Electrophoretic Deposition of Hydroxyapatite Film Containing Re-Doped MoS2 Nanoparticles
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.1.1. Surface Treatment
2.1.2. Titanium Anodization
2.1.3. Electrophoretic Deposition
2.2. Characterization
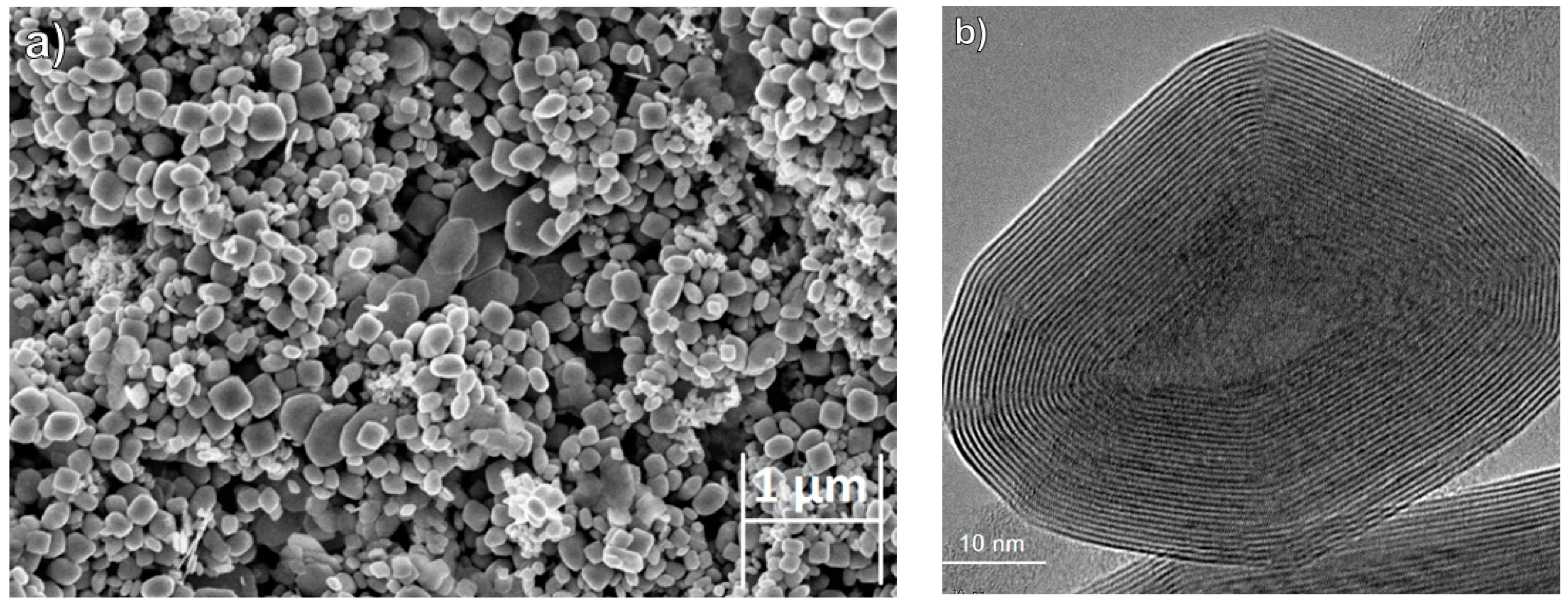
2.2.1. High-Resolution Scanning Electron Microscopy (HRSEM) and High-Resolution Transmission Electron Microcopy (HRTEM)
2.2.2. X-ray Diffraction (XRD)
2.2.3. Raman Spectroscopy
2.2.4. Zeta Potential Measurements
2.2.5. Tribological Testing
3. Results and Discussion
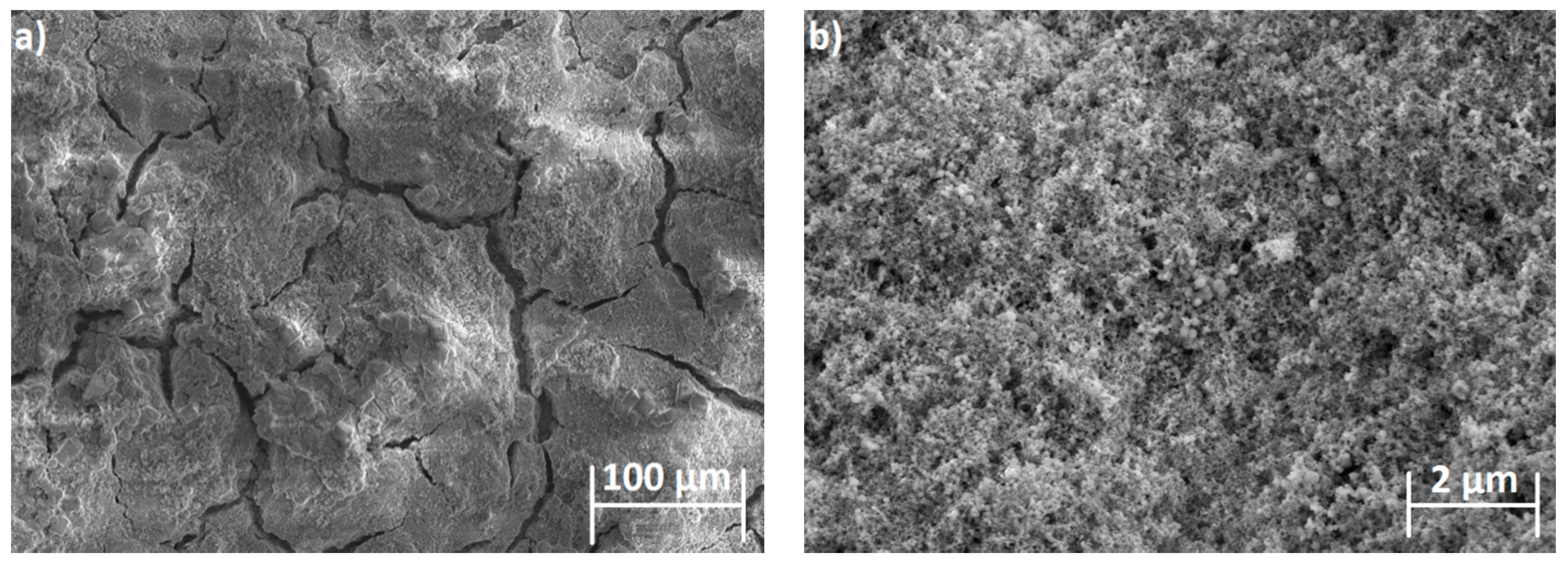
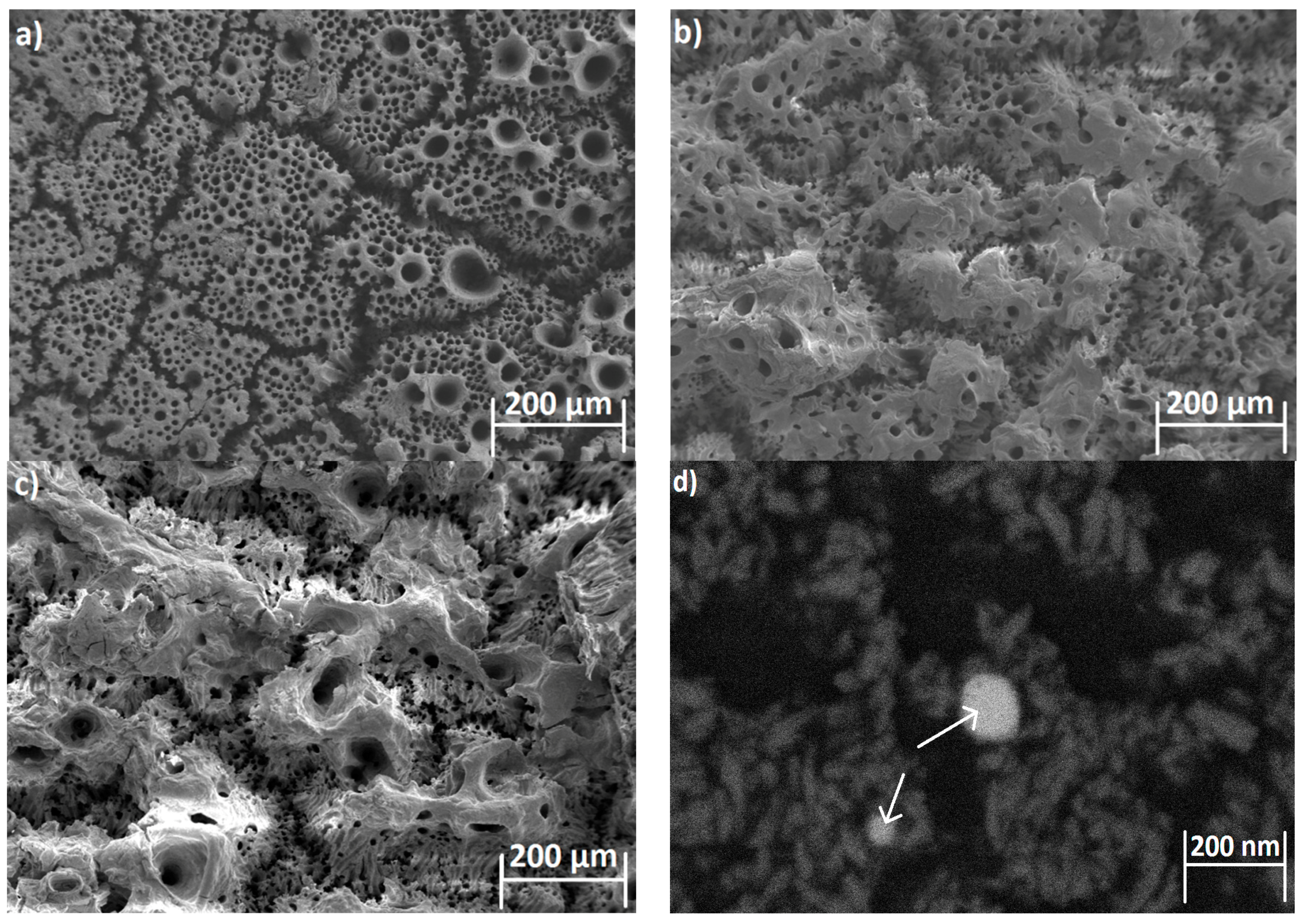
3.1. SEM Analysis
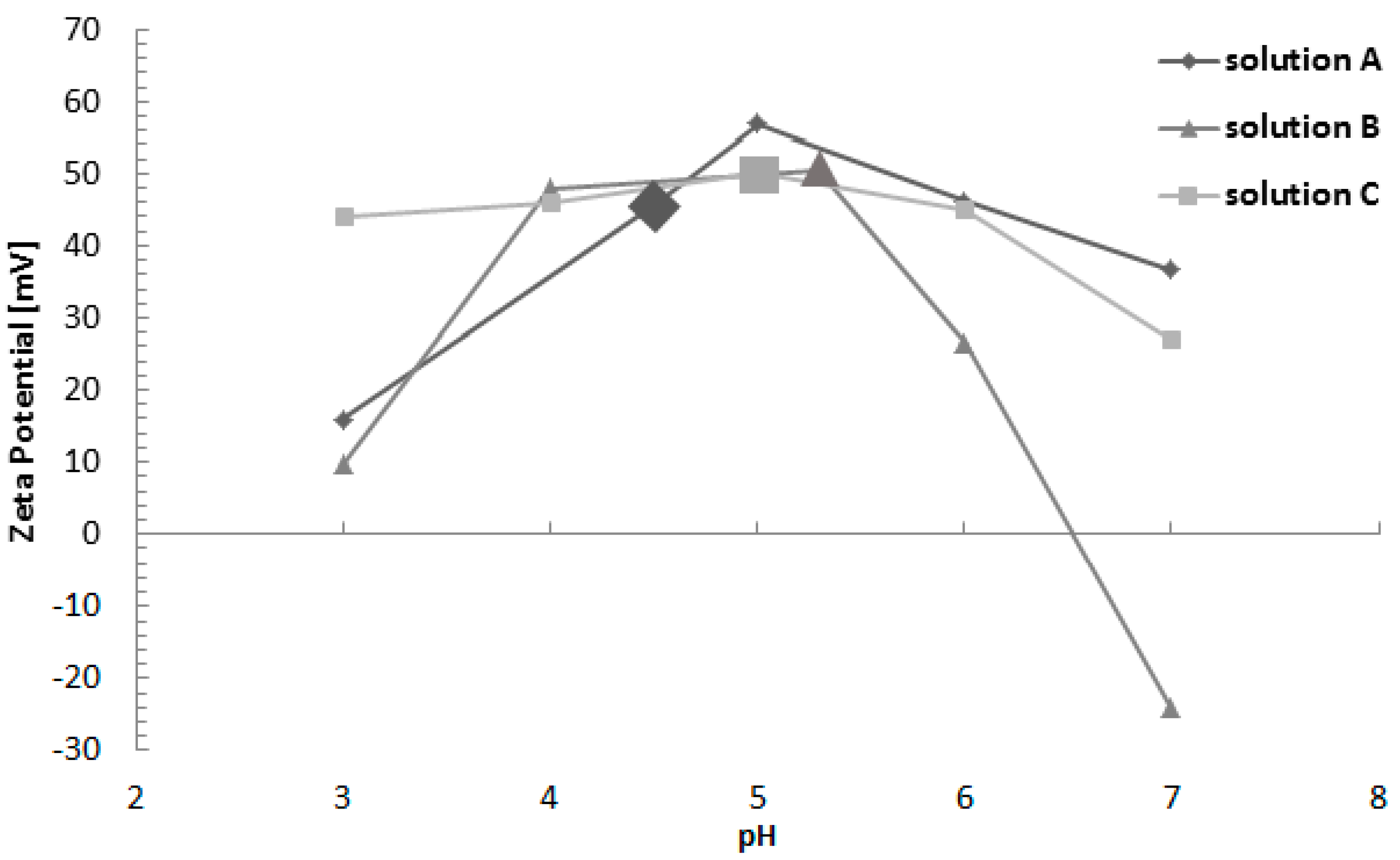
3.2. Zeta Potential Measurements
3.3. X-ray Diffraction (XRD)
3.4. Raman Spectroscopy
3.5. Tribological Testing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clauses, F.J. Solid Lubricants and Self-Lubricating Solids; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Basnyat, P.; Luster, B.; Kertzman, Z.; Stadler, S.; Kohli, P.; Aouadi, S.; Xu, J.; Mishra, S.R.; Eryilmaz, O.L.; Erdemir, A. Mechanical and tribological properties of CrAlN-Ag self-lubricating films. Surf. Coat. Technol. 2007, 202, 1011–1016. [Google Scholar] [CrossRef]
- Fusaro, R.L. Self-lubricating polymer composites and polymer transfer film lubrication for space applications. Tribol. Int. 1990, 23, 105–122. [Google Scholar] [CrossRef]
- Li, J.L.; Xiong, D.S. Tribological properties of nickel-based self-lubricating composite at elevated temperature and counterface material selection. Wear 2008, 265, 533–539. [Google Scholar] [CrossRef]
