Analysis of Elafin
2.7. Statistical Analysis
3. Results
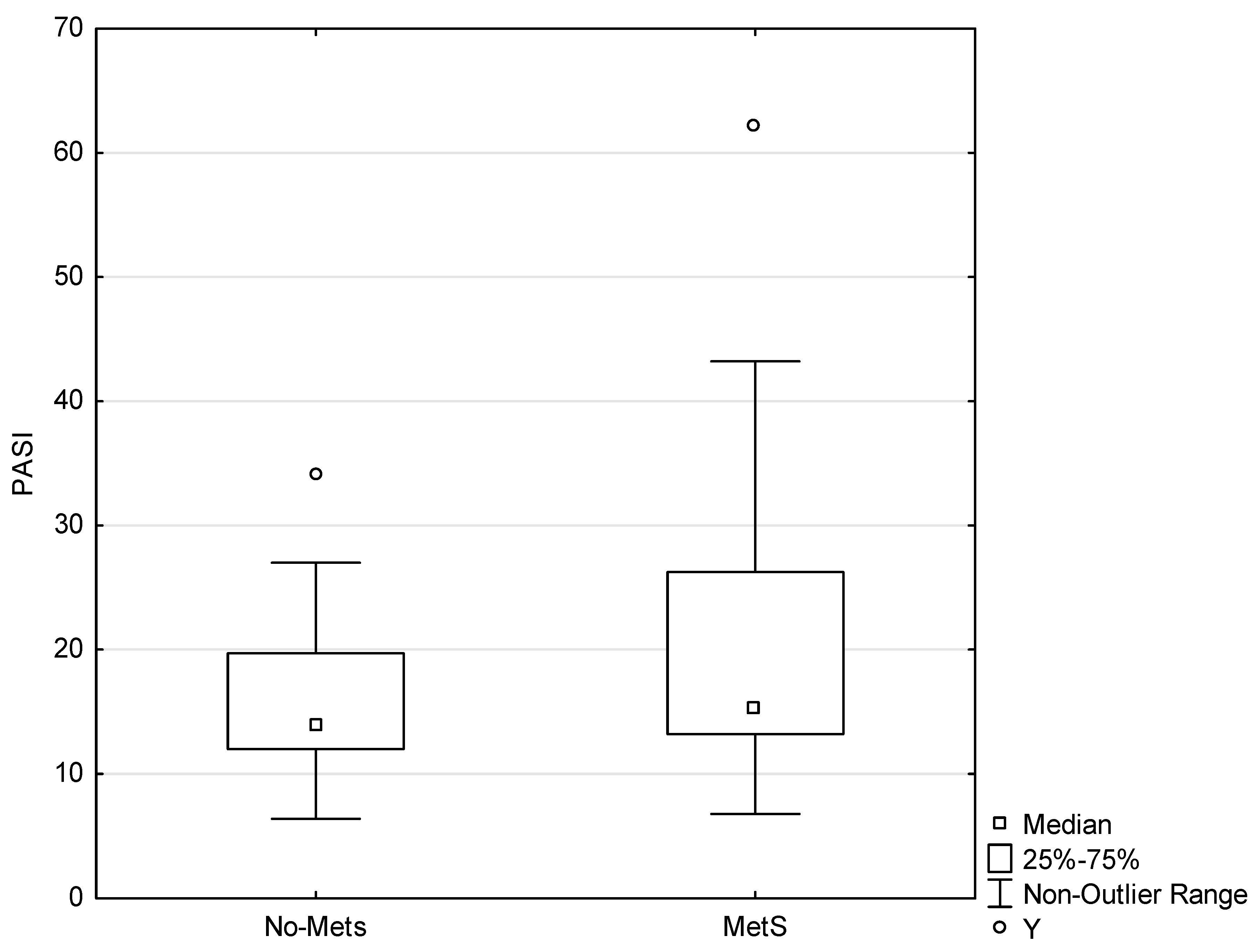
3.1. Participants Data
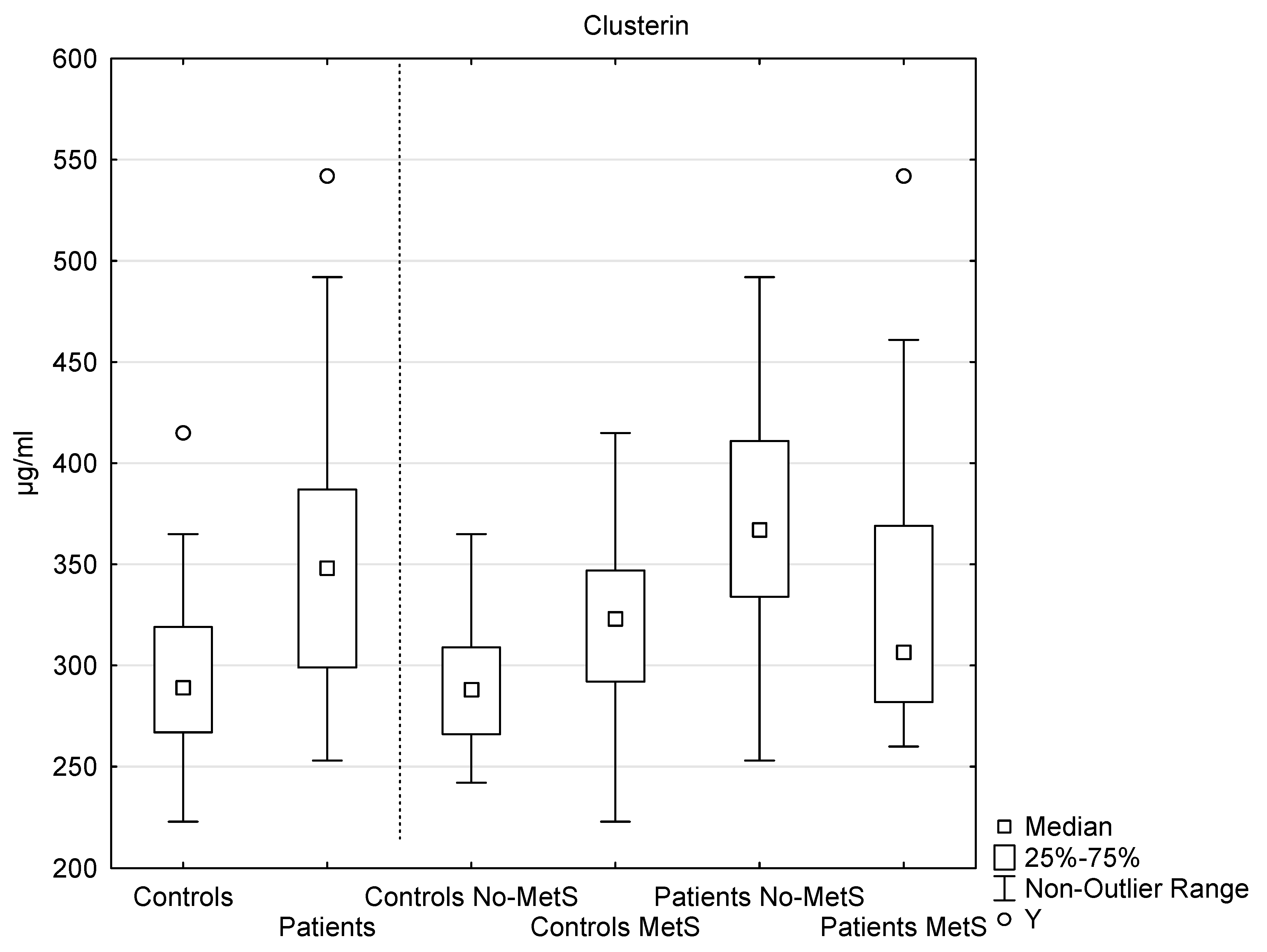
3.2. Clusterin Analysis
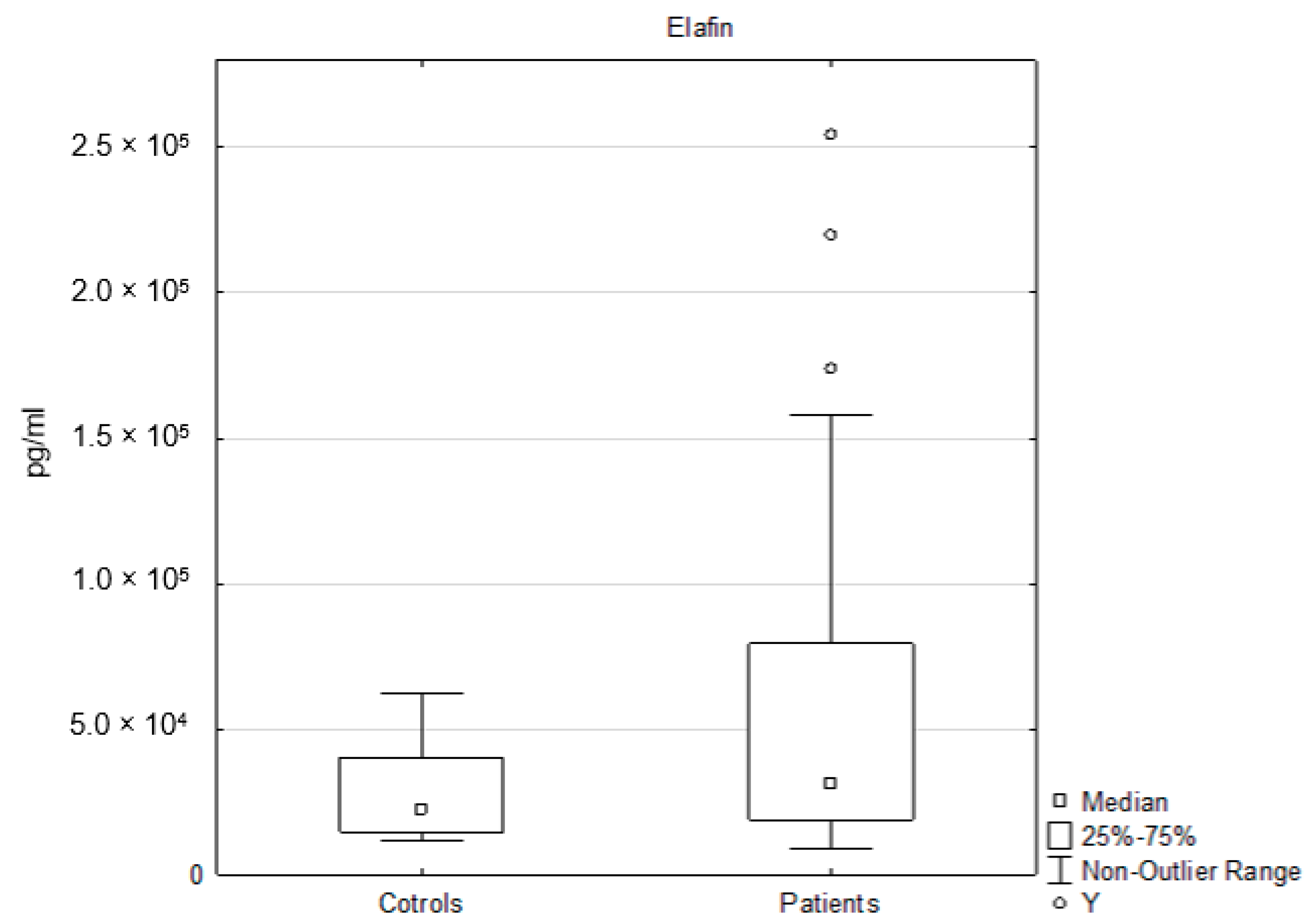
3.3. Elafin Analysis
3.4. Relationships Between the Evaluated Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017, 34, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Perez-Chada, L.M.; Merola, J.F. Comorbidities associated with psoriatic arthritis: Review and update. Clin. Immunol. 2020, 214, 108397. [Google Scholar] [CrossRef] [PubMed]
- Huang, P. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Diane, A.; Pierce, W.D.; Kelly, S.E.; Sokolik, S.; Borthwick, F.; Jacome-Sosa, M.; Mangat, R.; Pradillo, J.M.; Allan, S.M.; Ruth, M.R.; et al. Mechanisms of Comorbidities Associated with the Metabolic Syndrome: Insights from the JCR:LA-cp Corpulent Rat Strain. Front. Nutr. 2016, 3. [Google Scholar] [CrossRef]
- De Felice, C.; Marulli, G.C.; Ardigò, M.; Berardesca, E. Biological markers in the etiology of psoriasis: Targeted treatment options. Biol. Targets Ther. 2007, 1, 11–18. [Google Scholar]
- Coimbra, S. Biomarkers of psoriasis severity and therapy monitoring. World J. Dermatol. 2014, 3, 15. [Google Scholar] [CrossRef]
- Qian, M.; Song, N.-J. Serum calprotectin correlates with risk and disease severity in psoriasis patients and the decrease of calprotectin predicts better response to tumor necrosis factor inhibitors. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4299–4309. [Google Scholar]
