Abstract
Lysophosphatidylcholine (LPC) is increasingly recognized as a key marker/factor positively associated with cardiovascular and neurodegenerative diseases. However, findings from recent clinical lipidomic studies of LPC have been controversial. A key issue is the complexity of the enzymatic cascade involved in LPC metabolism. Here, we address the coordination of these enzymes and the derangement that may disrupt LPC homeostasis, leading to metabolic disorders. LPC is mainly derived from the turnover of phosphatidylcholine (PC) in the circulation by phospholipase A2 (PLA2). In the presence of Acyl-CoA, lysophosphatidylcholine acyltransferase (LPCAT) converts LPC to PC, which rapidly gets recycled by the Lands cycle. However, overexpression or enhanced activity of PLA2 increases the LPC content in modified low-density lipoprotein (LDL) and oxidized LDL, which play significant roles in the development of atherosclerotic plaques and endothelial dysfunction. The intracellular enzyme LPCAT cannot directly remove LPC from circulation. Hydrolysis of LPC by autotaxin, an enzyme with lysophospholipase D activity, generates lysophosphatidic acid, which is highly associated with cancers. Although enzymes with lysophospholipase A1 activity could theoretically degrade LPC into harmless metabolites, they have not been found in the circulation. In conclusion, understanding enzyme kinetics and LPC metabolism may help identify novel therapeutic targets in LPC-associated diseases.
1. Introduction
1.1. General Features of Lysophosphatidylcholine
Lysophosphatidylcholine (LPC), also called lysolecithins, is a class of lipid biomolecule derived by the cleaving of phosphatidylcholine (PC) via the action of phospholipase A2 (PLA2) [1,2] and/or by the transfer of fatty acids to free cholesterol via lecithin-cholesterol acyltransferase (LCAT) [3]. In healthy individuals, the plasma level of LPC ranges from 125 to 143 nmole/mL, but its level increases in cardiovascular diseases, diabetes, ovarian cancer, and renal failure [4,5,6]. PC, also called lecithins (lécithine), was originally derived from the Greek word “lekithos” (λεκιθος, egg yolk), and in 1847, Theodore Nicolas Gobley [7] published a description of their chemical structure. PC is a major component of biological membranes found in animal and plant cells [8]. Although LPCs can be produced in the circulation when PLA2 cleaves PCs, they can be converted back to PCs by the enzyme lysophosphatidylcholine acyltransferase (LPCAT) in the presence of Acyl-CoA. These two pathways are part of the Lands cycle [9], which is one of the body’s mechanisms for the cyclical synthesis and degradation of PC. The details of these processes are described in this review. LPCATs are intracellular enzymes found in body tissues such as lung [10], liver [11], and adipose tissue [12], but these intracellular enzymes are unlikely to interact directly with extracellular circulating LPC, which is positively associated with diseases [4,5,6].
1.2. Effects of Lysophosphatidylcholines
In the liver, LPCs upregulate genes involved in cholesterol biosynthesis and downregulate genes involved in hepatic fatty acid oxidation [13]. Higher concentrations of LPCs disrupt mitochondrial integrity and enhance cytochrome C release in hepatocytes (Table 1). In the vascular system, LPC induces prolonged endothelial activation and atherogenesis [14,15]. It modulates inflammatory chemokine expression from endothelial cells [16,17,18,19], impairs arterial relaxation [20], increases oxidative stress [21,22], and inhibits endothelial cell migration and proliferation [23,24]. Rich in oxidized low-density lipoprotein (OxLDL), LPCs have been identified as a group of proinflammatory lipids that are critically involved in the pathogenesis of atherosclerosis [25] and other inflammatory diseases such as multiple sclerosis [26,27]. Overproduction of LPC can result from the overexpression or enhanced activity of enzymes such as lipoprotein-associated phospholipase A2 (Lp-PLA2) in circulation [28,29]. In contrast, effectively clearing LPC, through either Lands cycle remodeling inside cells or the direct degradation of circulating LPC, is essential for maintaining LPC levels.

Table 1.
Summary of reported effects of LPC on various cell types.
1.3. Lysophosphatidylcholine Signaling through Receptors
LPC activates multiple signaling pathways that are involved in oxidative stress and inflammatory responses. The signaling cascade is triggered through G protein–coupled receptor G2A [30,31,32,33] and Toll-like receptors [34]. However, whether G2A is a canonical receptor for LPC is debated [35]. It is notable that LPC is not an agonist of the platelet-activating factor receptor, and the LPC used in previous studies may have been contaminated with PAF-like lipids [36]. Exogenous LPC induces pro-inflammatory effects such as upregulated gene expression for smooth muscle/fibroblast-directed growth factors and adhesion molecules in endothelial cells [37,38], increased release of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) from adipocytes [39,40], enhanced secretion of interferon-γ from peripheral blood mononuclear leucocytes [16], and increased activation of B cells [16] and macrophages [30,34,41]. In addition, LPC enhances the Foxp3 expression and suppressive function of naturally occurring regulatory T cells (nTregs) [42]; these actions are believed to be mediated through G2A signaling [42]. nTregs are responsible for preventing immune responses through various means, including the production of anti-inflammatory cytokines [43]. In 2014, researchers reported that LPC promotes and stabilizes macrophage polarization strongly toward the M1 phenotype, and that blocking the G2A receptor reduces the impact of LPC on macrophage polarization [44]. In the central nervous system, LPC induces demyelination in white matter of the spinal cord by activating G protein-coupled receptor 17 (Gpr17) signaling. Highly expressed in brain tissue, GPR17 reduces the intracellular cAMP level and induces pro-apoptotic gene XIAP-associated factor 1 (Xaf1) expression, which in turn inhibits oligodendrocyte survival and precursor cell differentiation [45]. In another study, LPC impaired the barrier function of the endothelium in the brain microvasculature and induced inflammation through G protein-coupled receptor4 (Gpr4) [46]. LPC mediates pericyte loss, vascular barrier disruption, demyelination, and motor function defects; all of these effects can be decreased by iloprost, an analog of prostacyclin [47]. The mechanism for demyelination involves the integration of LPC into the cell membrane, which induces permeability and necrotic cell death [48].
1.4. Recent Clinical Findings of Lysophosphatidylcholines
As described above, LPC may alter the physiology of the vascular endothelium, pericytes, and neuron cells in vitro and in vivo, indicating it may be a compelling risk factor and may be associated with the pathogenesis and prognosis of cardiovascular diseases. However, findings from recent clinical lipidomics studies have been controversial and somewhat confusing. For example, plasma LPCs showed an inverse relationship with cardiovascular diseases [51,52,53]. In other studies, the LPC:PC ratio decreased either in plasma or cerebrospinal fluid from patients with Alzheimer’s disease [54,55,56]. To help clarify the controversy and delineate the role of LPC in these diseases, we have provided a timely and important updated review on LPC homeostasis. In this article, we discuss whether excess LPC has a cause-effect association with human diseases. Specifically, we review LPC production in circulation and its transport and reconversion to PC within cells.
2. Lysophosphatidylcholine and Human Diseases
2.1. Lysophosphatidylcholine and Cardiovascular Diseases
Cardiovascular diseases are a class of diseases that include atherosclerosis, diabetes, metabolic syndrome, myocardial infarction, and angina. According to the World Health Organization, cardiovascular diseases account for 17.7 million deaths (31% of all global deaths) each year and are now the leading cause of death in the world [57]. Atherosclerosis is a pathological process that involves plaque build-up in the walls of the arteries [58]. LPC levels in the circulation are associated with the development of atherosclerotic plaques and endothelial cell dysfunction [58,59,60,61]. Some LPC species can be diagnostic markers for myocardial infarction [62]. LPC content is increased in circulating modified low-density lipoprotein (LDL) [63], enzymatically degraded LDL [64], and oxidized LDL [64,65]. In addition, LPC promotes fatty acid-induced insulin resistance [66] and inhibits endothelial progenitor cell revitalization [67]. LPC and LDL levels are increased in the plasma of patients with familial hyperlipidemia and diabetes [65,68,69] (Table 2), and treatment with simvastatin reduces Lp-PLA2 and LPC content [68]. Interestingly, several recent lipidomic profiling studies showed a negative correlation between LDL levels and the occurrence of cardiovascular diseases [51,52,53]. In addition, diabetes is an important risk factor for cardiovascular diseases; however, conflicting results have been reported on the correlation of LPC and diseases [4,70,71,72,73].

Table 2.
LPC levels in circulation, LDL, or tissue.
2.2. Lysophosphatidylcholine and Brain Diseases
Oligodendrocytes are myelin-producing cells that provide metabolic support for neurons and prevent neurodegeneration [78]. Myelination defects are seen in many brain diseases such as multiple sclerosis, stroke, schizophrenia, and Alzheimer’s disease [47,79]. LPC mediates pericyte loss, vascular barrier disruption, demyelination, and motor function defects [45,46,47]. In addition, LPC enhances the neurotoxicity of amyloid β1–42 peptide oligomer formation and neurotoxic protein aggregation, indicating that inhibiting LPC generation may be important in treating neurodegenerative diseases [80,81]. In one study, LPC levels were significantly increased in patients with repetitive mild traumatic brain injury [82]. However, other studies have shown that plasma levels of LPC were decreased in patients with Alzheimer’s disease [54,55] and that the LPC-to-PC ratio was also decreased either in plasma or in cerebrospinal fluid from patients with Alzheimer’s disease [56,77].
2.3. Brief Summary of Lysophosphatidylcholine in Human Disease
Through Notch1 and/or ERK1/2 signaling, LPC induces monocyte chemoattractant protein-1 and inflammatory cytokine expression and damages endothelial cells [18,19,50]. In addition, LPC activates monocytes and polarizes macrophage activation toward the M1 phenotype [30,34,41,44], leading to the development of atherosclerosis and cardiovascular diseases. In the brain, LPC promotes oligodendrocyte demyelination and pericyte loss, and impairs barrier function of the endothelium [45,46,47,48], leading to neurodegenerative diseases. LPC levels are determined by different mechanisms, including LPC production, clearance, and degradation. Overproduction of LPC and/or increasing LPC levels in LDL particles, or in tissue, are positively correlated with disease development.
3. Mechanisms for Increased Circulating Lysophosphatidylcholine Levels
3.1. Increased Degradation of Phosphatidylcholine by Lipoprotein-Associated Phospholipase A2
PC synthesized in the liver is the most abundant lipid component (up to 70% mole ratio) of plasma very low density lipoprotein (VLDL) and also makes up close to 40% of nascent high-density lipoprotein (HDL) [83,84]. In the liver, PC is involved in VLDL secretion [85,86,87] and HDL metabolism [88,89]. After being secreted into the blood stream, PC on lipoprotein particles is degraded at the Sn-2 position of an oxidized fatty acid by the hydrolysis of Lp-PLA2 (Figure 1). LPC is then produced under a variety of physiological and pathological conditions. In atherosclerotic plaque, macrophages produce Lp-PLA2, which is then secreted into the circulatory system [90]. ApoCIII, OxLDL, serum amyloid A, and leukocyte activation are associated with the regulation and activation of Lp-PLA2 expression [91,92,93]. In contrast, nitro-oleic acid downregulates Lp-PLA2 expression [94]. Extensive clinical evidence indicates that the quantity and activity of Lp-PLA2 are positively correlated with cardiovascular events [27,95,96,97]. Quantifying plasma Lp-PLA2 is useful for identifying plaque instability, acute coronary syndrome, and other cardiovascular diseases [98,99,100]. Moreover, Lp-PLA2 is a predictor for incident ischemic stroke severity, early neurological deterioration in patients with acute ischemic stroke, and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage [101,102,103]. However, inhibiting Lp-PLA2 by darapladib, a synthetic specific small molecular weight inhibitor of platelet-activating factor-acetylhydrolase (PAF-AH), did not yield promising results in clinical trials [104,105]. Although Lp-PLA2 has the anti-inflammatory function of degrading PAF [106,107] and thus reduces platelet activation, it also has the proinflammatory properties of increasing LPC and oxidized non-esterified fatty acids levels, which may be associated with the development of atherosclerosis [16,108]. Another potential future strategy for inhibiting Lp-PLA2 may involve the use of combined RNA interference (RNAi), which ameliorated atherosclerosis in apolipoprotein E-deficient mice [109].