- Skeldon, P.; Wang, H.W.; Thompson, G.E. Formation and characterization of self-lubricating MoS2 precursor films on anodized aluminum. Wear 1997, 206, 187–196. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal chemical approach to the formulation of self-lubricating nanocomposite coatings. Surf. Coat. Technol. 2005, 200, 1792–1796. [Google Scholar] [CrossRef]
- Moghadam, A.D.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and grapheme—A review. Compos. Part B 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Liu, E.Y.; Wang, W.Z.; Gao, Y.M.; Jia, J.H. Tribological properties of Ni-based self-lubricating composites with addition of silver and molybdenum disulfide. Tribol. Int. 2013, 57, 235–241. [Google Scholar] [CrossRef]
- Lian, Y.; Deng, J.; Li, S.; Yan, G.; Lei, S. Friction and wear behavior of WS2/Zr self-lubricating soft coatings in dry sliding against 40Cr-hardened steel balls. Tribol. Lett. 2014, 53, 237–246. [Google Scholar] [CrossRef]
- Petit, R. The use of hydroxyapatite in orthopaedic surgery: A ten-year review. Eur. J. Orthop. Surg. Traumatol. 1999, 9, 71–74. [Google Scholar] [CrossRef]
- Mucalo, M. Hydroxyapatite (HAp) for Biomedical Applications; Woodhead Publishing Series in Biomaterials; Elsevier: Cambridge, MA, USA, 2015; ISBN 978-1-78242-041-5. [Google Scholar]
- Lahiri, D.; Singh, V.; Keshri, A.K.; Seal, S.; Agarwal, A. Carbon nanotube toughened hydroxyapatite by spark plasma sintering: Microstructural evolution and multiscale tribological properties. Carbon 2010, 48, 3103–3120. [Google Scholar] [CrossRef]