- Zlibut, A.; Agoston-Coldea, L.; Mocan, T.; Bocsan, I.C.; Mocan, L. Biomarkers in Metabolic Syndrome. Ultimat. Guide Insulin 2019. [Google Scholar] [CrossRef]
- Michel, D.; Chatelain, G.; North, S.; Brun, G. Stress-induced transcription of the clusterin/apoJ gene. Biochem. J. 1997, 328, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Howe, P.H. Regulation of Clusterin Gene Expression by Transforming Growth Factor β. J. Biol. Chem. 1997, 272, 26620–26626. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Lourda, M.; Antonelou, M.H.; Kletsas, D.; Gorgoulis, V.G.; Papassideri, I.S.; Zou, Y.; Margaritis, L.H.; Boothman, D.A.; Gonos, E.S. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin. Cancer Res. 2009, 15, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Chiba, M.; Murata, T.; Matsuura, A. Heparan sulfate is a clearance receptor for aberrant extracellular proteins. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Essabbani, A.; Margottin-Goguet, F.; Chiocchia, G. Identification of Clusterin Domain Involved in NF-κB Pathway Regulation. J. Biol. Chem. 2009, 285, 4273–4277. [Google Scholar] [CrossRef]
- Shim, Y.-J.; Kang, B.-H.; Choi, B.-K.; Park, I.-S.; Min, B.-H. Clusterin induces the secretion of TNF-α and the chemotactic migration of macrophages. Biochem. Biophys. Res. Commun. 2012, 422, 200–205. [Google Scholar] [CrossRef]
