Abstract
The Hildebrand solubility parameters have been calculated for eight ionic liquids. Retention data from the inverse gas chromatography measurements of the activity coefficients at infinite dilution were used for the calculation. From the solubility parameters, the enthalpies of vaporization of ionic liquids were estimated. Results are compared with solubility parameters estimated by different methods.
1. Introduction
Ionic liquids (ILs) are a relatively new class of salts with a melting temperature below 373.15 K. In general, ILs are composed of organic cations with either inorganic or organic anions. Ionic liquids have unique properties, namely, a wide liquid range, stability at high temperatures and negligible vapor pressure. Because of the last mentioned property, the inverse gas chromatography (IGC) is a suitable method for measuring thermodynamic properties of pure substances and their mixtures [1]. From the IGC measurements, the activity coefficients at infinite dilution, Flory-Huggins interaction parameters as well as the Hildebrand solubility parameters can be determined. By this method the solubility parameters were determined previously for different ionic liquids [2–6].
The Hildebrand solubility parameters have numerous applications including gas-liquid solubility, solvent extraction and many others as described in detail in the literature [7,8]. The solubility parameter is the square root of the cohesive energy density, which is defined as the ratio of the energy of vaporization, ΔvapU, to the molar volume, υ:
Because ILs have negligible vapor pressure, experimental measurements of their energy of vaporization are difficult. For this reason, experimental data of ΔvapU are unavailable. Alternative methods have been considered for estimation of the solubility parameters of ionic liquids: From melting temperatures of ILs [9], from intrinsic viscosity measurements [10], from the activation energy of viscosity [11,12], from surface tension measurements [13], from Kamlet-Taft equation [14], using non random hydrogen bonding (NRHB) and PC-SAFT models [15], from lattice energy density [16].
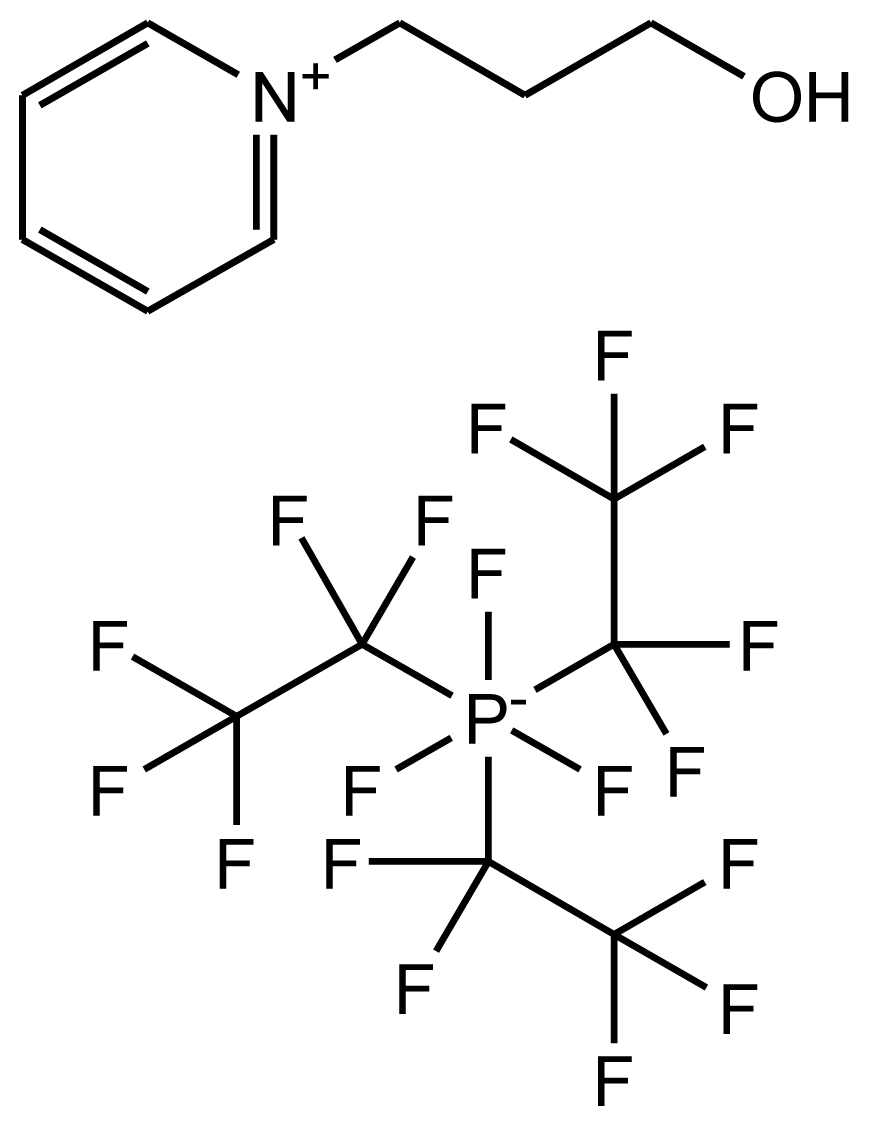
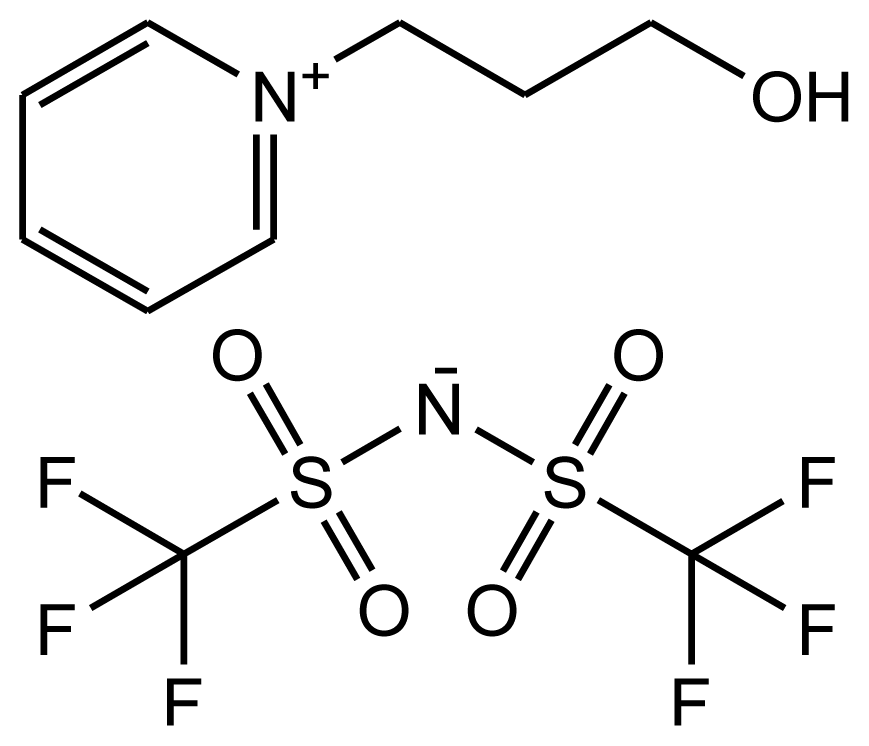
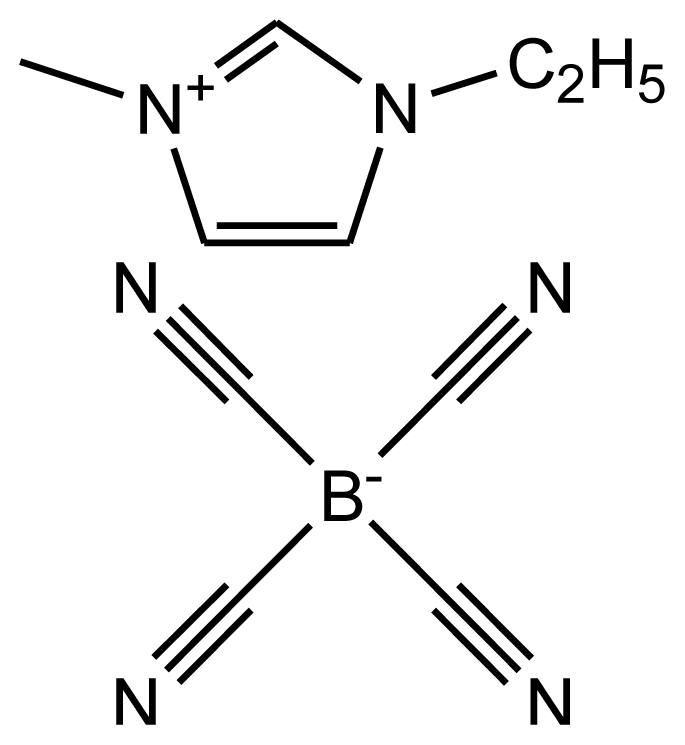
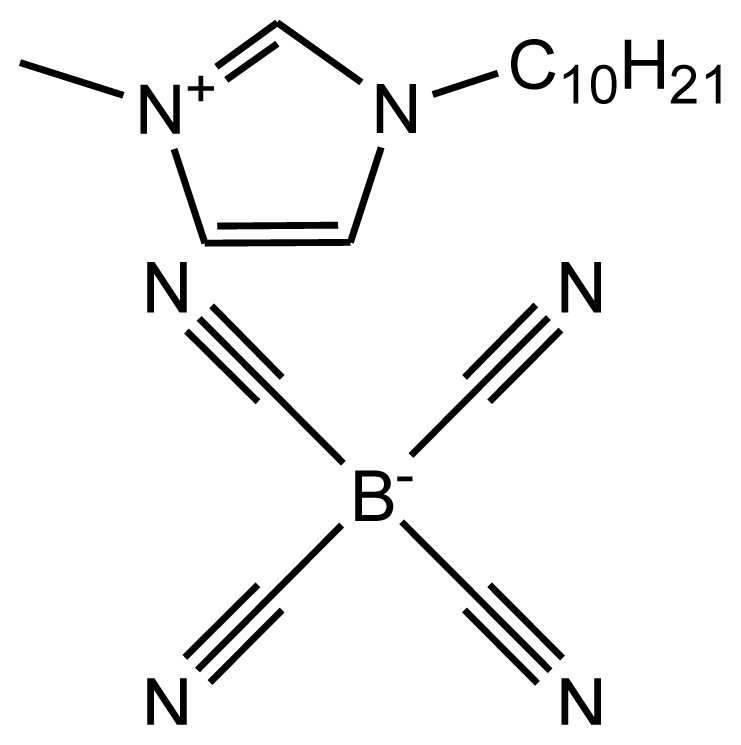
This paper provides information on the Hildebrand solubility parameters determined for eight ionic liquids as a function of temperature and the enthalpies of vaporization calculated from the values of the solubility parameters. The solubility parameters were calculated using the experimental data from the activity coefficients at infinite dilution measurements. The list of investigated ionic liquids is shown in Table 1. The values of the activity coefficients at infinite dilution for the investigated ionic liquids were published earlier [17–24].