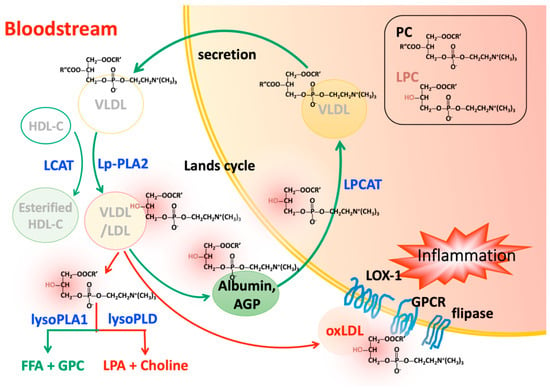
Figure 1.
Phosphatidylcholines (PCs) are synthesized in the liver and secreted as components of very low density lipoprotein (VLDL). PCs are degraded via hydrolysis by Lp-PLA2 or conversion by LCAT. The metabolites, LPCs, can be transported back to the liver by albumin or AGP and then cleared by LPCAT in the presence of acyl-CoA. The actions of these two distinct enzymes form a cycle of PC degradation and regeneration called the Lands cycle. Excess circulating LPCs may be released or carried on OxLDL to exert harmful effects on various cells though LOX-1, lipid flippase, and G protein-coupled receptor signaling. LPCs can also undergo further hydrolysis by lysoPLD such as autotaxin to become LPA, another important inflammatory mediator. Green arrows indicate occurrence under normal physiologic conditions. Red arrows indicate the promotion of inflammation.
3.2. Increased Degradation of Phosphatidylcholine by Lecithin-Cholesterol Acyltransferase
LCAT is secreted from the liver and catalyzes the transfer of the fatty acids at position sn-2 of PC to free cholesterol in plasma, which results in the formation of cholesterol esters and LPC on the surface of HDL and LDL [110,111] (Figure 1). In the brain, LCAT is synthesized by primary astrocytes and is activated by apolipoprotein E (apoE) secreted by glial cells [112]. The effect of LCAT on human atherogenesis is controversial [113,114]. Genetic deficiency of LCAT leads to the accumulation of nascent pre-β HDL in the circulation and development of atherosclerosis and acute coronary syndrome [114,115,116,117,118,119,120,121]. In golden Syrian hamsters, the loss of LCAT activity led to dyslipidemia and atherosclerosis [122]. In addition, proteomic studies in LCAT deficiency showed that plasma levels of HDL-C, apoAI, and apoAI were decreased, leading to corneal opacity, hemolytic anemia, and renal disease [123]. In contrast, overexpression of LCAT is associated with the formation of large apoE-rich HDL and liver cholesterol [124,125]. Higher LCAT activity is correlated with insulin resistance and nonalcoholic fatty liver disease [126]. The LDL-particle size can be reduced and positively associated with atherosclerotic cardiovascular disease [127]. However, in some cases, increased LCAT levels are associated with a reduced coronary atheroma burden [128]. Interestingly, LCAT levels did not differ in LCAT-transgenic mice and wild-type mice [129]. The detailed mechanisms are unclear.
3.3. Hypoxia Condition Regulates Glycolysis and Lysophosphatidylcholine Overproduction
Hypoxia induces a reprogramming of cell respiration and carbohydrate metabolism [130]. Under hypoxic conditions, hypoxia-inducible factor (HIF)-1 alters the expression profile of glycolytic molecules and regulates glycolysis [131]. In addition, HIF-1 in the liver activates sterol regulatory element binding protein-1 and stearoyl-coenzyme A desaturase, which are key regulatory genes in the biosynthesis of triglycerides and phospholipids [132]. Trzepizur et al. examined serum lipid levels in 2018 fasting patients and found that nocturnal intermittent hypoxia and obstructive sleep apnea severity were associated with higher triglyceride and lower HDL-C levels [133]. In cardiovascular diseases, intermittent hypoxia leads to cardiomyocyte apoptosis and inflammation through protein O-GlcNAc glycosylation and phosphorylation of the p38 mitogen-activated protein kinase [134]. Some lipidomic studies showed that hypoxia stimulation caused a prominent increase in the amount of LPC [135]. In addition, findings from a lipid consumption study also suggested that LPC provides a more accessible nutrient source for cell proliferation under hypoxia [136]. In AbPPSwe/PSIdE9 mice, chronic intermittent hypoxia triggered earlier learning memory impairment. In a symptomatic N5 TgCRND8 mouse model of Alzheimer’s disease, cytosolic phospholipase A2α activity progressively increased; overall LPC levels progressively rose. The authors concluded that disruptions in Lands cycle metabolism were linked to the onset of symptoms and a progressive behavioral decline in mice with pre-existing Aβ pathology [137]. However, the detailed effects of hypoxia on lipid metabolism in humans are not well understood.
4. Transportation of Lysophosphatidylcholine in the Circulatory System
Plasma LPC is rapidly cleared from circulation by transporters such as albumin and alpha-1 acid glycoprotein (AGP) to the liver for the synthesis of PC [138], or it accumulates in the brain for the production of acetylcholine [139] (Figure 1). Although overproduction of LPC can occur through different mechanisms as mentioned above, it can also accumulate in oxidized LDL and is associated with vascular inflammation [140,141].
4.1. Albumin
About 80% of LPC is bound to albumin [142,143]. Hypoalbuminemia due to proteinuria results in a decrease in albumin-LPC binding and an increase in LPC levels in VLDL, intermediate-density lipoprotein, and LDL [144]. Increased levels of LPC in LDL of hyperlipidemic patients were associated with nephrotic syndrome [145]. In another study, albumin was found to be protective in LPC-induced attenuation of vasodilation [146]. Moreover, albumin counteracted LPC-induced renal vasoconstriction [147], suggesting that albumin is a potent buffer for the effects of LPC.
4.2. Alpha-1 Acid Glycoprotein
The concentration of AGP in the plasma increases under inflammatory conditions. Similar to the function of antitrypsin, AGP exerts anti-inflammatory effects by inhibiting platelet aggregation, preventing superoxide production, and attenuating TNF-α effects on cells [148]. In addition, AGP maintains capillary permeability and prevents apoptosis in ischemia/reperfusion injury. With a higher binding affinity to LPC, AGP complements albumin as a lysophospholipid-scavenging protein, especially in inflammatory conditions in which albumin-sequestering capacity is weakened [138].
4.3. Transmembrane Transporter Protein
P4-ATPases are lipid flippases expressed on the eukaryotic plasma membrane. They function in translocating phospholipids from the exoplasm to the cytosolic area against a concentration gradient via ATP hydrolysis [149,150]. In a reverse process, LPC can be exported by the action of ATP-binding cassette transporter A7 (ABCA7) in the presence of apolipoprotein AI (apoAI) and apoE [151,152]. Mutations of ABCA7 are associated with neurodegenerative diseases [153]. The possible mechanism may involve a scenario in which excess LPC synergistically enhances Aβ1-42-induced neuronal apoptosis [154,155].
4.4. Oxidized Low Density Liopoprotein
Human lipoproteins, including VLDL, LDL, and HDL, contain LPC. However, the levels of LPC in these lipoproteins are debated [65,156,157]. Using electrospray ionization and matrix-assisted laser desorption/ionization mass spectrometry, Stübiger et al. reported that LPC concentration was increased in patients with familial hypercholesterolemia or familial combined hyperlipidemia [65]. Specifically, the amount of bioactive lipid LPC is increased up to 5 times in circulating modified LDL or OxLDL [63,65,158]. Because of the atherogenicity of LPC, OxLDL modulates dendritic cell phenotypic and functional maturation [159,160], triggers adipocyte activation and plasminogen activator inhibitor-1 secretion [40], leads to endothelial damage by inhibiting Ca2+ influx and NO synthesis [161], and promotes human artery smooth muscle cells proliferation and migration [162]. These pathophysiological implications make LPC a promising target for biomarker and treatment of atherosclerosis and cardiovascular disorders [162,163].
5. Lysophosphatidylcholine Turnover
5.1. Lysophosphatidylcholine Clearance by Acyltransferases in Various Tissues
Under normal physiological conditions, LPC is cleared by enzymes such as acyl-CoA:LPCAT [164], located in the endoplasmic reticulum within alveolar type II cells in the lung [10], in lipid droplets [12], in red blood cells [164], in hepatocytes [11,165], and in other cell types (Figure 1). At least four LPCAT subtypes have been identified [166]. Among them, overexpression of the LPCAT1 gene may contribute to the progression and metastasis of human cancers, such as hepatocellular carcinoma [167], oral squamous cell carcinoma [168], breast cancer [169], prostate cancer [170], and colorectal cancer [171]. LPCAT2 supports lipid droplet production, and its overexpression inhibits the function of chemotherapeutic agents for colorectal cancer [172]. Expression of the LPCAT2 gene is upregulated in breast and cervical cancers [173]. LPCAT3 is regulated by peroxisome proliferator-activated receptor δ. Transient liver-specific knockdown of LPCAT3 in mice attenuated the fatty acid metabolic pathway [11,165]. In another study, LPCAT3 knockdown resulted in LPC accumulation in the liver but promoted VLDL secretion and microsomal triglyceride transfer protein expression [174]. In addition, LPCAT3 deficiency reduced lipid adsorption in small intestine [175]. LPCAT4 is also called acyl-CoA:lysophosphatidylethanolamine acyltransferase 2 and is primarily expressed in the brain [176]. In colorectal cancer, LPCAT4 levels are elevated [177]. Tumor necrosis factor-α and transforming growth factor-β1 induced the expression of LPCAT2 and LPCAT4 [178,179].
5.2. Degradation of Lysophosphatidylcholine by Lysophospholipases in the Circulation
The hydrolysis of LPC can be catalyzed by lysophospholipases A1, C, or D, according to the cleavage site (Figure 2). In neutrophils in humans, phospholipase B-like 1 exhibits weak lysophospholipase A1 activity [180]. Autotaxin has lysophospholipase D activity; the product resulting from the action of autotaxin—lysophosphatidic acid (LPA)—is associated with cancer and other inflammatory diseases. To date, no enzyme has been documented to exhibit lysophospholipase C activity.
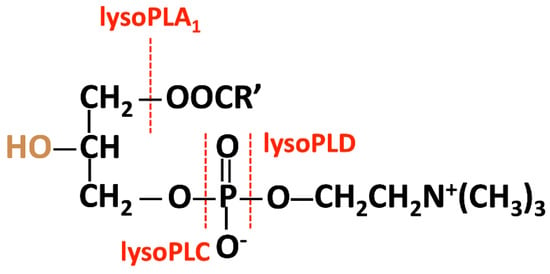
Figure 2.
The hydrolysis of LPC is catalyzed by lysophospholipases A1, C, or D, according to the cleavage site.
5.2.1. Enzymes with Lysophospholipases A1 Activity
Galectin-10: Also known as Charcot-Leyden crystal protein, galectin-10 was first described by Charcot and Robin more than 150 years ago. Galectin-10 is associated with eosinophil- or basophil-mediated inflammation involved with allergy responses [181,182]. Initially, galectin-10 was falsely considered to have weak lipase activity [183] but was later shown to bind a pancreatic-like lysophospholipase in human eosinophils and to inhibit lipolytic activity [184,185]. Highly expressed in eosinophils, galectin-10 is associated with the formation of Charcot-Leyden crystals in lymphocytes; however, the function of the crystals is not fully understood [186].