- McDonald, J.F.; Nelsestuen, G.L. Potent Inhibition of Terminal Complement Assembly by Clusterin: Characterization of Its Impact on C9 Polymerization†. Biochemistry 1997, 36, 7464–7473. [Google Scholar] [CrossRef]
- Seo, H.-Y.; Kim, M.K.; Jung, Y.-A.; Jang, B.K.; Yoo, E.K.; Kim, M.K.; Lee, I.K. Clusterin Decreases Hepatic SREBP-1c Expression and Lipid Accumulation. Endocrinology 2013, 154, 1722–1730. [Google Scholar] [CrossRef]
- Won, J.C.; Park, C.-Y.; Oh, S.W.; Lee, E.S.; Youn, B.S.; Kim, M.S. Plasma Clusterin (ApoJ) Levels Are Associated with Adiposity and Systemic Inflammation. PLoS ONE 2014, 9, e103351. [Google Scholar] [CrossRef]
- Gil, S.Y.; Youn, B.S.; Byun, K.; Huang, H.; Namkoong, C.; Jang, P.-G.; Lee, J.-Y.; Jo, Y.-H.; Kang, G.M.; Kim, H.-K.; et al. Clusterin and LRP2 are critical components of the hypothalamic feeding regulatory pathway. Nat. Commun. 2013, 4, 1862. [Google Scholar] [CrossRef]
- Williams, S.E.; Brown, T.I.; Roghanian, A.; Sallenave, J.-M. SLPI and elafin: One glove, many fingers. Clin. Sci. 2005, 110, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Guyot, N.; Zani, M.-L.; Berger, P.; Dallet-Choisy, S.; Moreau, T. Proteolytic susceptibility of the serine protease inhibitor trappin-2 (pre-elafin): Evidence for tryptase-mediated generation of elafin. Biol. Chem. 2005, 386. [Google Scholar] [CrossRef] [PubMed]