Table 1.
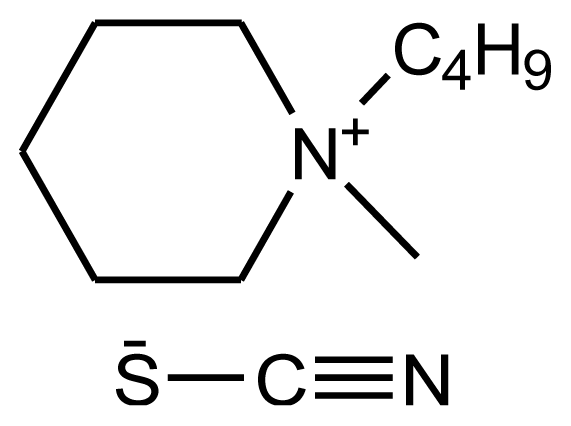
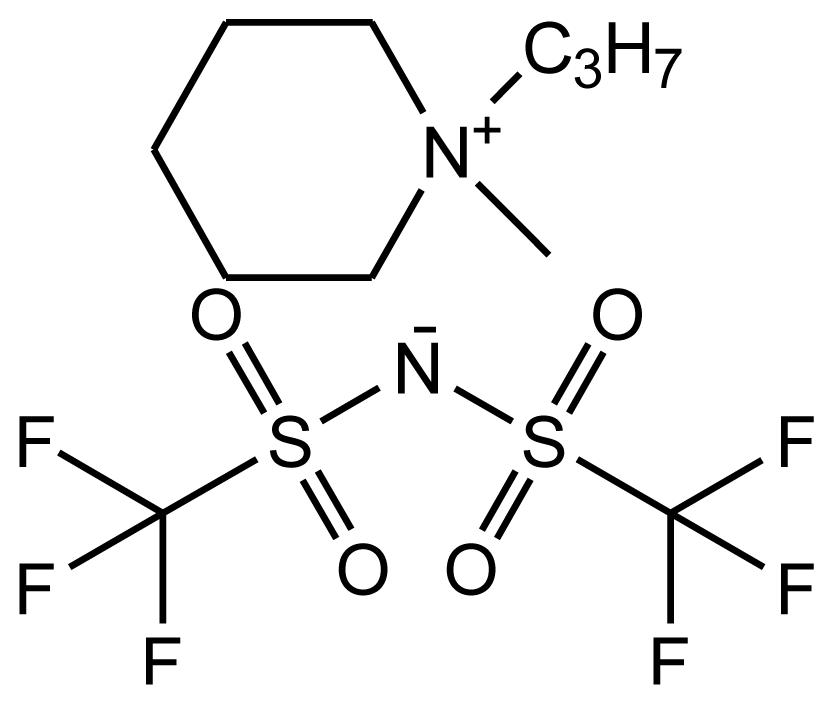
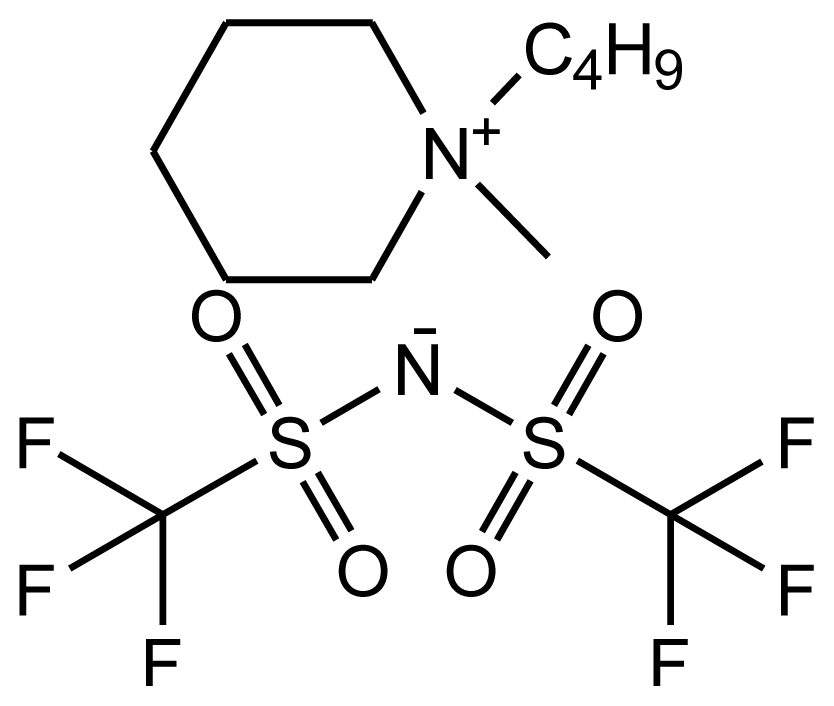
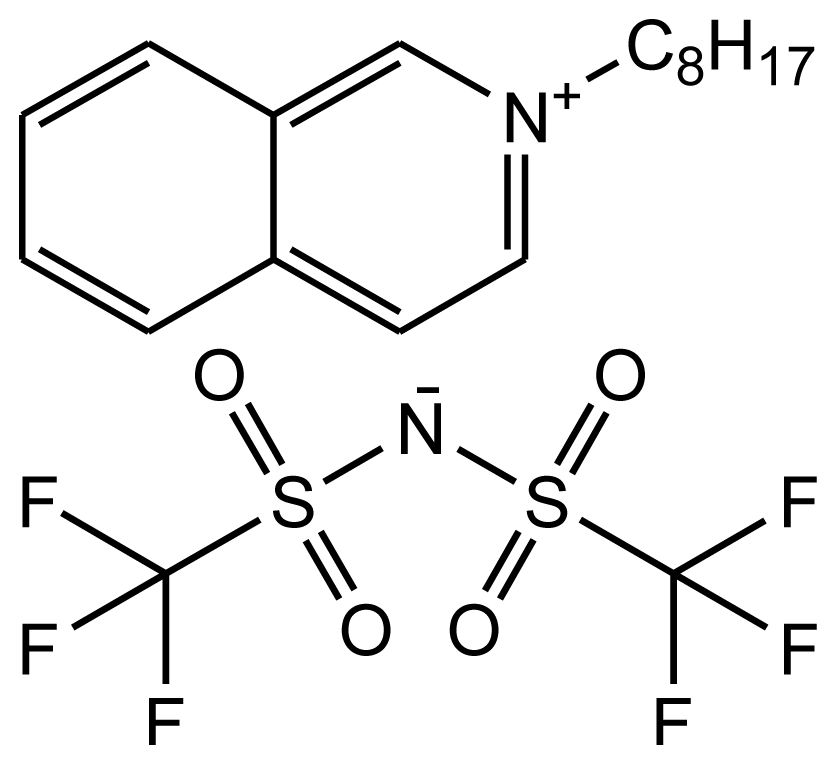
Abbreviations, names, sources, purities and structures of investigated ionic liquids.
2. Results and Discussion
The Hildebrand solubility parameters were calculated for the ionic liquids presented (with abbreviations and structures) in Table 1. The results are presented in Table 2. For ionic liquids based on [FAP]− and [NTf2]− anions with the same cation, [N-C3OHPY]+, the solubility parameter is higher for IL with [NTf2]− anion. Estimated enthalpy of vaporization is higher for [N-C3OHPY][FAP] than for [N-C3OHPY][NTf2], the higher molar mass and more complex structure of [FAP]− anion causes higher enthalpy of vaporization. For ionic liquids [bmPIP][SCN] and [bmPIP][NTf2] the solubility parameter as well as the enthalpy of vaporization is higher for ionic liquid with [SCN]− anion. In this case the structure of [SCN]− anion is much simpler than for [NTf2]− and the molar mass is lower, but very strong interaction of thiocyanate group increases the enthalpy of vaporization. With an increase of the alkyl chain in the cation structure of an ionic liquid the solubility parameter decreases. Due to increase of molar mass and alkyl chain length the enthalpy of vaporization also increases. This is typical behavior observed with increasing of alkyl chain length for example in linear alkanes or alkylbenzenes. This effect is visible in two pairs of ionic liquids, namely [emim][TCB]−[dmim][TCB] and [pmPIP][NTf2]−[bmPIP][NTf2].

Table 2.
Hildebrand solubility parameters, δ2 and standard enthalpies of vaporization for the investigated ionic liquids.