- Lahiri, D.; Singh, V.; Benaduce, A.P.; Seal, S.; Kos, L.; Agarwal, A. Boron nitride nanotube reinforced hydroxyapatite composite: Mechanical and tribological performance and in-vitro biocompatibility to osteoblasts. J. Mech. Behav. Biomed. 2011, 4, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, I.; Gal-Or, L. Electrophoretic deposition of hydroxyapatite. J. Mater. Sci. Mater. Med. 1997, 8, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.T.; Wonga, P.K.; Cheng, F.T.; Man, H.C. Characterization and corrosion behavior of hydroxyapatite coatings on Ti6Al4V fabricated by electrophoretic deposition. Appl. Surf. Sci. 2009, 255, 6736–6744. [Google Scholar] [CrossRef]
- Sridhar, T.M.; Eliaz, N.; Kamachi Mudali, U.; Raj, B. Electrophoretic deposition of hydroxyapatite coatings and corrosion aspects of metallic implants. Corros. Rev. 2002, 20, 255–293. [Google Scholar] [CrossRef]
- Theiss, F.; Apelt, D.; Brand, B.; Kutter, A.; Zlinszky, K.; Bohner, M. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 2005, 26, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lugo, V.; Angeles-Chavez, C.; Mondragon, G.; Recillas-Gispert, S.; Castano, V.M. Synthesis and structural characterization of hydroxyapatite obtained from CaO and CaHPO4 by a hydrothermal method. Mater. Res. Innov. 2005, 9, 20–22. [Google Scholar] [CrossRef]
- Kuo, M.C.; Yen, S.K. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater. Sci. Eng. C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Legeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; Legeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 44, 16399–16404. [Google Scholar] [CrossRef]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.R.; Tenne, R. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar] [CrossRef]