- Elgharib, I.; Khashaba, S.A.; Elsaid, H.H.; Sharaf, M.M. Serum elafin as a potential inflammatory marker in psoriasis. Int. J. Dermatol. 2018, 58, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Fujioka, A.; Tajima, S.; Ishibashi, A.; Hirose, S. Elafin is induced in epidermis in skin disorders with dermal neutrophilic infiltration: Interleukin-1β and tumour necrosis factor-α stimulate its secretion in vitro. Br. J. Dermatol. 2000, 143, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M. EVE and beyond, retro and prospective insights. Am. J. Physiol. Content 1999, 277, L5–L12. [Google Scholar] [CrossRef]
- Guyot, N.; Butler, M.W.; McNally, P.; Weldon, S.; Greene, C.M.; Levine, R.L.; O’Neill, S.J.; Taggart, C.C.; McElvaney, N.G. Elafin, an Elastase-specific Inhibitor, Is Cleaved by Its Cognate Enzyme Neutrophil Elastase in Sputum from Individuals with Cystic Fibrosis. J. Biol. Chem. 2008, 283, 32377–32385. [Google Scholar] [CrossRef]
- Simpson, A.J.; Maxwell, A.; Govan, J.; Haslett, C.; Sallenave, J.-M. Elafin (elastase-specific inhibitor) has anti-microbial activity against Gram-positive and Gram-negative respiratory pathogens. FEBS Lett. 1999, 452, 309–313. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Motta, J.-P.; Aubry, C.; Kharrat, P.; Martin, L.; Sallenave, J.-M.; Deraison, C.; Vergnolle, N.; Langella, P. Serine protease inhibitors protect better than IL-10 and TGF-β anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb. Cell Factories 2015, 14, 26. [Google Scholar] [CrossRef]