Table 3 presents comparison of the Hildebrand solubility parameters determined by different methods for selected ionic liquids based on [NTf2]− anion. Camper et al. presents different values of δ for ionic liquid [emim][NTf2] estimated from the IL melting point [9] and from lattice energy density [16]. These values differ about 2.4 times and are inconsistent with δ obtained by other methods. Solubility parameters determined from enthalpy of vaporization are in good agreement with values of δ obtained by IGC for [emim][NTf2] and [hmim][NTf2] and with values of δ estimated from surface tension for [bmim][NTf2] and [bmPYR][NTf2]. Kilaru et al. estimated solubility parameters from activation energy of viscosity using the equation presented below [11]:

Table 3.
Hildebrand solubility parameters, δ2 determined by different methods for selected ionic liquids based on [NTf2]− anion at T = 298.15 K.
where: μ is the dynamic viscosity of IL (in units of mPa·s), υ is the molar volume (in units of cm3·mol−1), h is Planck constant (in units of J·s), NA is Avogadro constant (in units of mol−1), and Kv is a proportionality constant. They calculated Kv value of 7.8 for ILs based on [NTf2]− anion from solubility parameters determined from intrinsic viscosity [10]. Consequently the solubility parameters estimated from Equation 2 are consistent with those estimated from intrinsic viscosity. In this work Kv value of 5.23 was obtained from the solubility parameters determined from experimental enthalpy of vaporization (the procedure is described in Supporting Information). Based on this value the solubility parameters were determined for [N-C3OHPY][NTf2], [pmPIP][NTf2] and [bmPIP][NTf2] ionic liquids for which the molar volumes and viscosities were determined (see Table 3S). Results are presented in Table 4. The differences in results are in the range from 3 to 10%.

Table 4.
Hildebrand solubility parameters, δ2 determined by different methods for [N-C3OHPY][NTf2], [pmPIP][NTf2] and [bmPIP][NTf2] ionic liquids.
3. Calculation of Solubility Parameters
3.1. Experimental Procedure
On the basis of the experimental data from the activity coefficients at infinite dilution measurements, the Hildebrand solubility parameters have been calculated using the equations presented below. The activity coefficients at infinite dilution for all investigated ionic liquids were measured using inverse gas chromatography. Detailed descriptions of materials, apparatus and methods used in each experiment are presented in the relevant papers [17–24].
3.2. Theoretical Basis
Retention data were used for the calculation of Hildebrand solubility parameters, δ2. According to the Flory-Huggins theory the interaction parameter at infinite dilution can be determined using the following expression:
where R denotes the gas constant, T the temperature, P1* the saturated vapor pressure of the solute at temperature T, B11 the second virial coefficient of pure solute, V1* and V2* the molar volume of the solute and solvent respectively, M1 the molar mass of solute, ρ1 and ρ2 density of solute and solvent respectively, Vg specific retention volume which is given by:
where m2 denotes the mass of the solvent on the column packing and VN the net retention volume of the solute given by:
where tR and tG are the retention times for the solute and an unretained gas, respectively, Uo is the column outlet flow rate, J23 the pressure correction term given by:
where Pi and Po denote the inlet and the outlet pressure, respectively. The column outlet flow rate corrected for the vapor pressure of water Uo is given by:
where Tf is the temperature at the column outlet, Pw is the vapor pressure of water at Tf and U is the flow rate measured with the flow meter. The interaction parameter χ12∞ may be expressed as a function of δ1 and δ2 which denote the solubility parameters of the solute and of the solvent, respectively, by:
Equation 8 can be rewritten as:
The solubility parameters δ1 of the solutes were calculated using following equation:
where ΔvapH denotes enthalpy of vaporization and υ the molar volume. The thermophysical properties required in calculations were calculated using equations and constants taken from the literature [30].
Values of χ12∞ were determined from Equation 2 and are presented in Table 1S. If the left side of Equation 9 is plotted against δ1, a straight line having a slope of 2δ2/RT and an intercept of −δ22 /RT is obtained. The solubility parameter of the solvent δ2 (ionic liquid) can be calculated from the slope. Example of calculations is presented in the Supporting Information. Hildebrand solubility parameters of the investigated ionic liquids and the estimated enthalpies of vaporization calculated using Equation 10 are listed in Table 2.
4. Conclusions
The Hildebrand solubility parameters estimated by different methods are divergent. The most reliable results are from the experiment especially from the enthalpies of vaporization. As presented in Table 3, solubility parameters calculated from enthalpies of vaporization and determined by IGC are in good consistency for [emim][NTf2] and [hmim][NTf2] ionic liquids. Therefore, the inverse gas chromatography is an appropriate method to determine Hildebrand solubility parameters of ionic liquids. While the ionic liquids have negligible vapor pressure, experimental measurements of their enthalpy of vaporization are difficult; therefore, this property can be estimated from the solubility parameters.
Acknowledgements
Funding for this research was provided by the Ministry of Sciences and Higher Education in years 2008–2011 (Grant No. N209 096435).