Phospholipase B-like 1: The membrane-bound protein from neutrophils exhibited weak phospholipase activity for various phospholipids, including LPC [180]; the investigators suggested that phospholipase B-like 1 may play a role in the response against microorganisms and inflammation. Phospholipase B-like 1 is highly expressed on leukocytes in patients with ischemic stroke [187,188], but the detailed mechanisms are not clear.
Lysophospholipase I (encoded by the LYPLA1 gene) was first cloned from human brain tissue [189,190]. Similar to lysophospholipase I, the paralog lysophospholipase II (encoded by the LYPLA2 gene) is a cytosolic enzyme that is transported through the cell membrane by palmitoylation [191]. Interference by using small molecules such as palmostatin B inhibits Ras localization and signaling through lysophospholipase acylation [192]. Both lysophospolipase I and II are now classified as EC 3.1.2.22 (UniProt, release 2019_01) and have been renamed acyl-protein thioesterase 1 and 2 (APT-1/APT-2) because they have depalmitoylating activity but low lysophospholipase activity [192,193,194]. Although the alternative names are APT-1/APT-2 and lysophospholipase I/II (LysoPLA I/LysoPLA II), the major functions of these enzymes differ from those of lysophospholipase A1 (lysoPLA1), which is classified as EC 3.1.1.5. Instead, the depalmitoylating activity of APT-1/APT-2 is associated with membrane protein localization and signaling such as Ras [192].
5.2.2. Enzymes with Lysophospholipases D Activity
Autotaxin: Autotaxin, also called ecto-nucleotide pyrophosphatase/phosphodiesterase-2, is a secreted exo-enzyme that produces most of the extracellular lipid mediator, LPA [195,196]. Autotaxin hydrolyzes phosphodiester bonds of nucleoside triphosphates, lysophospholipids, and cholinephosphate esters [197]. The unique lysophospholipase D activity of autotaxin is determined by a characteristic bimetallic active site and a deep lipid-binding pocket [198]. Originally isolated from human melanoma A2058 cells and defined as an “autocrine motility factor” [199], autotaxin plays a major role in the development of the embryonic vasculature and neural tissue [200,201,202] and in wound healing [203]. However, autotaxin also stimulates tumor cell motility and contributes to the progression of breast cancer [204]. In addition, autotaxin promotes bone cancer metastasis [205], increases the proliferation of thyroid cancer cells [206], and provides cells with resistance to chemotherapy [207]. Moreover, patients with liver fibrosis or hepatocellular carcinoma have increased levels of autotaxin [208,209].
LPA signaling and function: The LPA concentration in plasma ranges between 100 to 164 nM, which is about 1000 times less than that of LPC [210,211,212]. Extracellular LPA is generated via several mechanisms, including the action of phospholipases (group IIA secretory phospholipase A2; sPLA2-IIA and phosphatidylserine-specific phospholipase A1; and PS-PLA1) and removal of the choline moiety from LPC by autotaxin [213,214]. Platelets are a major source of LPA because of the presence of autotaxin in their α-granules, and secreted autotaxin is responsible for the basal concentration of LPA in blood [215]. There are six LPA-associated G protein-coupled receptors (GPCRs), LPA1–6 or LPAR1–6, involved in autotaxin–LPA axis signaling [216,217]. LPA induces a variety of responses such as cell proliferation and migration and cytokine production via GPCR signaling and the effects on ion channels under normal and pathological conditions [218]. LPA plays an important physiological role in the functioning of the immune system. It promotes the homing of lymphocytes to secondary lymphoid tissue through high endothelial venules [219] and stimulates the polarization and transendothelial migration of naïve T cells [220].
LPA and diseases: LPA signaling is associated with metabolic and inflammatory disorders including obesity, insulin resistance, atherosclerosis, and myocardial infarction [221,222,223]. Either by autotaxin overexpression or by supplemented LPA intake, LPA impairs paraoxonase/arylesterase activity and inhibits scavenger receptor BI expression but promotes matrix metalloproteinase-9 activation in THP-1 cells, which results in foam cell formation and atherosclerosis [222,224,225]. Treatment targeting LPA receptors and the downstream signaling pathway attenuates atherosclerosis progression in LDL-receptor deficient mice [226]. In addition, the LPA inactivator, phospholipid phosphatase 3 (PLPP3), is repressed in advanced stages of human atherosclerosis [227]. In mice, inactivation of PLPP3 led to myocardial dysfunction and heart failure [228]. These data indicate that LPA signaling may be an important therapeutic target. LPA stimulates angiogenesis, cancer cell growth, and metastasis [229,230]. High levels of autotaxin/LPA correlate with breast cancer, type I endometrial cancer, and formation of other tumors [231,232,233]. LPA impairs autophagy and regulates tumor progression in various cancer cells [234,235,236,237]. LPA and autotaxin are also highly expressed in the central nervous system, promoting lymphocyte circulation and maturation [219]. Dysregulation of LPA contributes to the pathogenesis of Alzheimer’s disease and mild cognitive impairment in patients with type 2 diabetes [238,239,240].
6. Conclusions
LPC, via G protein-coupled receptor signaling, has harmful effects on various cells that include enhancing inflammatory responses, disrupting mitochondrial integrity, and inducing apoptosis. However, the optimal level of LPC in the plasma has not been established, and the mechanisms underlying LPC’s harmful effects are not well understood. Levels of LPC in LDL positively correlate with disease development. An increase in LPC levels is determined primarily by enzyme activity—Lp-PLA2 for LPC production. LPCAT contributes to reducing LPC levels; however, the overexpression of LPCAT is associated with cancer. Direct degradation of LPC by autotaxin with lysophospholipase D activity produces LPA, which is another mediator that is highly associated with cancers. Enzymes with lysophospholipaseA1 activity can degrade LPC into harmless materials, but no enzyme with strong lysophospholipase A1 activity has been identified. Targeting LPC may be an important therapeutic option for treating cardiovascular and neurodegenerative diseases. The use of RNAi to inhibit Lp-PLA2 or enzyme lysophospholipase A1 activity may potentially be an effective future strategy. In summary, gaining a better understanding of the enzymes and non-enzyme proteins involved in LPC metabolism and how their levels correlate with disease conditions may be useful in identifying novel therapeutic targets for LPC-associated diseases.
Author Contributions
S.-H.L., F.P. wrote and reviewed the manuscripts; M.-L.C. organized and constructed tables and figures; G.K.M. and C.-H.C. reviewed the final draft of the manuscript and critically revised the manuscript for important intellectual content; L.-Y.K. conceptualized the manuscript.
Funding
This work was supported in part by grants from the Vascular and Medicinal Research Fund, Texas Heart Institute (#765-64050) to C.-H.C.; the Kaohsiung Medical University grant (KMU-TP105D14, KMU-DK107012) to L.-Y.K., (KMUH106-6R64, KMUH107-7R66) to C.-H.C.; and Taiwan Ministry of Science and Technology (MOST 105-2320-B-037-004-MY3, MOST 107-2314-B-037-114-MY3, MOST107-2911-I-037-505) to L.-Y.K., (MOST106-2321-B-037-003, 107-2321-B-037-002, 106-2314-B-037-069-) to C.-H.C. respectively.
Acknowledgments
In addition, we thank for Günter Schwarz from Cologne University, and Shau-Ku Huang from Johns Hopkins University School of Medicine, Baltimore, MD, USA for revising and final editing of this paper. The authors thank Rebecca Bartow, PhD, of the Texas Heart Institute in Houston, Texas, USA for editorial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AGP | Alpha-1 acid glycoprotein |
| HDL | High-density lipoprotein |
| LCAT | Lecithin-cholesterol acyltransferase |
| LDL | Low-density lipoprotein |
| LPA | Lysophosphatidic acid |
| LPC | Lysophosphatidylcholine |
| LPCAT | Lysophosphatidylcholine acyltransferase |
| VLDL | Very low density lipoprotein |
References
- Ridgway, N.; McLeod, R. Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Gauster, M.; Rechberger, G.; Sovic, A.; Horl, G.; Steyrer, E.; Sattler, W.; Frank, S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J. Lipid Res. 2005, 46, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.; Gansevoort, R.T.; Dikkeschei, B.D.; de Zeeuw, D.; de Jong, P.E.; van Tol, A. Role of elevated lecithin: Cholesterol acyltransferase and cholesteryl ester transfer protein activities in abnormal lipoproteins from proteinuric patients. Kidney Int 1993, 44, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Rabini, R.A.; Galassi, R.; Fumelli, P.; Dousset, N.; Solera, M.L.; Valdiguie, P.; Curatola, G.; Ferretti, G.; Taus, M.; Mazzanti, L. Reduced Na(+)-K(+)-ATPase activity and plasma lysophosphatidylcholine concentrations in diabetic patients. Diabetes 1994, 43, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Okita, M.; Gaudette, D.C.; Mills, G.B.; Holub, B.J. Elevated levels and altered fatty acid composition of plasma lysophosphatidylcholine(lysoPC) in ovarian cancer patients. Int. J. Cancer 1997, 71, 31–34. [Google Scholar] [CrossRef]
- Sasagawa, T.; Suzuki, K.; Shiota, T.; Kondo, T.; Okita, M. The significance of plasma lysophospholipids in patients with renal failure on hemodialysis. J. Nutr. Sci. Vitaminol. 1998, 44, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. A brief history of choline. Ann. Nutr. Metab. 2012, 61, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Watson, H. Biological membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, W.; Kazachkov, M.; Chen, G.; Chen, Q.; Carlsson, A.S.; Stymne, S.; Weselake, R.J.; Zou, J. Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell 2012, 24, 4652–4669. [Google Scholar] [CrossRef]
- Chen, X.; Hyatt, B.A.; Mucenski, M.L.; Mason, R.J.; Shannon, J.M. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11724–11729. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.Q.; Bonacci, T.M.; Bredt, D.S.; Li, S.; Bensch, W.R.; Moller, D.E.; Kowala, M.; Konrad, R.J.; Cao, G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J. Biol. Chem. 2008, 283, 8258–8265. [Google Scholar] [CrossRef]
- Moessinger, C.; Kuerschner, L.; Spandl, J.; Shevchenko, A.; Thiele, C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 2011, 286, 21330–21339. [Google Scholar] [CrossRef] [PubMed]