- Salihbegovic, E.M.; Hadzigrahic, N.; Cickusic, A.J. Psoriasis and Metabolic Syndrome. Med. Arch. 2015, 69, 85–87. [Google Scholar] [CrossRef]
- Borská, L.; Kremláček, J.; Andrys, C.; Krejsek, J.; Hamakova, K.; Borsky, P.; Palička, V.; Řeháček, V.; Málková, A.; Fiala, Z. Systemic Inflammation, Oxidative Damage to Nucleic Acids, and Metabolic Syndrome in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2017, 18, 2238. [Google Scholar] [CrossRef]
- Narendra, G.; Roshin, N.A.; Thimmappa, V.; Shivanna, R. Metabolic syndrome in patients with psoriasis: A hospital-based case–control study. Clin. Dermatol. Rev. 2018, 2, 64–68. [Google Scholar] [CrossRef]
- Das, S.; Manna, A.; Ahmad, N.; Banerjee, D.; Mondal, S.; Tayal, P. Psoriasis and metabolic syndrome: Co-incidence or correlation. Med. J. Dr. D.Y. Patil Univ. 2016, 9, 177. [Google Scholar] [CrossRef]
- Al-Mutairi, N.; Al-Farag, S.; Al-Mutairi, A.; Al-Shiltawy, M. Comorbidities associated with psoriasis: An experience from the Middle East. J. Dermatol. 2010, 37, 146–155. [Google Scholar] [CrossRef]
- Buquicchio, R.; Foti, C.; Loconsole, F.; Polimeno, L.; Ventura, M.T. Clusterin serum level: How does it affect psoriatic patients? J. Biol. Regul. Homeost. Agents 2017, 31, 785–789. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28958138 (accessed on 1 May 2020).