Appendix Electronic Supporting Information
Table S1, interaction parameters, χ12∞ Example of calculation of the solubility parameter. Calculation of the Kv constant from Equation 2; Table S2, data used in calculation of Kv constant; Table S3, densities and viscosities for [N-C3OHPY][NTf2], [pmPIP][NTf2] and [bmPIP][NTf2] ionic liquids.

Table S1.
Interaction parameters, χ 12∞.
| χ 12∞ | ||||||
|---|---|---|---|---|---|---|
| [N-C3OHPY][FAP] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | n-nonane | n-decane |
| 308.15 | 3.62 | 3.96 | 4.32 | 4.68 | 5.04 | 5.42 |
| 318.15 | 3.50 | 3.83 | 4.17 | 4.52 | 4.86 | 5.24 |
| 328.15 | 3.39 | 3.71 | 4.04 | 4.38 | 4.72 | 5.08 |
| 338.15 | 3.27 | 3.58 | 3.91 | 4.24 | 4.57 | 4.93 |
| 348.15 | 3.18 | 3.48 | 3.78 | 4.11 | 4.44 | 4.78 |
| 358.15 | 3.09 | 3.37 | 3.67 | 3.99 | 4.30 | 4.64 |
| T/K | cyclopentane | cyclohexane | cycloheptane | cyclooctane | 1-pentene | 1-hexene |
| 308.15 | 3.19 | 3.55 | 3.81 | 4.08 | 2.75 | 3.07 |
| 318.15 | 3.08 | 3.42 | 3.67 | 3.93 | 2.66 | 2.97 |
| 328.15 | 2.97 | 3.29 | 3.54 | 3.80 | 2.58 | 2.88 |
| 338.15 | 2.86 | 3.17 | 3.42 | 3.67 | 2.50 | 2.80 |
| 348.15 | 2.75 | 3.05 | 3.30 | 3.55 | 2.43 | 2.72 |
| 358.15 | 2.67 | 2.94 | 3.19 | 3.43 | 2.36 | 2.64 |
| T/K | 1-heptene | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 308.15 | 3.40 | 3.79 | 2.01 | 2.33 | 2.69 | 0.482 |
| 318.15 | 3.31 | 3.68 | 1.96 | 2.28 | 2.63 | 0.495 |
| 328.15 | 3.22 | 3.58 | 1.92 | 2.23 | 2.57 | 0.505 |
| 338.15 | 3.14 | 3.48 | 1.87 | 2.18 | 2.51 | 0.519 |
| 348.15 | 3.06 | 3.38 | 1.83 | 2.13 | 2.45 | 0.528 |
| 358.15 | 2.97 | 3.29 | 1.79 | 2.08 | 2.40 | 0.537 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 308.15 | 0.732 | 1.12 | 0.944 | 1.01 | 1.06 | 1.01 |
| 318.15 | 0.743 | 1.12 | 0.950 | 1.02 | 1.07 | 0.979 |
| 328.15 | 0.751 | 1.12 | 0.955 | 1.03 | 1.07 | 0.944 |
| 338.15 | 0.761 | 1.12 | 0.961 | 1.04 | 1.08 | 0.913 |
| 348.15 | 0.769 | 1.12 | 0.966 | 1.04 | 1.08 | 0.882 |
| 358.15 | 0.777 | 1.11 | 0.971 | 1.05 | 1.09 | 0.855 |
| T/K | ethanol | 1-propanol | 1-butanol | water | thiophene | tetrahydrofuran |
| 308.15 | 0.873 | 1.01 | 1.20 | 2.95 | 0.557 | −0.967 |
| 318.15 | 0.832 | 0.965 | 1.14 | 2.85 | 0.564 | −0.859 |
| 328.15 | 0.796 | 0.916 | 1.08 | 2.77 | 0.572 | −0.776 |
| 338.15 | 0.761 | 0.878 | 1.03 | 2.69 | 0.578 | −0.683 |
| 348.15 | 0.730 | 0.836 | 0.974 | 2.61 | 0.585 | −0.601 |
| 358.15 | 0.699 | 0.803 | 0.926 | 2.54 | 0.591 | −0.529 |
| T/K | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | 2-pentanone | 3-pentanone |
| 308.15 | −0.0104 | 0.168 | 1.29 | 2.14 | ||
| 318.15 | 0.083 | 0.245 | 1.32 | 2.15 | −1.09 | −0.988 |
| 328.15 | 0.170 | 0.324 | 1.36 | 2.15 | −1.01 | −0.907 |
| 338.15 | 0.262 | 0.390 | 1.39 | 2.16 | −0.933 | −0.831 |