- Hollie, N.I.; Cash, J.G.; Matlib, M.A.; Wortman, M.; Basford, J.E.; Abplanalp, W.; Hui, D.Y. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim. Biophys. Acta 2014, 1841, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fang, P.; Sun, Y.; Jiang, X.; Wang, H.; Yang, X.F. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J. Biol. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.; Tran, K.; Pierce, G.N.; Chan, A.C.; Karmin, O.; Choy, P.C. Lysophosphatidylcholine stimulates the release of arachidonic acid in human endothelial cells. J. Biol. Chem. 1998, 273, 6830–6836. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Schafer-Elinder, L.; Wu, R.; Claesson, H.E.; Frostegard, J. Lysophosphatidylcholine (LPC) induces proinflammatory cytokines by a platelet-activating factor (PAF) receptor-dependent mechanism. Clin. Exp. Immunol. 1999, 116, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Takahara, N.; Kashiwagi, A.; Maegawa, H.; Shigeta, Y. Lysophosphatidylcholine stimulates the expression and production of MCP-1 by human vascular endothelial cells. Metabolism 1996, 45, 559–564. [Google Scholar] [CrossRef]
- Murugesan, G.; Sandhya Rani, M.R.; Gerber, C.E.; Mukhopadhyay, C.; Ransohoff, R.M.; Chisolm, G.M.; Kottke-Marchant, K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J. Mol. Cell. Cardiol. 2003, 35, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Lee, J.J.; Chen, Y.J.; Lin, S.I.; Lin, L.D.; Jein-Wen Liou, E.; Huang, W.L.; Chan, C.P.; Huang, C.C.; Jeng, J.H. Lysophosphatidylcholine induces cytotoxicity/apoptosis and IL-8 production of human endothelial cells: Related mechanisms. Oncotarget 2017, 8, 106177–106189. [Google Scholar] [CrossRef]
- Kugiyama, K.; Kerns, S.A.; Morrisett, J.D.; Roberts, R.; Henry, P.D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature 1990, 344, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Kim, J.A.; Park, M.H.; Jung, S.C.; Suh, S.H.; Pang, M.G.; Kim, Y.J. Lysophosphatidylcholine induces endothelial cell injury by nitric oxide production through oxidative stress. J. Matern. Fetal Neonatal Med. 2009, 22, 325–331. [Google Scholar] [CrossRef]
- Li, B.; Tian, S.; Liu, X.; He, C.; Ding, Z.; Shan, Y. Sulforaphane protected the injury of human vascular endothelial cell induced by LPC through up-regulating endogenous antioxidants and phase II enzymes. Food Funct. 2015, 6, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.; Colles, S.M.; Damron, D.S.; Graham, L.M. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, Y.; Kawashima, S.; Yamashita, T.; Ueyama, T.; Ishido, S.; Hotta, H.; Hirata, K.; Yokoyama, M. Lysophosphatidylcholine inhibits endothelial cell migration and proliferation via inhibition of the extracellular signal-regulated kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ruebsaamen, K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis 2010, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zahednasab, H.; Balood, M.; Harirchian, M.H.; Mesbah-Namin, S.A.; Rahimian, N.; Siroos, B. Increased autotaxin activity in multiple sclerosis. J. Neuroimmunol. 2014, 273, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Lehto, L.J.; Albors, A.A.; Sierra, A.; Tolppanen, L.; Eberly, L.E.; Mangia, S.; Nurmi, A.; Michaeli, S.; Grohn, O. Lysophosphatidyl Choline Induced Demyelination in Rat Probed by Relaxation along a Fictitious Field in High Rank Rotating Frame. Front. Neurosci. 2017, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J.; O’Reilly, D.S.; Caslake, M.J.; McMahon, A.D.; Ford, I.; Cooney, J.; Macphee, C.H.; Suckling, K.E.; Krishna, M.; Wilkinson, F.E.; et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 2000, 343, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Gao, P.; Orfei, L.; Watson, S.; Di, E.A.; Kaptoge, S.; Ballantyne, C.; Cannon, C.P.; Criqui, M.; Cushman, M.; et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: Collaborative analysis of 32 prospective studies. Lancet 2010, 375, 1536–1544. [Google Scholar] [PubMed]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.G.; Yang, L.V.; Riedinger, M.; Au, M.; Witte, O.N. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Hao, F.; Xu, X.; Chisolm, G.M.; Cui, M.Z. Lysophosphatidylcholine activates a novel PKD2-mediated signaling pathway that controls monocyte migration. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Waibel, M.; Radu, C.G.; Yang, L.V.; Witte, O.N.; Schulze-Osthoff, K.; Wesselborg, S.; Lauber, K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 2008, 283, 5296–5305. [Google Scholar] [CrossRef]
- Carneiro, A.B.; Iaciura, B.M.; Nohara, L.L.; Lopes, C.D.; Veas, E.M.; Mariano, V.S.; Bozza, P.T.; Lopes, U.G.; Atella, G.C.; Almeida, I.C.; et al. Lysophosphatidylcholine triggers TLR2- and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-kappaB translocation and MAPK/ERK phosphorylation. PLoS ONE 2013, 8, e76233. [Google Scholar] [CrossRef] [PubMed]
- Witte, O.N.; Kabarowski, J.H.; Xu, Y.; Le, L.Q.; Zhu, K. Retraction. Science 2005, 307, 206. [Google Scholar] [CrossRef] [PubMed]
- Marathe, G.K.; Silva, A.R.; de Castro Faria Neto, H.C.; Tjoelker, L.W.; Prescott, S.M.; Zimmerman, G.A.; McIntyre, T.M. Lysophosphatidylcholine and lyso-PAF display PAF-like activity derived from contaminating phospholipids. J. Lipid Res. 2001, 42, 1430–1437. [Google Scholar] [PubMed]
- Kume, N.; Gimbrone, M.A. Jr. Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J. Clin. Investig. 1994, 93, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Kume, N.; Cybulsky, M.I.; Gimbrone, M.A. Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Investig. 1992, 90, 1138–1144. [Google Scholar] [CrossRef]
- Sato, A.; Kumagai, T.; Ebina, K. A synthetic biotinylated peptide, BP21, inhibits the induction of mRNA expression of inflammatory substances by oxidized- and lyso-phosphatidylcholine. Drug Dev. Res. 2014, 75, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Kuniyasu, A.; Tokunaga, M.; Yamamoto, T.; Inoue, S.; Obama, K.; Kawahara, K.; Nakayama, H. Oxidized LDL and lysophosphatidylcholine stimulate plasminogen activator inhibitor-1 expression through reactive oxygen species generation and ERK1/2 activation in 3T3-L1 adipocytes. Biochim. Biophys. Acta 2011, 1811, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Homma, S.; Millman, I. Identification of the serum factor required for in vitro activation of macrophages. Role of vitamin D3-binding protein (group specific component, Gc) in lysophospholipid activation of mouse peritoneal macrophages. J. Immunol. 1991, 147, 273–280. [Google Scholar] [PubMed]
- Hasegawa, H.; Lei, J.; Matsumoto, T.; Onishi, S.; Suemori, K.; Yasukawa, M. Lysophosphatidylcholine enhances the suppressive function of human naturally occurring regulatory T cells through TGF-beta production. Biochem. Biophys. Res. Commun. 2011, 415, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Qiu, C.; Zhao, L. Lysophosphatidylcholine perpetuates macrophage polarization toward classically activated phenotype in inflammation. Cell. Immunol. 2014, 289, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Sun, Y.; Lin, L.; You, N.; Liu, X.; Li, H.; Ma, Y.; Cao, L.; Han, Y.; Liu, M.; et al. Olig2-Targeted G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Survival in Response to Lysolecithin-Induced Demyelination. J. Neurosci. 2016, 36, 10560–10573. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Huang, F.; Naikawadi, R.P.; Kim, K.S.; Said, T.; Lum, H. Lysophosphatidylcholine impairs endothelial barrier function through the G protein-coupled receptor GPR4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L91–L101. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, R.; Kuroda, M.; Matoba, K.; Lin, H.; Takahashi, C.; Koyama, Y.; Yamashita, T. Prostacyclin prevents pericyte loss and demyelination induced by lysophosphatidylcholine in the central nervous system. J. Biol. Chem. 2015, 290, 11515–11525. [Google Scholar] [CrossRef] [PubMed]
- Plemel, J.R.; Michaels, N.J.; Weishaupt, N.; Caprariello, A.V.; Keough, M.B.; Rogers, J.A.; Yukseloglu, A.; Lim, J.; Patel, V.V.; Rawji, K.S.; et al. Mechanisms of lysophosphatidylcholine-induced demyelination: A primary lipid disrupting myelinopathy. Glia 2018, 66, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Takahara, N.; Kashiwagi, A.; Nishio, Y.; Harada, N.; Kojima, H.; Maegawa, H.; Hidaka, H.; Kikkawa, R. Oxidized lipoproteins found in patients with NIDDM stimulate radical-induced monocyte chemoattractant protein-1 mRNA expression in cultured human endothelial cells. Diabetologia 1997, 40, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, Y.; Song, F.; Xu, S.; Nian, L.; Zhou, X.; Wang, S. TSG attenuates LPC-induced endothelial cells inflammatory damage through notch signaling inhibition. IUBMB Life 2016, 68, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Ganna, A.; Salihovic, S.; Sundstrom, J.; Broeckling, C.D.; Hedman, A.K.; Magnusson, P.K.; Pedersen, N.L.; Larsson, A.; Siegbahn, A.; Zilmer, M.; et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014, 10, e1004801. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, D.H.; Kim, J.K.; Park, M.J.; Yan, J.J.; Song, D.K.; Vaziri, N.D.; Noh, J.W. Lysophosphatidylcholine, oxidized low-density lipoprotein and cardiovascular disease in Korean hemodialysis patients: Analysis at 5 years of follow-up. J. Korean Med. Sci. 2013, 28, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, J.; Liu, Y.; Wu, R.; Yang, H.; Hu, X.; Ling, X. Studies on diagnostic biomarkers and therapeutic mechanism of Alzheimer’s disease through metabolomics and hippocampal proteomics. Eur. J. Pharm. Sci. 2017, 105, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Klavins, K.; Koal, T.; Dallmann, G.; Marksteiner, J.; Kemmler, G.; Humpel, C. The ratio of phosphatidylcholines to lysophosphatidylcholines in plasma differentiates healthy controls from patients with Alzheimer’s disease and mild cognitive impairment. Alzheimers Dement. 2015, 1, 295–302. [Google Scholar] [CrossRef]