- Rosentsveig, R.; Tenne, R.; Gorodnev, A.; Feuerstein, N.; Friedman, H.; Fleischer, N.; Tannous, J.; Dassenoy, F. Fullerene-like MoS2 nanoparticles and their tribological behavior. Tribol. Lett. 2009, 36, 175–182. [Google Scholar] [CrossRef]
- André, B.; Gustavsson, F.; Svahn, F.; Jacobson, S. Performance and tribofilm formation of a low-friction coating incorporating inorganic fullerene like nano-particles. Surf. Coat. Technol. 2012, 206, 2325–2329. [Google Scholar] [CrossRef]
- Alberdi, A.; Hatto, P.; Díaz, B.; Csillag, S. Tribological behavior of nanocomposite coatings based on fullerene-like structures. Vacuum 2011, 85, 1087–1092. [Google Scholar] [CrossRef]
- Polcar, T.; Mohan, D.B.; Silviu Sandu, C.; Radnoczi, G.; Cavaleiro, A. Properties of nanocomposite film combining hard TiN matrix with embedded fullerene-like WS2 nanoclusters. Thin Solid Films 2011, 519, 3191–3195. [Google Scholar] [CrossRef]
- Yadgarov, L.; Petrone, V.; Rosentsveig, R.; Feldman, Y.; Tenne, R.; Senatore, A. Tribological studies of rhenium doped fullerene-like MoS2 nanoparticles in boundary, mixed and elasto-hydrodynamic lubrication conditions. Wear 2013, 297, 1103–1110. [Google Scholar] [CrossRef]
- Chhetri, M.; Gupta, U.; Yadgarov, L.; Rosentsveig, R.; Tenne, R.; Rao, C.N.R. Beneficial effect of Re doping on the electrochemical HER activity of MoS2 fullerenes. Dalton Trans. 2015, 44, 16399–16404. [Google Scholar] [CrossRef] [PubMed]
- Yadgarov, L.; Rosentsveig, R.; Leitus, G.; Albu-Yaron, A.; Moshkovich, A.; Perfilyev, V.; Vasic, R.; Frenkel, A.I.; Enyashin, A.N.; Seifert, G.; et al. Controlled doping of MS2 (M = W, Mo) nanotubes and fullerene-like nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Sedova, A.; Ron, R.; Goldbart, O.; Elianov, O.; Yadgarov, L.; Kampf, N.; Rosentsveig, R.; Shumalinsky, D.; Lobik, L.; Shay, B.; et al. Re-doped fullerene-like MoS2 nanoparticles in relationship with soft lubrication. Nanomater. Energy 2014, 4, 30–38. [Google Scholar] [CrossRef]
- Xu, F.; Kobayashi, T.; Yang, Z.; Sekine, T.; Chang, H.; Wang, N.; Xia, Y.; Zhu, Y. Low cytotoxicity and genotoxicity of two-dimensional MoS2 and WS2. ACS Nano 2017, 11, 8114–8121. [Google Scholar] [CrossRef] [PubMed]
- Appel, J.H.; Li, D.O.; Podlevsky, J.D.; Debnath, A.; Green, A.A.; Wang, Q.H.; Chae, J. Low cytotoxicity and genotoxicity of two-dimensional MoS2 and WS2. ACS Biomater. Sci. Eng. 2016, 2, 361–367. [Google Scholar] [CrossRef]
- Goldman, E.B.; Zak, A.; Tenne, R.; Kartvelishvily, E.; Levin-Zaidman, S.; Neumann, Y.; Stiubea-Cohen, R.; Palmon, A.; Hovav, A.H.; Aframian, D.J. Biocompatibility of Tungsten Disulfide Inorganic Nanotubes and Fullerene-Like Nanoparticles with Salivary Gland Cells. Tissue Eng. Part A 2014, 21, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Shuster-Meiseles, T.; Levin-Zaidman, S.; Rudich, A.; Rudich, Y. Low Cytotoxicity of Inorganic Nanotubes and Fullerene-Like Nanostructures in Human Bronchial Epithelial Cells: Relation to Inflammatory Gene Induction and Antioxidant Response. Environ. Sci. Technol. 2014, 48, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Schmuki, P. Nanoporous materials high-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Yadgarov, L.; Stroppa, D.G.; Rosentsveig, R.; Ron, R.; Enyashin, A.N.; Houben, L.; Tenne, R. Investigation of Rhenium-Doped MoS2 Nanoparticles with Fullerene-Like Structure. Z. Anorg. Allg. Chem. 2012, 638, 2610–2616. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Lebugle, A.; Sfihi, H.; Barroug, A. Nanocrystalline apatites in biological systems: Characterisation, structure and properties. Materialwiss. Werkstofftech. 2007, 38, 996–1002. [Google Scholar] [CrossRef]
- Chen, J.S.; Juang, H.Y.; Hon, M.H. Second order Raman spectrum of MoS2. J. Mater. Sci. Mater. Med. 1998, 9, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Kendall, B. Nanocrystalline Apatite-Based Biomaterials: Synthesis; Processing and Characterization; Nova Science Publishers: New York, NY, USA, 2008; pp. 93–143. [Google Scholar]
- Chen, J.M.; Wang, C.S. Second order Raman spectrum of MoS2. Solid State Commun. 1974, 14, 857–860. [Google Scholar] [CrossRef]
- Sourisseau, C.; Fouassier, M.; Alba, M.; Ghorayeb, A.; Gorochov, O. Resonance Raman, inelastic neutron scattering and lattice dynamics studies of 2H-WS2. Mater. Sci. Eng. B 1989, 3, 119–123. [Google Scholar] [CrossRef]
- Grandfield, K.; Sun, F.; FitzPatrick, M.; Cheong, M.; Zhitomirsky, I. Electrophoretic deposition of polymer-carbon nanotube–hydroxyapatite composites. Surf. Coat. Technol. 2009, 203, 1481–1487. [Google Scholar] [CrossRef]
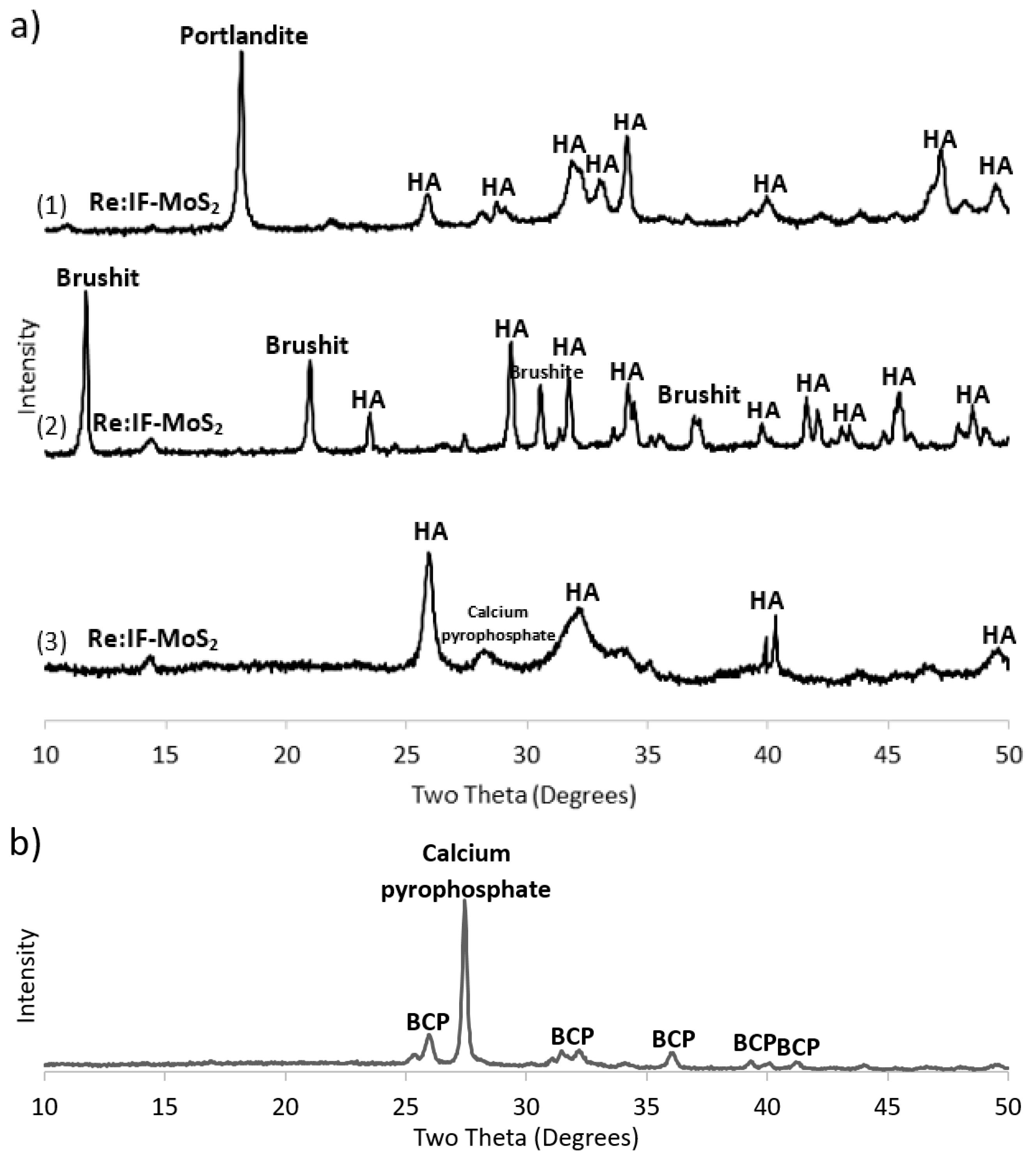
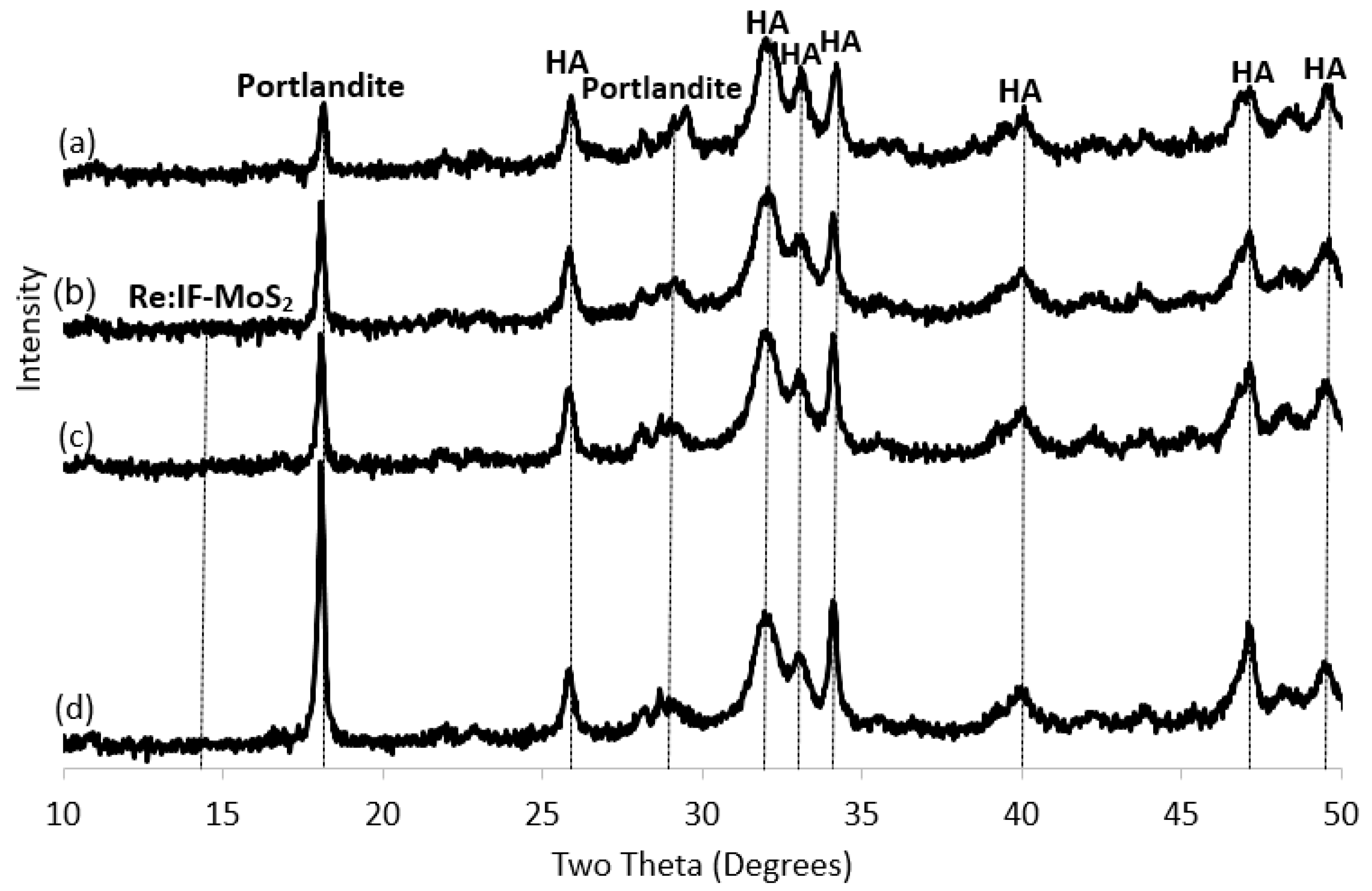
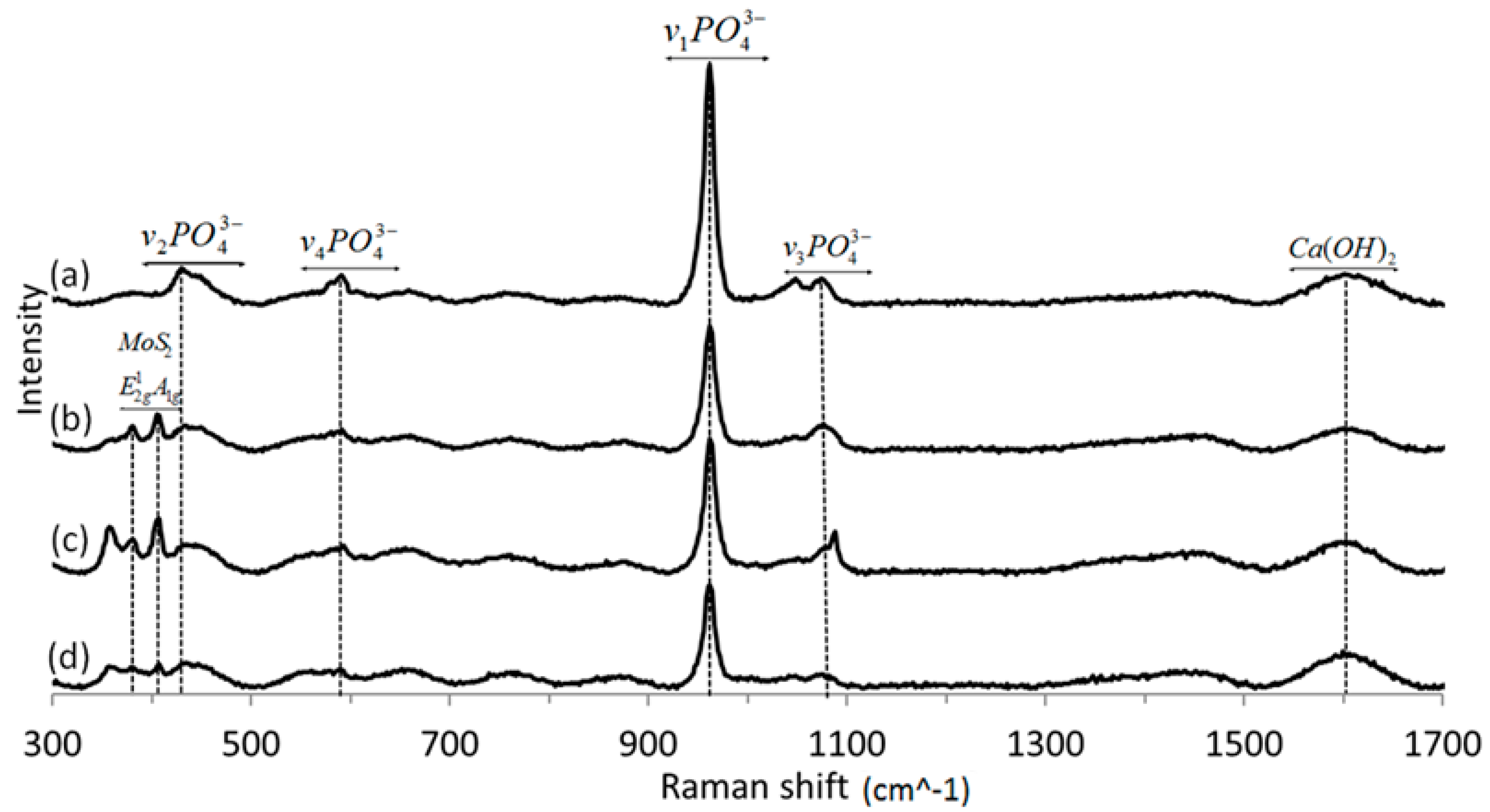

| EPD Films | HA | Portlandite | Brushite | Calcium Pyrophosphate | β-TCP | Re:IF-MoS2 |
|---|---|---|---|---|---|---|
| Film obtained from solution A | 74.8 wt % | 25 wt % | 0.2 wt % | |||
| Film obtained from solution B | 17.2 wt % | 81.3 wt % | 1.5 wt % | |||
| Film obtained from solution C | 81.1 wt % | 17.5 wt % | 1.4 wt % | |||