- Ataseven, A.; Kesli, R.; Kurtipek, G.S.; Ozturk, P. Assessment of Lipocalin 2, Clusterin, Soluble Tumor Necrosis Factor Receptor-1, Interleukin-6, Homocysteine, and Uric Acid Levels in Patients with Psoriasis. Dis. Markers 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; Arias-Santiago, S.; Perandrés-López, R.; Orgaz-Molina, J.; Castellote, L.; Buendía-Eisman, A.; Ruiz, J.; Naranjo, R.; Navarro, P.; Sancho, J.; et al. Decreased Plasma Levels of Clusterin in Patients With Psoriasis. Actas Dermo-Sifiliográficas (English Edition) 2013, 104, 497–503. [Google Scholar] [CrossRef]
- Devauchelle, V.; Essabbani, A.; De Pinieux, G.; Germain, S.; Tourneur, L.; Mistou, S.; Margottin-Goguet, F.; Anract, P.; Migaud, H.; Le Nen, D.; et al. Characterization and functional consequences of underexpression of clusterin in rheumatoid arthritis. J. Immunol. 2006, 177, 6471–6479. [Google Scholar] [CrossRef]
- Sol, I.S.; Kim, Y.H.; Lee, K.E.; Hong, J.Y.; Na Kim, M.; Kim, Y.S.; Oh, M.S.; Kim, M.J.; Yoon, S.H.; Park, Y.A.; et al. Serum clusterin level in children with atopic dermatitis. Allergy Asthma Proc. 2016, 37, 335–339. [Google Scholar] [CrossRef]
- Vranová, H.P.; Hényková, E.; Mareš, J.; Kaiserová, M.; Menšíková, K.; Vaštík, M.; Hluštík, P.; Zapletalová, J.; Strnad, M.; Stejskal, D.; et al. Clusterin CSF levels in differential diagnosis of neurodegenerative disorders. J. Neurol. Sci. 2016, 361, 117–121. [Google Scholar] [CrossRef]
- Giang, J.; Seelen, M.A.J.; Van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Kim, T.-G.; Kim, D.S.; Kim, H.-P.; Lee, M.-G. The pathophysiological role of dendritic cell subsets in psoriasis. BMB Rep. 2014, 47, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.H.; Kwon, H.-S.; Moon, K.-A.; Park, S.Y.; Park, S.; Lee, K.Y.; Ha, E.H.; Kim, T.; Moon, H.-B.; Lee, H.; et al. Clusterin Modulates Allergic Airway Inflammation by Attenuating CCL20-Mediated Dendritic Cell Recruitment. J. Immunol. 2016, 196, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Savkovic, V.; Gantzer, H.; Reiser, U.; Selig, L.; Gaiser, S.; Sack, U.; Klöppel, G.; Mössner, J.; Keim, V.; Horn, F.; et al. Clusterin is protective in pancreatitis through anti-apoptotic and anti-inflammatory properties. Biochem. Biophys. Res. Commun. 2007, 356, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Cunin, P.; Beauvillain, C.; Miot, C.; Augusto, J.-F.; Preisser, L.; Blanchard, S.; Pignon, P.; Scotet, M.; Garo, E.; Fremaux, I.; et al. Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses. Cell Death Dis. 2016, 7, e2215. [Google Scholar] [CrossRef]
- Santarlasci, V.; Maggi, L.; Capone, M.; Frosali, F.; Querci, V.; De Palma, R.; Liotta, F.; Cosmi, L.; Maggi, E.; Romagnani, S.; et al. TGF-β indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur. J. Immunol. 2009, 39, 207–215. [Google Scholar] [CrossRef]
- Jenne, D.E.; Lowin, B.; Peitsch, M.C.; Bottcher, A.; Schmitz, G.; Tschopp, J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J. Biol. Chem. 1991, 266, 11030–11036. [Google Scholar]
- Balantinou, E.; Trougakos, I.P.; Chondrogianni, N.; Margaritis, L.H.; Gonos, E.S. Transcriptional and posttranslational regulation of clusterin by the two main cellular proteolytic pathways. Free. Radic. Biol. Med. 2009, 46, 1267–1274. [Google Scholar] [CrossRef]