- Khan, M.; Lamelas, P.; Musa, H.; Paty, J.; McCready, T.; Nieuwlaat, R.; Ng, E.; Lopez-Jaramillo, P.; Lopez-Lopez, J.; Yusoff, K.; et al. Development, Testing, and Implementation of a Training Curriculum for Nonphysician Health Workers to Reduce Cardiovascular Disease. Glob. Heart 2018, 13, 93–100. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Li, Y.; Kuo, Y.M.; Andrews, A.J.; Nanayakkara, G.; Johnson, C.; Fu, H.; Shan, H.; Du, F.; et al. Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Bjorkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Lavi, S.; McConnell, J.P.; Rihal, C.S.; Prasad, A.; Mathew, V.; Lerman, L.O.; Lerman, A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: Association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 2007, 115, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Mannheim, D.; Herrmann, J.; Versari, D.; Gossl, M.; Meyer, F.B.; McConnell, J.P.; Lerman, L.O.; Lerman, A. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke 2008, 39, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Ward-Caviness, C.K.; Xu, T.; Aspelund, T.; Thorand, B.; Montrone, C.; Meisinger, C.; Dunger-Kaltenbach, I.; Zierer, A.; Yu, Z.; Helgadottir, I.R.; et al. Improvement of myocardial infarction risk prediction via inflammation-associated metabolite biomarkers. Heart 2017, 103, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Zakiev, E.R.; Sukhorukov, V.N.; Melnichenko, A.A.; Sobenin, I.A.; Ivanova, E.A.; Orekhov, A.N. Lipid composition of circulating multiple-modified low density lipoprotein. Lipids Health Dis. 2016, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Orso, E.; Matysik, S.; Grandl, M.; Liebisch, G.; Schmitz, G. Human native, enzymatically modified and oxidized low density lipoproteins show different lipidomic pattern. Biochim. Biophys. Acta 2015, 1851, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Stubiger, G.; Aldover-Macasaet, E.; Bicker, W.; Sobal, G.; Willfort-Ehringer, A.; Pock, K.; Bochkov, V.; Widhalm, K.; Belgacem, O. Targeted profiling of atherogenic phospholipids in human plasma and lipoproteins of hyperlipidemic patients using MALDI-QIT-TOF-MS/MS. Atherosclerosis 2012, 224, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Lim, Y.M.; Quan, W.; Kim, J.R.; Chung, K.W.; Kang, M.; Kim, S.; Park, S.Y.; Han, J.S.; Park, S.Y.; et al. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J. Lipid Res. 2011, 52, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Jang, H.H.; Lee, S.R.; Lee, K.H.; Woo, J.S.; Kim, J.B.; Kim, W.-S.; Min, B.I.; Cho, K.H.; Kim, K.S.; et al. Impact of lysophosphatidylcholine on survival and function of UEA-1+acLDL+ endothelial progenitor cells in patients with coronary artery disease. Heart Vessel. 2015, 30, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Iwase, M.; Sonoki, K.; Sasaki, N.; Ohdo, S.; Higuchi, S.; Hattori, H.; Iida, M. Lysophosphatidylcholine contents in plasma LDL in patients with type 2 diabetes mellitus: Relation with lipoprotein-associated phospholipase A2 and effects of simvastatin treatment. Atherosclerosis 2008, 196, 931–936. [Google Scholar] [CrossRef]
- Sonoki, K.; Iwase, M.; Sasaki, N.; Ohdo, S.; Higuchi, S.; Matsuyama, N.; Iida, M. Relations of lysophosphatidylcholine in low-density lipoprotein with serum lipoprotein-associated phospholipase A2, paraoxonase and homocysteine thiolactonase activities in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2009, 86, 117–123. [Google Scholar] [CrossRef]
- Petersen, K.S.; Keogh, J.B.; Lister, N.; Weir, J.M.; Meikle, P.J.; Clifton, P.M. Association between dairy intake, lipids and vascular structure and function in diabetes. World J. Diabetes 2017, 8, 202–212. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef] [PubMed]
- Kopprasch, S.; Dheban, S.; Schuhmann, K.; Xu, A.; Schulte, K.M.; Simeonovic, C.J.; Schwarz, P.E.; Bornstein, S.R.; Shevchenko, A.; Graessler, J. Detection of Independent Associations of Plasma Lipidomic Parameters with Insulin Sensitivity Indices Using Data Mining Methodology. PLoS ONE 2016, 11, e0164173. [Google Scholar] [CrossRef]
- Menegaut, L.; Masson, D.; Abello, N.; Denimal, D.; Truntzer, C.; Ducoroy, P.; Lagrost, L.; de Pais Barros, J.P.; Athias, A.; Petit, J.M.; et al. Specific enrichment of 2-arachidonoyl-lysophosphatidylcholine in carotid atheroma plaque from type 2 diabetic patients. Atherosclerosis 2016, 251, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guaqueta, A.M.; Posada-Duque, R.; Cortes, N.C.; Arias-Londono, J.D.; Cardona-Gomez, G.P. Changes in the hippocampal and peripheral phospholipid profiles are associated with neurodegeneration hallmarks in a long-term global cerebral ischemia model: Attenuation by Linalool. Neuropharmacology 2018, 135, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Yamamoto, S.; Hayasaka, T.; Konishi, Y.; Yamaguchi-Okada, M.; Goto-Inoue, N.; Sugiura, Y.; Setou, M.; Namba, H. Imaging mass spectrometry revealed the production of lyso-phosphatidylcholine in the injured ischemic rat brain. Neuroscience 2010, 168, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.; Wahlund, L.O.; Teerlink, T.; Blomberg, M.; Veerhuis, R.; van Kamp, G.J.; Scheltens, P.; Scheffer, P.G. Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer’s disease. J. Neural Transm. 2003, 110, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Dong, L.; Zhou, H.; Li, Q.; Huang, G.; Bai, S.J.; Liao, L. G-protein-coupled receptor Gpr17 regulates oligodendrocyte differentiation in response to lysolecithin-induced demyelination. Sci. Rep. 2018, 8, 4502. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.M.; Michikawa, M.; Kim, S.U.; Nagai, A. Lysophosphatidylcholine increases the neurotoxicity of Alzheimer’s amyloid beta1-42 peptide: Role of oligomer formation. Neuroscience 2015, 292, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Brinet, D.; Michno, W.; Baskurt, M.; Zetterberg, H.; Blenow, K.; Hanrieder, J. Novel Trimodal MALDI Imaging Mass Spectrometry (IMS3) at 10 mum Reveals Spatial Lipid and Peptide Correlates Implicated in Abeta Plaque Pathology in Alzheimer’s Disease. ACS Chem. Neurosci. 2017, 8, 2778–2790. [Google Scholar] [CrossRef] [PubMed]
- Tzekov, R.; Dawson, C.; Orlando, M.; Mouzon, B.; Reed, J.; Evans, J.; Crynen, G.; Mullan, M.; Crawford, F. Sub-Chronic Neuropathological and Biochemical Changes in Mouse Visual System after Repetitive Mild Traumatic Brain Injury. PLoS ONE 2016, 11, e0153608. [Google Scholar] [CrossRef] [PubMed]
- Agren, J.J.; Kurvinen, J.P.; Kuksis, A. Isolation of very low density lipoprotein phospholipids enriched in ethanolamine phospholipids from rats injected with Triton WR 1339. Biochim. Biophys. Acta 2005, 1734, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Skipski, V.P.; Barclay, M.; Barclay, R.K.; Fetzer, V.A.; Good, J.J.; Archibald, F.M. Lipid composition of human serum lipoproteins. Biochem. J. 1967, 104, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.M.; Vance, D.E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988, 263, 2998–3004. [Google Scholar] [PubMed]
- Rinella, M.E.; Elias, M.S.; Smolak, R.R.; Fu, T.; Borensztajn, J.; Green, R.M. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J. Lipid Res. 2008, 49, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Fast, D.G.; Vance, D.E. Nascent VLDL phospholipid composition is altered when phosphatidylcholine biosynthesis is inhibited: Evidence for a novel mechanism that regulates VLDL secretion. Biochim. Biophys. Acta 1995, 1258, 159–168. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Devlin, C.; Tabas, I.; Vance, D.E. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase alpha in mice decreases plasma high density and very low density lipoproteins. J. Biol. Chem. 2004, 279, 47402–47410. [Google Scholar] [CrossRef]
- Noga, A.A.; Vance, D.E. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J. Biol. Chem. 2003, 278, 21851–21859. [Google Scholar] [CrossRef]
- Hakkinen, T.; Luoma, J.S.; Hiltunen, M.O.; Macphee, C.H.; Milliner, K.J.; Patel, L.; Rice, S.Q.; Tew, D.G.; Karkola, K.; Yla-Herttuala, S. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2909–2917. [Google Scholar] [CrossRef]
- Han, X.; Wang, T.; Zhang, J.; Liu, X.; Li, Z.; Wang, G.; Song, Q.; Pang, D.; Ouyang, H.; Tang, X. Apolipoprotein CIII regulates lipoprotein-associated phospholipase A2 expression via the MAPK and NFkappaB pathways. Biol. Open 2015, 4, 661–665. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, P.; Zhang, L.; Osman, H.; Mohler, E.R., 3rd; Macphee, C.; Zalewski, A.; Postle, A.; Wilensky, R.L. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis 2007, 191, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, Z.; Liu, H.; Xia, Y.F.; Liu, X.M.; Luo, B.B.; Wang, W.K.; Li, B.; Gao, F.; Zhang, C.; et al. Serum amyloid A stimulates lipoprotein-associated phospholipase A2 expression in vitro and in vivo. Atherosclerosis 2013, 228, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ji, Y.; Li, Z.; Han, X.; Guo, N.; Song, Q.; Quan, L.; Wang, T.; Han, W.; Pang, D.; et al. Nitro-oleic acid downregulates lipoprotein-associated phospholipase A2 expression via the p42/p44 MAPK and NFkappaB pathways. Sci. Rep. 2014, 4, 4905. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, W.; Ran, X.; Yu, J.; Li, H.; Zhao, L.; Zeng, H.; Cao, Y.; Zeng, Z.; Wan, Z. Lipoprotein-associated phospholipase A2 and risks of coronary heart disease and ischemic stroke in the general population: A systematic review and meta-analysis. Clin. Chim. Acta 2017, 471, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Esenwa, C.C.; Elkind, M.S. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat. Rev. Neurol. 2016, 12, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Talmud, P.J.; Holmes, M.V. Deciphering the causal role of sPLA2s and Lp-PLA2 in coronaryheart disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Chen, W. Chemiluminescence immunoassay for sensing lipoprotein-associated phospholipase A2 in cardiovascular risk evaluation. Clin. Chim. Acta 2019, 488, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Niu, D.; Zheng, D.; Zhang, Q.; Li, W. Predictive value of combining the level of lipoprotein-associated phospholipase A2 and antithrombin III for acute coronary syndrome risk. Biomed. Rep. 2018, 9, 517–522. [Google Scholar] [CrossRef]
- Lyngbakken, M.N.; Myhre, P.L.; Rosjo, H.; Omland, T. Novel biomarkers of cardiovascular disease: Applications in clinical practice. Crit. Rev. Clin. Lab. Sci. 2018, 56, 33–60. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.; Shi, H.; Huang, Q.; Zhou, J. Relation between lipoprotein-associated phospholipase A2 mass and incident ischemic stroke severity. Neurol. Sci. 2018, 39, 1591–1596. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S.; Ren, L.; Lei, Z.; Lan, T.; Cai, J.; Li, C. Lp-PLA2 as a risk factor of early neurological deterioration in acute ischemic stroke with TOAST type of large arterial atherosclerosis. Neurol Res. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.Y.; Cai, H.P.; Ge, H.L.; Yu, L.H.; Lin, Y.X.; Kang, D.Z. Assessment of lipoprotein-associated phospholipase A2 level and its changes in the early stages as predictors of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, R.L.; Shi, Y.; Mohler, E.R., 3rd; Hamamdzic, D.; Burgert, M.E.; Li, J.; Postle, A.; Fenning, R.S.; Bollinger, J.G.; Hoffman, B.E.; et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat. Med. 2008, 14, 1059–1066. [Google Scholar] [CrossRef]
- Serruys, P.W.; Garcia-Garcia, H.M.; Buszman, P.; Erne, P.; Verheye, S.; Aschermann, M.; Duckers, H.; Bleie, O.; Dudek, D.; Botker, H.E.; et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008, 118, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H. Platelet-activating factor acetylhydrolase: Is it good or bad for you? Curr. Opin. Lipidol. 2004, 15, 337–341. [Google Scholar] [CrossRef]