| Film obtained from solution A after annealing | 73.6 wt % | 20.4 wt % | 5.9 wt % | 0.1 wt % |
| EPD films | HA | Portlandite | Calcium Oxide | Re:IF-MoS2 |
|---|---|---|---|---|
| Film obtained from solution A without Re:IF-MoS2 (3 h) | 87.8 wt % | 4.6 wt % | 7.6 wt % | |
| Film obtained from solution A (2 h) | 82.6 wt % | 7.4 wt % | 9.1 wt % | 0.3 wt % |
| Film obtained from solution A (3 h) | 80.4 wt % | 11.3 wt % | 8.0 wt % | 0.3 wt % |
| Film obtained from solution A (4 h) | 77.8 wt % | 13.6 wt % | 8.3 wt % | 0.3 wt % |
| Tested Film | Initial Coefficient of Friction | Final Coefficient of Friction (after 20 Cycles) | Initial Roughness (μm) |
|---|---|---|---|
| Titanium after surface treatment | 0.50 ± 0.01 | 0. 60 ± 0.02 | 0.23 ± 0.03 |
| Titanium after anodization | 0.15 ± 0.01 | 0.23 ± 0.03 | 0.50 ± 0.05 |
| Film of HA with Re:IF-MoS2 NP obtained from solution A on anodized titanium | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.45 ± 0.4 |
| Film of HA with Re:IF-MoS2 NP obtained from solution B on anodized titanium | 0.21 ± 0.02 | 0.43 ± 0.08 | 0.37 ± 0.03 |
| Film of HA with Re:IF-MoS2 NP obtained from solution C on anodized titanium | 0.37 ± 0.23 | 0.30 ± 0.18 | 0.52 ± 0.02 |
| Film of HA with Re:IF-MoS2 NP obtained from solution A on anodized titanium after annealing | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.49 ± 0.7 |
| Tested Film | Initial Coefficient of Friction | Final Coefficient of Friction (after 100 Cycles) | Initial Roughness (μm) |
|---|---|---|---|
| Pure HA film obtained from solution A without NP after 3 h deposition | 0.66 ± 0.08 | 0.78 ± 0.04 | 1.59 ± 0.28 |