| 348.15 | 0.335 | 0.450 | 1.43 | 2.16 | −0.860 | −0.759 |
| 358.15 | 0.414 | 0.504 | 1.45 | 2.16 | −0.792 | −0.694 |
| T/K | acetone | |||||
| 308.15 | −1.66 | |||||
| 318.15 | −1.56 | |||||
| 328.15 | −1.47 | |||||
| 338.15 | −1.38 | |||||
| 348.15 | −1.30 | |||||
| 358.15 | −1.23 | |||||
| [N-C3OHPY][NTf2] | ||||||
| T/K | n-pentane | n-hexane | 3-methylpentane | 2,2-dimethylbutane | n-heptane | n-octane |
| 318.15 | 3.67 | 4.04 | 3.90 | 3.81 | 4.45 | 4.86 |
| 328.15 | 3.59 | 3.94 | 3.80 | 3.71 | 4.34 | 4.73 |
| 338.15 | 3.50 | 3.84 | 3.70 | 3.61 | 4.22 | 4.61 |
| 348.15 | 3.43 | 3.76 | 3.62 | 3.53 | 4.13 | 4.50 |
| 358.15 | 3.35 | 3.67 | 3.54 | 3.44 | 4.03 | 4.40 |
| T/K | 2,2,4-trimethylpentane | n-nonane | n-decane | cyclopentane | cyclohexane | methylcyclohe xane |
| 318.15 | 4.40 | 5.27 | 5.70 | 3.08 | 3.45 | 3.78 |
| 328.15 | 4.30 | 5.13 | 5.54 | 2.99 | 3.35 | 3.68 |
| 338.15 | 4.20 | 5.00 | 5.41 | 2.91 | 3.26 | 3.58 |
| 348.15 | 4.11 | 4.89 | 5.29 | 2.84 | 3.18 | 3.49 |
| 358.15 | 4.03 | 4.77 | 5.16 | 2.78 | 3.10 | 3.41 |
| T/K | cycloheptane | cyclooctane | 1-pentene | 1-hexene | cyclohexene | 1-heptene |
| 318.15 | 3.73 | 4.03 | 2.89 | 3.28 | 2.75 | 3.67 |
| 328.15 | 3.63 | 3.92 | 2.83 | 3.20 | 2.68 | 3.59 |
| 338.15 | 3.53 | 3.81 | 2.76 | 3.12 | 2.62 | 3.51 |
| 348.15 | 3.44 | 3.71 | 2.71 | 3.06 | 2.57 | 3.44 |
| 358.15 | 3.36 | 3.63 | 2.65 | 3.00 | 2.52 | 3.38 |
| T/K | 1-octene | 1-decene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 318.15 | 4.09 | 4.91 | 2.10 | 2.48 | 2.89 | 0.886 |
| 328.15 | 4.00 | 4.80 | 2.08 | 2.45 | 2.84 | 0.887 |
| 338.15 | 3.91 | 4.70 | 2.05 | 2.41 | 2.79 | 0.888 |
| 348.15 | 3.83 | 4.61 | 2.03 | 2.39 | 2.76 | 0.888 |
| 358.15 | 3.75 | 4.51 | 2.01 | 2.35 | 2.71 | 0.888 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 318.15 | 1.18 | 1.62 | 1.38 | 1.51 | 1.51 | 0.896 |
| 328.15 | 1.18 | 1.61 | 1.38 | 1.51 | 1.51 | 0.850 |
| 338.15 | 1.18 | 1.60 | 1.38 | 1.51 | 1.51 | 0.805 |
| 348.15 | 1.18 | 1.58 | 1.38 | 1.51 | 1.51 | 0.761 |
| 358.15 | 1.18 | 1.58 | 1.38 | 1.51 | 1.51 | 0.719 |
| T/K | ethanol | 1-propanol | 1-butanol | water | acetic acid | thiophene |
| 318.15 | 0.885 | 1.02 | 1.23 | 2.21 | −0.614 | 0.754 |
| 328.15 | 0.836 | 0.968 | 1.17 | 2.13 | −0.534 | 0.756 |
| 338.15 | 0.788 | 0.918 | 1.11 | 2.06 | −0.464 | 0.756 |
| 348.15 | 0.745 | 0.868 | 1.05 | 1.99 | −0.397 | 0.757 |
| 358.15 | 0.698 | 0.822 | 0.995 | 1.94 | −0.334 | 0.756 |
| T/K | tetrahydrofuran | 1,4-dioxane | methyl tert-butyl ether | methyl tert-pentyl ether | diethyl ether | di-n-propyl ether |
| 318.15 | 0.125 | −0.205 | 1.26 | 1.69 | 1.34 | 2.43 |
| 328.15 | 0.166 | −0.157 | 1.29 | 1.70 | 1.35 | 2.40 |
| 338.15 | 0.201 | −0.112 | 1.31 | 1.71 | 1.36 | 2.38 |
| 348.15 | 0.230 | −0.070 | 1.33 | 1.73 | 1.37 | 2.36 |