- Marathe, G.K.; Pandit, C.; Lakshmikanth, C.L.; Chaithra, V.H.; Jacob, S.P.; D’Souza, C.J. To hydrolyze or not to hydrolyze: The dilemma of platelet-activating factor acetylhydrolase. J. Lipid Res. 2014, 55, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Benitez, S.; Camacho, M.; Arcelus, R.; Vila, L.; Bancells, C.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Increased lysophosphatidylcholine and non-esterified fatty acid content in LDL induces chemokine release in endothelial cells. Relationship with electronegative LDL. Atherosclerosis 2004, 177, 299–305. [Google Scholar] [PubMed]
- Zhang, H.; Zhou, W.; Cao, C.; Zhang, W.; Liu, G.; Zhang, J. Amelioration of atherosclerosis in apolipoprotein E-deficient mice by combined RNA interference of lipoprotein-associated phospholipase A2 and YKL-40. PLoS ONE 2018, 13, e0202797. [Google Scholar] [CrossRef] [PubMed]
- Jonas, A. Regulation of lecithin cholesterol acyltransferase activity. Prog. Lipid Res. 1998, 37, 209–234. [Google Scholar] [CrossRef]
- Calabresi, L.; Simonelli, S.; Gomaraschi, M.; Franceschini, G. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis 2012, 222, 299–306. [Google Scholar] [CrossRef]
- Hirsch-Reinshagen, V.; Donkin, J.; Stukas, S.; Chan, J.; Wilkinson, A.; Fan, J.; Parks, J.S.; Kuivenhoven, J.A.; Lutjohann, D.; Pritchard, H.; et al. LCAT synthesized by primary astrocytes esterifies cholesterol on glia-derived lipoproteins. J. Lipid Res. 2009, 50, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, L.; Franceschini, G. Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc. Med. 2010, 20, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kunnen, S.; Van Eck, M. Lecithin:cholesterol acyltransferase: Old friend or foe in atherosclerosis? J. Lipid Res. 2012, 53, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Ossoli, A.; Simonelli, S.; Vitali, C.; Franceschini, G.; Calabresi, L. Role of LCAT in Atherosclerosis. J. Atheroscler. Thromb. 2016, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, F.; Baldassarre, D.; Castelnuovo, S.; Ossoli, A.; Amato, M.; van Capelleveen, J.; Hovingh, G.K.; De Groot, E.; Bochem, A.; Simonelli, S.; et al. Complete and partial lecithin: Cholesterol acyltransferase deficiency is differentially associated with atherosclerosis. Circulation 2018, 138, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Alarcon, G.; Perez-Mendez, O.; Herrera-Maya, G.; Garcia-Sanchez, C.; Martinez-Rios, M.A.; Pena-Duque, M.A.; Posadas-Sanchez, R.; Posadas-Romero, C.; Escobedo, G.; Fragoso, J.M. CETP and LCAT Gene Polymorphisms Are Associated with High-Density Lipoprotein Subclasses and Acute Coronary Syndrome. Lipids 2018, 53, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Schaefer, E.J.; Horvath, K.V.; Yamashita, S.; Miller, M.; Franceschini, G.; Calabresi, L. Role of LCAT in HDL remodeling: Investigation of LCAT deficiency states. J. Lipid Res. 2007, 48, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, L.; Baldassarre, D.; Simonelli, S.; Gomaraschi, M.; Amato, M.; Castelnuovo, S.; Frigerio, B.; Ravani, A.; Sansaro, D.; Kauhanen, J.; et al. Plasma lecithin:cholesterol acyltransferase and carotid intima-media thickness in European individuals at high cardiovascular risk. J. Lipid Res. 2011, 52, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.; Tietge, U.J.; Kwakernaak, A.J.; Dikkeschei, B.D.; Perton, F.; Tio, R.A. Alterations in plasma lecithin:cholesterol acyltransferase and myeloperoxidase in acute myocardial infarction: Implications for cardiac outcome. Atherosclerosis 2014, 234, 185–192. [Google Scholar] [CrossRef]
- Dullaart, R.P.; Perton, F.; Sluiter, W.J.; de Vries, R.; van Tol, A. Plasma lecithin: Cholesterol acyltransferase activity is elevated in metabolic syndrome and is an independent marker of increased carotid artery intima media thickness. J. Clin. Endocrinol. Metab. 2008, 93, 4860–4866. [Google Scholar] [CrossRef]
- Dong, Z.; Shi, H.; Zhao, M.; Zhang, X.; Huang, W.; Wang, Y.; Zheng, L.; Xian, X.; Liu, G. Loss of LCAT activity in the golden Syrian hamster elicits pro-atherogenic dyslipidemia and enhanced atherosclerosis. Metabolism 2018, 83, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, S.; Ossoli, A.; Banfi, C.; Pavanello, C.; Calabresi, L.; Gianazza, E. A proteomic approach to identify novel disease biomarkers in LCAT deficiency. J. Proteom. 2018. [Google Scholar] [CrossRef] [PubMed]
- Furbee, J.W. Jr.; Parks, J.S. Transgenic overexpression of human lecithin: Cholesterol acyltransferase (LCAT) in mice does not increase aortic cholesterol deposition. Atherosclerosis 2002, 165, 89–100. [Google Scholar] [CrossRef]
- Vaisman, B.L.; Klein, H.G.; Rouis, M.; Berard, A.M.; Kindt, M.R.; Talley, G.D.; Meyn, S.M.; Hoyt, R.F. Jr.; Marcovina, S.M.; Albers, J.J.; et al. Overexpression of human lecithin cholesterol acyltransferase leads to hyperalphalipoproteinemia in transgenic mice. J. Biol. Chem. 1995, 270, 12269–12275. [Google Scholar] [CrossRef]
- Nass, K.J.; van den Berg, E.H.; Gruppen, E.G.; Dullaart, R.P.F. Plasma lecithin:cholesterol acyltransferase and phospholipid transfer protein activity independently associate with nonalcoholic fatty liver disease. Eur J. Clin. Investig. 2018, 48, e12988. [Google Scholar] [CrossRef]
- Yokoyama, K.; Tani, S.; Matsuo, R.; Matsumoto, N. Association of lecithin-cholesterol acyltransferase activity and low-density lipoprotein heterogeneity with atherosclerotic cardiovascular disease risk: A longitudinal pilot study. BMC Cardiovasc. Disord. 2018, 18, 224. [Google Scholar] [CrossRef]
- Gebhard, C.; Rhainds, D.; He, G.; Rodes-Cabau, J.; Lavi, S.; Spence, J.D.; Title, L.; Kouz, S.; L’Allier, P.L.; Gregoire, J.; et al. Elevated level of lecithin:cholesterol acyltransferase (LCAT) is associated with reduced coronary atheroma burden. Atherosclerosis 2018, 276, 131–139. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, D.; Castro-Perez, J.M.; Ni, W.; Zhang, A.; Krsmanovic, M.L.; Xie, D.; Shah, V.; Stout, S.J.; McLaren, D.G.; et al. AAV8-mediated long-term expression of human LCAT significantly improves lipid profiles in hCETP;Ldlr(+/−) mice. J. Cardiovasc. Transl. Res. 2011, 4, 801–810. [Google Scholar] [CrossRef]
- Silva-Filho, A.F.; Sena, W.L.B.; Lima, L.R.A.; Carvalho, L.V.N.; Pereira, M.C.; Santos, L.G.S.; Santos, R.V.C.; Tavares, L.B.; Pitta, M.G.R.; Rego, M. Glycobiology Modifications in Intratumoral Hypoxia: The Breathless Side of Glycans Interaction. Cell. Physiol. Biochem. 2017, 41, 1801–1829. [Google Scholar] [CrossRef]
- Lu, H.; Forbes, R.A.; Verma, A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 2002, 277, 23111–23115. [Google Scholar] [CrossRef]
- Li, J.; Thorne, L.N.; Punjabi, N.M.; Sun, C.K.; Schwartz, A.R.; Smith, P.L.; Marino, R.L.; Rodriguez, A.; Hubbard, W.C.; O’Donnell, C.P.; et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005, 97, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Trzepizur, W.; Le Vaillant, M.; Meslier, N.; Pigeanne, T.; Masson, P.; Humeau, M.P.; Bizieux-Thaminy, A.; Goupil, F.; Chollet, S.; Ducluzeau, P.H.; et al. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest 2013, 143, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shang, J.; Deng, Y.; Yuan, X.; Zhu, D.; Liu, H. Alterations in left ventricular function during intermittent hypoxia: Possible involvement of O-GlcNAc protein and MAPK signaling. Int. J. Mol. Med. 2015, 36, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Vidalino, L.; Anesi, A.; Macchi, P.; Guella, G. A lipidomics investigation of the induced hypoxia stress on HeLa cells by using MS and NMR techniques. Mol. Biosyst. 2014, 10, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; de Stanchina, E.; Mathew, R.; White, E.P.; Thompson, C.B.; Rabinowitz, J.D. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef] [PubMed]
- Granger, M.W.; Liu, H.; Fowler, C.F.; Blanchard, A.P.; Taylor, M.W.; Sherman, S.P.M.; Xu, H.; Le, W.; Bennett, S.A.L. Distinct disruptions in Land’s cycle remodeling of glycerophosphocholines in murine cortex mark symptomatic onset and progression in two Alzheimer’s disease mouse models. J. Neurochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ojala, P.J.; Hermansson, M.; Tolvanen, M.; Polvinen, K.; Hirvonen, T.; Impola, U.; Jauhiainen, M.; Somerharju, P.; Parkkinen, J. Identification of alpha-1 acid glycoprotein as a lysophospholipid binding protein: A complementary role to albumin in the scavenging of lysophosphatidylcholine. Biochemistry 2006, 45, 14021–14031. [Google Scholar] [CrossRef] [PubMed]
- Spanner, S.; Ansell, G.B. Phospholipid transport and turnover in nervous tissue. Biochem. Soc. Trans. 1979, 7, 338–341. [Google Scholar] [CrossRef]
- Takeshita, S.; Inoue, N.; Gao, D.; Rikitake, Y.; Kawashima, S.; Tawa, R.; Sakurai, H.; Yokoyama, M. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J. Atheroscler. Thromb. 2000, 7, 238–246. [Google Scholar] [CrossRef]
- Uchida, Y.; Uchida, Y.; Kameda, N. Visualization of lipid components in human coronary plaques using color fluorescence angioscopy. Circ. J. 2010, 74, 2181–2186. [Google Scholar] [CrossRef]
- Switzer, S.; Eder, H.A. Transport of lysolecithin by albumin in human and rat plasma. J. Lipid Res. 1965, 6, 506–511. [Google Scholar] [PubMed]
- Kugiyama, K.; Sakamoto, T.; Misumi, I.; Sugiyama, S.; Ohgushi, M.; Ogawa, H.; Horiguchi, M.; Yasue, H. Transferable lipids in oxidized low-density lipoprotein stimulate plasminogen activator inhibitor-1 and inhibit tissue-type plasminogen activator release from endothelial cells. Circ. Res. 1993, 73, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.D.; Stroes, E.S.; Willekes-Koolschijn, N.; Rabelink, T.J.; Koomans, H.A.; Joles, J.A. Hypoalbuminemia increases lysophosphatidylcholine in low-density lipoprotein of normocholesterolemic subjects. Kidney Int. 1999, 55, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.S.; Joles, J.A.; Chang, P.C.; Koomans, H.A.; Rabelink, T.J. Impaired endothelial function in patients with nephrotic range proteinuria. Kidney Int. 1995, 48, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.D.; de Kimpe, S.; de Roos, R.; Rabelink, T.J.; Koomans, H.A.; Joles, J.A. Albumin restores lysophosphatidylcholine-induced inhibition of vasodilation in rat aorta. Kidney Int. 2001, 60, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.D.; Braam, B.; Willekes-Koolschijn, N.; Boer, P.; Koomans, H.A.; Joles, J.A. Hypoalbuminaemia enhances the renal vasoconstrictor effect of lysophosphatidylcholine. Nephrol. Dial. Transpl. 2003, 18, 1485–1492. [Google Scholar] [CrossRef]
- Van Molle, W.; Libert, C.; Fiers, W.; Brouckaert, P. Alpha 1-acid glycoprotein and alpha 1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J. Immunol. 1997, 159, 3555–3564. [Google Scholar] [PubMed]