| HA film with Re:IF-MoS2 NP obtained from solution A after 2 h | 0.75 ± 0.05 | 0.63 ± 0.03 | 0.49 ± 0.05 |
| HA film with Re:IF-MoS2 NP obtained from solution A after 3 h | 0.53 ± 0.03 | 0.55 ± 0.04 | 0.57 ± 0.17 |
| HA film with Re:IF-MoS2 NP obtained from solution A after 4 h | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.48 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalom, H.; Feldman, Y.; Rosentsveig, R.; Pinkas, I.; Kaplan-Ashiri, I.; Moshkovich, A.; Perfilyev, V.; Rapoport, L.; Tenne, R. Electrophoretic Deposition of Hydroxyapatite Film Containing Re-Doped MoS2 Nanoparticles. Int. J. Mol. Sci. 2018, 19, 657. https://doi.org/10.3390/ijms19030657
Shalom H, Feldman Y, Rosentsveig R, Pinkas I, Kaplan-Ashiri I, Moshkovich A, Perfilyev V, Rapoport L, Tenne R. Electrophoretic Deposition of Hydroxyapatite Film Containing Re-Doped MoS2 Nanoparticles. International Journal of Molecular Sciences. 2018; 19(3):657. https://doi.org/10.3390/ijms19030657
Chicago/Turabian StyleShalom, Hila, Yishay Feldman, Rita Rosentsveig, Iddo Pinkas, Ifat Kaplan-Ashiri, Alexey Moshkovich, Vladislav Perfilyev, Lev Rapoport, and Reshef Tenne. 2018. "Electrophoretic Deposition of Hydroxyapatite Film Containing Re-Doped MoS2 Nanoparticles" International Journal of Molecular Sciences 19, no. 3: 657. https://doi.org/10.3390/ijms19030657
APA StyleShalom, H., Feldman, Y., Rosentsveig, R., Pinkas, I., Kaplan-Ashiri, I., Moshkovich, A., Perfilyev, V., Rapoport, L., & Tenne, R. (2018). Electrophoretic Deposition of Hydroxyapatite Film Containing Re-Doped MoS2 Nanoparticles. International Journal of Molecular Sciences, 19(3), 657. https://doi.org/10.3390/ijms19030657