- Kratzer, A.; Giral, H.; Landmesser, U. High-density lipoproteins as modulators of endothelial cell functions: Alterations in patients with coronary artery disease. Cardiovasc. Res. 2014, 103, 350–361. [Google Scholar] [CrossRef]
- Nizard, P.; Tetley, S.; Le Dréan, Y.; Watrin, T.; Le Goff, P.; Wilson, M.; Michel, D. Stress-Induced Retrotranslocation of Clusterin/ApoJ into the Cytosol. Traffic 2007, 8, 554–565. [Google Scholar] [CrossRef]
- Fujita, H.; Yagishita, N.; Aratani, S.; Saito-Fujita, T.; Morota, S.; Yamano, Y.; Hansson, M.J.; Inazu, M.; Kokuba, H.; Sudo, K.; et al. The E3 ligase synoviolin controls body weight and mitochondrial biogenesis through negative regulation of PGC -1β. EMBO J. 2015, 34, 1042–1055. [Google Scholar] [CrossRef]
- Wei, J.; Yuan, Y.; Chen, L.; Xu, Y.; Zhang, Y.; Wang, Y.; Yang, Y.; Peek, C.B.; Diebold, L.; Yang, Y.; et al. ER-associated ubiquitin ligase HRD1 programs liver metabolism by targeting multiple metabolic enzymes. Nat. Commun. 2018, 9, 3659. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zoubeidi, A.; Beraldi, E.; E Gleave, M. GRP78 regulates clusterin stability, retrotranslocation and mitochondrial localization under ER stress in prostate cancer. Oncogene 2012, 32, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, B.; Dong, H.; Shi, J.; Fang, D. Icariin Induces Synoviolin Expression through NFE2L1 to Protect Neurons from ER Stress-Induced Apoptosis. PLoS ONE 2015, 10, e0119955. [Google Scholar] [CrossRef] [PubMed]
- Widenmaier, S.; Snyder, N.A.; Nguyen, T.B.; Arduini, A.; Lee, G.Y.; Arruda, A.P.; Saksi, J.; Bartelt, A.; Hotamisligil, G.S. NRF1 Is an ER Membrane Sensor that Is Central to Cholesterol Homeostasis. Cell 2017, 171, 1094–1109.e15. [Google Scholar] [CrossRef]
- Shimoura, N.; Nagai, H.; Fujiwara, S.; Jimbo, H.; Nishigori, C. Exacerbation and Prolongation of Psoriasiform Inflammation in Diabetic Obese Mice: A Synergistic Role of CXCL5 and Endoplasmic Reticulum Stress. J. Investig. Dermatol. 2018, 138, 854–863. [Google Scholar] [CrossRef]
- Sugiura, K.; Muro, Y.; Futamura, K.; Matsumoto, K.; Hashimoto, N.; Nishizawa, Y.; Nagasaka, T.; Saito, H.; Tomita, Y.; Usukura, J. The Unfolded Protein Response Is Activated in Differentiating Epidermal Keratinocytes. J. Investig. Dermatol. 2009, 129, 2126–2135. [Google Scholar] [CrossRef]
- Arnold, T.; Brandlhofer, S.; Vrtikapa, K.; Stangl, H.; Hermann, M.; Zwiauer, K.; Mangge, H.; Karwautz, A.; Huemer, J.; Koller, D.; et al. Effect of Obesity on Plasma Clusterin: A Proposed Modulator of Leptin Action. Pediatr. Res. 2011, 69, 237–242. [Google Scholar] [CrossRef]
- Kloučková, J.; Lacinová, Z.; Kaválková, P.; Trachta, P.; Kasalický, M.; Haluzíková, D.; Mráz, M.; Haluzík, M. Plasma Concentrations and Subcutaneous Adipose Tissue mRNA Expression of Clusterin in Obesity and Type 2 Diabetes Mellitus: The Effect of Short-Term Hyperinsulinemia, Very-Low-Calorie Diet and Bariatric Surgery. Physiol. Res. 2016, 481–492. [Google Scholar] [CrossRef]
- Wang, J.; Ortiz, C.W.; Mukhopadhyay, R.; Fontenot, L.; Koon, H.W. Mo2017 – Antimicrobial Peptide Elafin Reverses Obesity, Insulin Resistance, and Liver Steatosis Via Circulating Exosomal Mir181B-5P and Mir219-5P. Gastroenterology 2019, 156. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil Elastase, Proteinase 3, and Cathepsin G as Therapeutic Targets in Human Diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Lingling, S.; Zhong, L.; Hoo, R.; Xu, A. Pharmacological Inhibition of Neutrophil Elastase Attenuates Insulitis and Autoimmune Diabetes in Mice. Diabetes 2018, 67, 1732. [Google Scholar] [CrossRef]