- Lopez-Marques, R.L.; Theorin, L.; Palmgren, M.G.; Pomorski, T.G. P4-ATPases: Lipid flippases in cell membranes. Pflug. Arch. 2014, 466, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.P.; Vestergaard, A.L.; Mikkelsen, S.A.; Mogensen, L.S.; Chalat, M.; Molday, R.S. P4-ATPases as Phospholipid Flippases-Structure, Function, and Enigmas. Front. Physiol. 2016, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Linsel-Nitschke, P.; Jehle, A.W.; Shan, J.; Cao, G.; Bacic, D.; Lan, D.; Wang, N.; Tall, A.R. Potential role of ABCA7 in cellular lipid efflux to apoA-I. J. Lipid Res. 2005, 46, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Abe-Dohmae, S.; Ikeda, Y.; Matsuo, M.; Hayashi, M.; Okuhira, K.; Ueda, K.; Yokoyama, S. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 2004, 279, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, M.; Toda, Y.; Manucat, N.B.; Akatsu, H.; Fukumoto, M.; Kono, N.; Arai, H.; Kioka, N.; Ueda, K. Lysophosphatidylcholine export by human ABCA7. Biochim. Biophys. Acta 2017, 1862, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Holm, M.L.; Kanekiyo, T. ABCA7 and Pathogenic Pathways of Alzheimer’s Disease. Brain Sci. 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.X.; Zhu, H.Y.; Hu, Y.H. Effects of lysophosphatidylcholine on beta-amyloid-induced neuronal apoptosis. Acta Pharmacol. Sin. 2009, 30, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Serna, J.; Garcia-Seisdedos, D.; Alcazar, A.; Lasuncion, M.A.; Busto, R.; Pastor, O. Quantitative lipidomic analysis of plasma and plasma lipoproteins using MALDI-TOF mass spectrometry. Chem. Phys. Lipids 2015, 189, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Holcapek, M.; Cifkova, E.; Cervena, B.; Lisa, M.; Vostalova, J.; Galuszka, J. Determination of nonpolar and polar lipid classes in human plasma, erythrocytes and plasma lipoprotein fractions using ultrahigh-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2015, 1377, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Heermeier, K.; Schneider, R.; Heinloth, A.; Wanner, C.; Dimmeler, S.; Galle, J. Oxidative stress mediates apoptosis induced by oxidized low-density lipoprotein and oxidized lipoprotein(a). Kidney Int. 1999, 56, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Cocon, L.; Diaz, O.; Andre, P.; Lotteau, V. Modified lipoproteins provide lipids that modulate dendritic cell immune function. Biochimie 2013, 95, 103–108. [Google Scholar] [CrossRef]
- Coutant, F.; Perrin-Cocon, L.; Agaugue, S.; Delair, T.; Andre, P.; Lotteau, V. Mature dendritic cell generation promoted by lysophosphatidylcholine. J. Immunol. 2002, 169, 1688–1695. [Google Scholar] [CrossRef]
- Liang, G.H.; Park, S.; Kim, M.Y.; Kim, J.A.; Choi, S.; Suh, S.H. Modulation of nonselective cation current by oxidized LDL and lysophosphatidylcholine and its inhibitory contribution to endothelial damage. Life Sci. 2010, 86, 733–739. [Google Scholar] [CrossRef]
- Bao, L.; Qi, J.; Wang, Y.W.; Xi, Q.; Tserennadmid, T.; Zhao, P.F.; Qi, J.; Damirin, A. The atherogenic actions of LPC on vascular smooth muscle cells and its LPA receptor mediated mechanism. Biochem. Biophys. Res. Commun. 2018, 503, 1911–1918. [Google Scholar] [CrossRef]
- Tselmin, S.; Schmitz, G.; Julius, U.; Bornstein, S.R.; Barthel, A.; Graessler, J. Acute effects of lipid apheresis on human serum lipidome. Atheroscler. Suppl. 2009, 10, 27–33. [Google Scholar] [CrossRef]
- Soupene, E.; Fyrst, H.; Kuypers, F.A. Mammalian acyl-CoA:lysophosphatidylcholine acyltransferase enzymes. Proc. Natl. Acad. Sci. USA 2008, 105, 88–93. [Google Scholar] [CrossRef]
- Singh, A.B.; Liu, J. Identification of Hepatic Lysophosphatidylcholine Acyltransferase 3 as a Novel Target Gene Regulated by Peroxisome Proliferator-activated Receptor delta. J. Biol. Chem. 2017, 292, 884–897. [Google Scholar] [CrossRef]
- Shindou, H.; Shimizu, T. Acyl-CoA:lysophospholipid acyltransferases. J. Biol. Chem. 2009, 284, 1–5. [Google Scholar] [CrossRef]
- Morita, Y.; Sakaguchi, T.; Ikegami, K.; Goto-Inoue, N.; Hayasaka, T.; Hang, V.T.; Tanaka, H.; Harada, T.; Shibasaki, Y.; Suzuki, A.; et al. Lysophosphatidylcholine acyltransferase 1 altered phospholipid composition and regulated hepatoma progression. J. Hepatol. 2013, 59, 292–299. [Google Scholar] [CrossRef]
- Shida-Sakazume, T.; Endo-Sakamoto, Y.; Unozawa, M.; Fukumoto, C.; Shimada, K.; Kasamatsu, A.; Ogawara, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. Lysophosphatidylcholine acyltransferase1 overexpression promotes oral squamous cell carcinoma progression via enhanced biosynthesis of platelet-activating factor. PLoS ONE 2015, 10, e0120143. [Google Scholar] [CrossRef]
- Abdelzaher, E.; Mostafa, M.F. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) upregulation in breast carcinoma contributes to tumor progression and predicts early tumor recurrence. Tumour Biol. 2015, 36, 5473–5483. [Google Scholar] [CrossRef]
- Uehara, T.; Kikuchi, H.; Miyazaki, S.; Iino, I.; Setoguchi, T.; Hiramatsu, Y.; Ohta, M.; Kamiya, K.; Morita, Y.; Tanaka, H.; et al. Overexpression of lysophosphatidylcholine acyltransferase 1 and concomitant lipid alterations in gastric cancer. Ann. Surg. Oncol. 2016, 23 (Suppl. 2), S206–S213. [Google Scholar] [CrossRef]
- Mansilla, F.; da Costa, K.A.; Wang, S.; Kruhoffer, M.; Lewin, T.M.; Orntoft, T.F.; Coleman, R.A.; Birkenkamp-Demtroder, K. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) overexpression in human colorectal cancer. J. Mol. Med. (Berl.) 2009, 87, 85–97. [Google Scholar] [CrossRef]
- Cotte, A.K.; Aires, V.; Fredon, M.; Limagne, E.; Derangere, V.; Thibaudin, M.; Humblin, E.; Scagliarini, A.; de Barros, J.P.; Hillon, P.; et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun. 2018, 9, 322. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Garg, A. Enzymatic activity of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 11: Upregulated in breast and cervical cancers. J. Lipid Res. 2010, 51, 2143–2152. [Google Scholar] [CrossRef]
- Li, Z.; Ding, T.; Pan, X.; Li, Y.; Li, R.; Sanders, P.E.; Kuo, M.S.; Hussain, M.M.; Cao, G.; Jiang, X.C. Lysophosphatidylcholine acyltransferase 3 knockdown-mediated liver lysophosphatidylcholine accumulation promotes very low density lipoprotein production by enhancing microsomal triglyceride transfer protein expression. J. Biol. Chem. 2012, 287, 20122–20131. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Ding, T.; Lou, C.; Bui, H.H.; Kuo, M.S.; Jiang, X.C. Deficiency in lysophosphatidylcholine acyltransferase 3 reduces plasma levels of lipids by reducing lipid absorption in mice. Gastroenterology 2015, 149, 1519–1529. [Google Scholar] [CrossRef]
- Cao, J.; Shan, D.; Revett, T.; Li, D.; Wu, L.; Liu, W.; Tobin, J.F.; Gimeno, R.E. Molecular identification of a novel mammalian brain isoform of acyl-CoA:lysophospholipid acyltransferase with prominent ethanolamine lysophospholipid acylating activity, LPEAT2. J. Biol. Chem. 2008, 283, 19049–19057. [Google Scholar] [CrossRef]
- Kurabe, N.; Hayasaka, T.; Ogawa, M.; Masaki, N.; Ide, Y.; Waki, M.; Nakamura, T.; Kurachi, K.; Kahyo, T.; Shinmura, K.; et al. Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci. 2013, 104, 1295–1302. [Google Scholar] [CrossRef]
- Tanaka, N.; Matsubara, T.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012, 56, 118–129. [Google Scholar] [CrossRef]
- Matsubara, T.; Tanaka, N.; Sato, M.; Kang, D.W.; Krausz, K.W.; Flanders, K.C.; Ikeda, K.; Luecke, H.; Wakefield, L.M.; Gonzalez, F.J. TGF-beta-SMAD3 signaling mediates hepatic bile acid and phospholipid metabolism following lithocholic acid-induced liver injury. J. Lipid Res. 2012, 53, 2698–2707. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, L.; Larsson, A.; Venge, P. The identification of a phospholipase B precursor in human neutrophils. FEBS J. 2009, 276, 175–186. [Google Scholar] [CrossRef]
- Dor, P.J.; Ackerman, S.J.; Gleich, G.J. Charcot-Leyden crystal protein and eosinophil granule major basic protein in sputum of patients with respiratory diseases. Am. Rev. Respir. Dis. 1984, 130, 1072–1077. [Google Scholar] [CrossRef]
- Ackerman, S.J.; Weil, G.J.; Gleich, G.J. Formation of Charcot-Leyden crystals by human basophils. J. Exp. Med. 1982, 155, 1597–1609. [Google Scholar] [CrossRef]
- Weller, P.F.; Goetzl, E.J.; Austen, K.F. Identification of human eosinophil lysophospholipase as the constituent of Charcot-Leyden crystals. Proc. Natl. Acad. Sci. USA 1980, 77, 7440–7443. [Google Scholar] [CrossRef]
- Ackerman, S.J.; Liu, L.; Kwatia, M.A.; Savage, M.P.; Leonidas, D.D.; Swaminathan, G.J.; Acharya, K.R. Charcot-Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J. Biol. Chem. 2002, 277, 14859–14868. [Google Scholar] [CrossRef]
- Holtsberg, F.W.; Ozgur, L.E.; Garsetti, D.E.; Myers, J.; Egan, R.W.; Clark, M.A. Presence in human eosinophils of a lysophospholipase similar to that found in the pancreas. Biochem. J. 1995, 309 (Pt 1), 141–144. [Google Scholar] [CrossRef]
- Su, J. A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research. Molecules 2018, 23, 2931. [Google Scholar] [CrossRef]
- Adamski, M.G.; Li, Y.; Wagner, E.; Yu, H.; Seales-Bailey, C.; Soper, S.A.; Murphy, M.; Baird, A.E. Expression profile based gene clusters for ischemic stroke detection. Genomics 2014, 104, 163–169. [Google Scholar] [CrossRef]
- Moore, D.F.; Li, H.; Jeffries, N.; Wright, V.; Cooper, R.A., Jr.; Elkahloun, A.; Gelderman, M.P.; Zudaire, E.; Blevins, G.; Yu, H.; et al. Using peripheral blood mononuclear cells to determine a gene expression profile of acute ischemic stroke: A pilot investigation. Circulation 2005, 111, 212–221. [Google Scholar] [CrossRef]
- Wang, A.; Yang, H.C.; Friedman, P.; Johnson, C.A.; Dennis, E.A. A specific human lysophospholipase: cDNA cloning, tissue distribution and kinetic characterization. Biochim. Biophys. Acta 1999, 1437, 157–169. [Google Scholar] [CrossRef]
- Hirano, T.; Kishi, M.; Sugimoto, H.; Taguchi, R.; Obinata, H.; Ohshima, N.; Tatei, K.; Izumi, T. Thioesterase activity and subcellular localization of acylprotein thioesterase 1/lysophospholipase 1. Biochim. Biophys. Acta 2009, 1791, 797–805. [Google Scholar] [CrossRef]
- Kong, E.; Peng, S.; Chandra, G.; Sarkar, C.; Zhang, Z.; Bagh, M.B.; Mukherjee, A.B. Dynamic palmitoylation links cytosol-membrane shuttling of acyl-protein thioesterase-1 and acyl-protein thioesterase-2 with that of proto-oncogene H-ras product and growth-associated protein-43. J. Biol. Chem. 2013, 288, 9112–9125. [Google Scholar] [CrossRef]
- Dekker, F.J.; Rocks, O.; Vartak, N.; Menninger, S.; Hedberg, C.; Balamurugan, R.; Wetzel, S.; Renner, S.; Gerauer, M.; Scholermann, B.; et al. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat. Chem. Biol. 2010, 6, 449–456. [Google Scholar] [CrossRef]
- Tian, L.; McClafferty, H.; Knaus, H.G.; Ruth, P.; Shipston, M.J. Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels. J. Biol. Chem. 2012, 287, 14718–14725. [Google Scholar] [CrossRef]
- Abrami, L.; Dallavilla, T.; Sandoz, P.A.; Demir, M.; Kunz, B.; Savoglidis, G.; Hatzimanikatis, V.; van der Goot, F.G. Identification and dynamics of the human ZDHHC16-ZDHHC6 palmitoylation cascade. Elife 2017, 6, e27826. [Google Scholar] [CrossRef]
- Benesch, M.G.; Ko, Y.M.; McMullen, T.P.; Brindley, D.N. Autotaxin in the crosshairs: Taking aim at cancer and other inflammatory conditions. FEBS Lett. 2014, 588, 2712–2727. [Google Scholar] [CrossRef]
- Nakamura, K.; Takeuchi, T.; Ohkawa, R.; Okubo, S.; Yokota, H.; Tozuka, M.; Aoki, J.; Arai, H.; Ikeda, H.; Ohshima, N.; et al. Serum lysophospholipase D/autotaxin may be a new nutritional assessment marker: Study on prostate cancer patients. Ann. Clin. Biochem. 2007, 44 (Pt 6), 549–556. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Strater, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-function and signaling. J. Lipid Res. 2014, 55, 1010–1018. [Google Scholar] [CrossRef]
- Stracke, M.L.; Krutzsch, H.C.; Unsworth, E.J.; Arestad, A.; Cioce, V.; Schiffmann, E.; Liotta, L.A. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 1992, 267, 2524–2529. [Google Scholar]
- Tanaka, M.; Okudaira, S.; Kishi, Y.; Ohkawa, R.; Iseki, S.; Ota, M.; Noji, S.; Yatomi, Y.; Aoki, J.; Arai, H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 2006, 281, 25822–25830. [Google Scholar] [CrossRef]
- van Meeteren, L.A.; Ruurs, P.; Stortelers, C.; Bouwman, P.; van Rooijen, M.A.; Pradere, J.P.; Pettit, T.R.; Wakelam, M.J.; Saulnier-Blache, J.S.; Mummery, C.L.; et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006, 26, 5015–5022. [Google Scholar] [CrossRef]
- Moolenaar, W.H.; Houben, A.J.; Lee, S.J.; van Meeteren, L.A. Autotaxin in embryonic development. Biochim. Biophys. Acta 2013, 1831, 13–19. [Google Scholar] [CrossRef]
- Brindley, D.N. Lipid phosphate phosphatases and related proteins: Signaling functions in development, cell division, and cancer. J. Cell. Biochem. 2004, 92, 900–912. [Google Scholar] [CrossRef]
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 2009, 15, 539–550. [Google Scholar] [CrossRef]
- David, M.; Wannecq, E.; Descotes, F.; Jansen, S.; Deux, B.; Ribeiro, J.; Serre, C.M.; Gres, S.; Bendriss-Vermare, N.; Bollen, M.; et al. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS ONE 2010, 5, e9741. [Google Scholar] [CrossRef]
- Kehlen, A.; Englert, N.; Seifert, A.; Klonisch, T.; Dralle, H.; Langner, J.; Hoang-Vu, C. Expression, regulation and function of autotaxin in thyroid carcinomas. Int. J. Cancer 2004, 109, 833–838. [Google Scholar] [CrossRef]
- Brindley, D.N.; Lin, F.T.; Tigyi, G.J. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta 2013, 1831, 74–85. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ikeda, H.; Nakamura, K.; Ohkawa, R.; Masuzaki, R.; Tateishi, R.; Yoshida, H.; Watanabe, N.; Tejima, K.; Kume, Y.; et al. Autotaxin as a novel serum marker of liver fibrosis. Clin. Chim. Acta 2011, 412, 1201–1206. [Google Scholar] [CrossRef]
- Kaffe, E.; Katsifa, A.; Xylourgidis, N.; Ninou, I.; Zannikou, M.; Harokopos, V.; Foka, P.; Dimitriadis, A.; Evangelou, K.; Moulas, A.N.; et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology 2017, 65, 1369–1383. [Google Scholar] [CrossRef]
- Nakamura, K.; Kishimoto, T.; Ohkawa, R.; Okubo, S.; Tozuka, M.; Yokota, H.; Ikeda, H.; Ohshima, N.; Mizuno, K.; Yatomi, Y. Suppression of lysophosphatidic acid and lysophosphatidylcholine formation in the plasma in vitro: Proposal of a plasma sample preparation method for laboratory testing of these lipids. Anal. Biochem. 2007, 367, 20–27. [Google Scholar] [CrossRef]
- Hosogaya, S.; Yatomi, Y.; Nakamura, K.; Ohkawa, R.; Okubo, S.; Yokota, H.; Ohta, M.; Yamazaki, H.; Koike, T.; Ozaki, Y. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: Strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 2008, 45 (Pt 4), 364–368. [Google Scholar] [CrossRef]
- Michalczyk, A.; Budkowska, M.; Dolegowska, B.; Chlubek, D.; Safranow, K. Lysophosphatidic acid plasma concentrations in healthy subjects: Circadian rhythm and associations with demographic, anthropometric and biochemical parameters. Lipids Health Dis. 2017, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, O.; Simon, M.F.; Viode, C.; Rugani, N.; Leballe, F.; Ragab, A.; Fournie, B.; Sarda, L.; Chap, H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 1995, 80, 919–927. [Google Scholar] [CrossRef]
- Aoki, J.; Taira, A.; Takanezawa, Y.; Kishi, Y.; Hama, K.; Kishimoto, T.; Mizuno, K.; Saku, K.; Taguchi, R.; Arai, H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 2002, 277, 48737–48744. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, R.; Houssin, A.; Peyruchaud, O. Platelets, autotaxin and lysophosphatidic acid signalling: Win-win factors for cancer metastasis. Br. J. Pharmacol. 2018, 175, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Knowlden, S.; Georas, S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 2014, 192, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Araiza, I.; Morales-Lazaro, S.L.; Canul-Sanchez, J.A.; Islas, L.D.; Rosenbaum, T. The role of lysophosphatidic acid on ion channel function and disease. J. Neurophysiol. 2018, 120, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Newton, R.; Klein, R.; Morita, Y.; Gunn, M.D.; Rosen, S.D. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 2008, 9, 415–423. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.C.; Krummel, M.F.; Rosen, S.D. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J. Immunol. 2012, 189, 3914–3924. [Google Scholar] [CrossRef]
- D’Souza, K.; Paramel, G.V.; Kienesberger, P.C. Lysophosphatidic Acid Signaling in Obesity and Insulin Resistance. Nutrients 2018, 10, 399. [Google Scholar] [CrossRef]
- Navab, M.; Chattopadhyay, A.; Hough, G.; Meriwether, D.; Fogelman, S.I.; Wagner, A.C.; Grijalva, V.; Su, F.; Anantharamaiah, G.M.; Hwang, L.H.; et al. Source and role of intestinally derived lysophosphatidic acid in dyslipidemia and atherosclerosis. J. Lipid Res. 2015, 56, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, X.Y.; Wang, N.D.; Ding, C.; Yang, Y.J.; You, Z.J.; Su, Q.; Chen, J.H. Serum lysophosphatidic acid concentrations measured by dot immunogold filtration assay in patients with acute myocardial infarction. Scand. J. Clin. Lab. Investig. 2003, 63, 497–503. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Deng, X.; Liu, Y.; Yang, X.; Wu, Q.; Yu, C. Lysophosphatidic acid directly induces macrophage-derived foam cell formation by blocking the expression of SRBI. Biochem. Biophys. Res. Commun. 2017, 491, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.B.; Yang, B.; Li, X.; Liu, H.; Lei, G. Lysophosphatidic Acid Promotes Expression and Activation of Matrix Metalloproteinase 9 (MMP9) in THP-1 Cells via Toll-Like Receptor 4/Nuclear Factor-kappaB (TLR4/NF-kappaB) Signaling Pathway. Med. Sci. Monit. 2018, 24, 4861–4868. [Google Scholar] [CrossRef] [PubMed]
- Kritikou, E.; van Puijvelde, G.H.; van der Heijden, T.; van Santbrink, P.J.; Swart, M.; Schaftenaar, F.H.; Kroner, M.J.; Kuiper, J.; Bot, I. Inhibition of lysophosphatidic acid receptors 1 and 3 attenuates atherosclerosis development in LDL-receptor deficient mice. Sci. Rep. 2016, 6, 37585. [Google Scholar] [CrossRef] [PubMed]
- Aldi, S.; Matic, L.P.; Hamm, G.; van Keulen, D.; Tempel, D.; Holmstrom, K.; Szwajda, A.; Nielsen, B.S.; Emilsson, V.; Ait-Belkacem, R.; et al. Integrated human evaluation of the lysophosphatidic acid pathway as a novel therapeutic target in atherosclerosis. Mol. Ther. Methods Clin. Dev. 2018, 10, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Escalante-Alcalde, D.; Bhuiyan, M.S.; Orr, A.W.; Kevil, C.; Morris, A.J.; Nam, H.; Dominic, P.; McCarthy, K.J.; Miriyala, S.; et al. Cardiac-specific inactivation of LPP3 in mice leads to myocardial dysfunction and heart failure. Redox. Biol. 2018, 14, 261–271. [Google Scholar] [CrossRef]
- Hisano, Y.; Hla, T. Bioactive lysolipids in cancer and angiogenesis. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Suh, D.S.; Lee, S.C.; Tigyi, G.J.; Kim, J.H. Role of autotaxin in cancer stem cells. Cancer Metastasis Rev. 2018, 37, 509–518. [Google Scholar] [CrossRef]
- Schmid, R.; Wolf, K.; Robering, J.W.; Strauss, S.; Strissel, P.L.; Strick, R.; Rubner, M.; Fasching, P.A.; Horch, R.E.; Kremer, A.E.; et al. ADSCs and adipocytes are the main producers in the autotaxin-lysophosphatidic acid axis of breast cancer and healthy mammary tissue in vitro. BMC Cancer 2018, 18, 1273. [Google Scholar] [CrossRef]
- Mazzocca, A.; Schonauer, L.M.; De Nola, R.; Lippolis, A.; Marrano, T.; Loverro, M.; Sabba, C.; Di Naro, E. Autotaxin is a novel molecular identifier of type I endometrial cancer. Med. Oncol. 2018, 35, 157. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G.J.; Yue, J.; Norman, D.D.; Szabo, E.; Balogh, A.; Balazs, L.; Zhao, G.; Lee, S.C. Regulation of tumor cell—Microenvironment interaction by the autotaxin-lysophosphatidic acid receptor axis. Adv. Biol. Regul. 2019, 71, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Genc, G.E.; Hipolito, V.E.B.; Botelho, R.J.; Gumuslu, S. Lysophosphatidic acid represses autophagy in prostate carcinoma cells. Biochem. Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Minami, K.; Otagaki, S.; Ishimoto, K.; Fukushima, K.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Lysophosphatidic acid receptor-2 (LPA2) and LPA5 regulate cellular functions during tumor progression in fibrosarcoma HT1080 cells. Biochem. Biophys. Res. Commun. 2018, 503, 2698–2703. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Lysophospholipid Signaling in the Epithelial Ovarian Cancer Tumor Microenvironment. Cancers 2018, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Fukushima, K.; Tanaka, K.; Minami, K.; Ishimoto, K.; Otagaki, S.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Involvement of LPA signaling via LPA receptor-2 in the promotion of malignant properties in osteosarcoma cells. Exp. Cell Res. 2018, 369, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govindarajulu, M.; Suppiramaniam, V.; Moore, T.; Dhanasekaran, M. Autotaxin(-)lysophosphatidic acid signaling in Alzheimer’s disease. Int. J. Mol. Sci. 2018, 19, 1827. [Google Scholar] [CrossRef]
- Shi, J.; Dong, Y.; Cui, M.Z.; Xu, X. Lysophosphatidic acid induces increased BACE1 expression and Abeta formation. Biochim. Biophys. Acta 2013, 1832, 29–38. [Google Scholar] [CrossRef]
- Zhang, J.B.; Cong, Y.N.; Li, Z.G.; Sun, H.R.; Zhang, J.S.; Wang, P.F.; Wu, Q.Z. Plasma Phospholipids are Associated with Mild Cognitive Impairment in Type 2 Diabetic Patients. Curr. Alzheimer Res. 2017, 14, 592–597. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).