- Tsai, Y.-S.; Tseng, Y.-T.; Chen, P.-S.; Lin, M.-C.; Wu, C.-C.; Huang, M.-S.; Wang, C.-C.; Chen, K.-S.; Lin, Y.-C.; Wang, T.-N. Protective effects of elafin against adult asthma. Allergy Asthma Proc. 2016, 37, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.A.; Hitt, M.; Xing, Z.; Wang, J.; Haslett, C.; Riemersma, R.A.; Webb, D.J.; Kotelevtsev, Y.V.; Sallenave, J.-M. Adenoviral Gene Delivery of Elafin and Secretory Leukocyte Protease Inhibitor Attenuates NF-κB-Dependent Inflammatory Responses of Human Endothelial Cells and Macrophages to Atherogenic Stimuli. J. Immunol. 2004, 172, 4535–4544. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.W.; Robertson, I.; Greene, C.M.; O’Neill, S.J.; Taggart, C.C.; McElvaney, N.G. Elafin Prevents Lipopolysaccharide-induced AP-1 and NF-κB Activation via an Effect on the Ubiquitin-Proteasome Pathway. J. Biol. Chem. 2006, 281, 34730–34735. [Google Scholar] [CrossRef]
- Motta, J.-P.; Magne, L.; Descamps, D.; Rolland, C.; Squarzoni–Dale, C.; Rousset, P.; Martin, L.; Cenac, N.; Balloy, V.; Huerre, M.; et al. Modifying the Protease, Antiprotease Pattern by Elafin Overexpression Protects Mice From Colitis. Gastroenterology 2011, 140, 1272–1282. [Google Scholar] [CrossRef]
| Controls | Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Median | Q1 | Q3 | N | Median | Q1 | Q3 | |
| Age | 40 | 50.5 | 36.9 | 57.4 | 45 | 52.9 | 40.5 | 67.6 |
| BMI * | 40 | 24.3 | 23.1 | 27.5 | 45 | 28 | 24.8 | 30.4 |
| Glc * | 40 | 4.26 | 3.63 | 4.66 | 45 | 4.77 | 4.40 | 5.46 |
| TAG | 40 | 1.23 | 0.84 | 1.64 | 45 | 1.57 | 0.98 | 2.02 |
| HDL * | 40 | 1.30 | 1.16 | 1.52 | 45 | 1.08 | 0.91 | 1.28 |
| Waist | 40 | 88 | 80 | 99 | 45 | 98 | 87 | 103 |
| BPsys | 40 | 130 | 120 | 140 | 45 | 130 | 125 | 140 |
| BPdia * | 40 | 80 | 75 | 85 | 45 | 90 | 80 | 95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmannova, D.; Borsky, P.; Borska, L.; Andrys, C.; Hamakova, K.; Rehacek, V.; Svadlakova, T.; Malkova, A.; Beranek, M.; Palicka, V.; et al. Metabolic Syndrome, Clusterin and Elafin in Patients with Psoriasis Vulgaris. Int. J. Mol. Sci. 2020, 21, 5617. https://doi.org/10.3390/ijms21165617
Holmannova D, Borsky P, Borska L, Andrys C, Hamakova K, Rehacek V, Svadlakova T, Malkova A, Beranek M, Palicka V, et al. Metabolic Syndrome, Clusterin and Elafin in Patients with Psoriasis Vulgaris. International Journal of Molecular Sciences. 2020; 21(16):5617. https://doi.org/10.3390/ijms21165617
Chicago/Turabian StyleHolmannova, Drahomira, Pavel Borsky, Lenka Borska, Ctirad Andrys, Kvetoslava Hamakova, Vit Rehacek, Tereza Svadlakova, Andrea Malkova, Martin Beranek, Vladimir Palicka, and et al. 2020. "Metabolic Syndrome, Clusterin and Elafin in Patients with Psoriasis Vulgaris" International Journal of Molecular Sciences 21, no. 16: 5617. https://doi.org/10.3390/ijms21165617
APA StyleHolmannova, D., Borsky, P., Borska, L., Andrys, C., Hamakova, K., Rehacek, V., Svadlakova, T., Malkova, A., Beranek, M., Palicka, V., Krejsek, J., & Fiala, Z. (2020). Metabolic Syndrome, Clusterin and Elafin in Patients with Psoriasis Vulgaris. International Journal of Molecular Sciences, 21(16), 5617. https://doi.org/10.3390/ijms21165617