| 358.15 | 0.260 | −0.032 | 1.35 | 1.74 | 1.38 | 2.35 |
| T/K | di-n-butyl ether | acetone | 2-pentanone | 3-pentanone | ||
| 318.15 | 3.30 | −0.351 | 0.193 | 0.253 | ||
| 328.15 | 3.25 | −0.314 | 0.217 | 0.277 | ||
| 338.15 | 3.20 | −0.284 | 0.242 | 0.301 | ||
| 348.15 | 3.16 | −0.255 | 0.261 | 0.322 | ||
| 358.15 | 3.12 | −0.229 | 0.281 | 0.341 | ||
| [emim][TCB] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | 2,2,4-trimethylpentane | n-nonane |
| 298.15 | 3.26 | 3.63 | 4.05 | 4.46 | 4.14 | 4.90 |
| 308.15 | 3.16 | 3.54 | 3.94 | 4.34 | 4.03 | 4.76 |
| 318.15 | 3.11 | 3.47 | 3.86 | 4.25 | 3.96 | 4.65 |
| 328.15 | 3.02 | 3.38 | 3.75 | 4.14 | 3.87 | 4.52 |
| 338.15 | 2.96 | 3.30 | 3.67 | 4.04 | 3.78 | 4.41 |
| 348.15 | 2.90 | 3.24 | 3.60 | 3.95 | 3.71 | 4.31 |
| 358.15 | 2.84 | 3.18 | 3.52 | 3.86 | 3.63 | 4.21 |
| T/K | n-decane | cyclopentane | cyclohexane | methylcyclohe xane | cycloheptane | cyclooctane |
| 298.15 | 5.32 | 2.64 | 3.01 | 3.35 | 3.23 | 3.49 |
| 308.15 | 5.18 | 2.57 | 2.92 | 3.24 | 3.13 | 3.38 |
| 318.15 | 5.06 | 2.52 | 2.86 | 3.17 | 3.07 | 3.31 |
| 328.15 | 4.92 | 2.44 | 2.77 | 3.08 | 2.98 | 3.21 |
| 338.15 | 4.81 | 2.39 | 2.70 | 3.00 | 2.90 | 3.14 |
| 348.15 | 4.69 | 2.34 | 2.64 | 2.94 | 2.83 | 3.06 |
| 358.15 | 4.58 | 2.28 | 2.57 | 2.88 | 2.77 | 2.98 |
| T/K | 1-pentene | 1-hexene | cyclohexene | 1-heptene | 1-octene | 1-hexyne |
| 298.15 | 2.42 | 2.80 | 2.21 | 3.18 | 3.61 | 1.50 |
| 308.15 | 2.37 | 2.73 | 2.16 | 3.10 | 3.52 | 1.49 |
| 318.15 | 2.34 | 2.69 | 2.14 | 3.06 | 3.46 | 1.49 |
| 328.15 | 2.29 | 2.62 | 2.09 | 2.99 | 3.37 | 1.48 |
| 338.15 | 2.25 | 2.57 | 2.04 | 2.92 | 3.30 | 1.48 |
| 348.15 | 2.22 | 2.53 | 2.01 | 2.89 | 3.24 | 1.47 |
| 358.15 | 2.15 | 2.48 | 1.98 | 2.83 | 3.18 | 1.47 |
| T/K | 1-heptyne | 1-octyne | benzene | toluene | ethylbenzene | o-xylene |
| 298.15 | 1.86 | 2.23 | 0.433 | 0.710 | 1.10 | 0.922 |
| 308.15 | 1.84 | 2.21 | 0.443 | 0.721 | 1.10 | 0.927 |
| 318.15 | 1.83 | 2.18 | 0.455 | 0.730 | 1.10 | 0.933 |
| 328.15 | 1.81 | 2.16 | 0.462 | 0.739 | 1.10 | 0.937 |
| 338.15 | 1.80 | 2.14 | 0.471 | 0.747 | 1.10 | 0.943 |
| 348.15 | 1.78 | 2.12 | 0.477 | 0.757 | 1.09 | 0.949 |
| 358.15 | 1.77 | 2.11 | 0.483 | 0.762 | 1.09 | 0.950 |
| T/K | m-xylene | p-xylene | methanol | ethanol | 1-propanol | 1-butanol |
| 298.15 | 1.08 | 1.02 | 0.968 | 1.04 | 1.11 | 1.29 |
| 308.15 | 1.08 | 1.03 | 0.886 | 0.944 | 1.01 | 1.17 |
| 318.15 | 1.09 | 1.03 | 0.812 | 0.856 | 0.909 | 1.06 |
| 328.15 | 1.09 | 1.04 | 0.739 | 0.770 | 0.816 | 0.953 |
| 338.15 | 1.10 | 1.05 | 0.674 | 0.693 | 0.734 | 0.861 |
| 348.15 | 1.10 | 1.06 | 0.612 | 0.620 | 0.660 | 0.780 |
| 358.15 | 1.10 | 1.06 | 0.553 | 0.551 | 0.591 | 0.701 |
| T/K | water | thiophene | tetrahydrofuran | methyl tert-butyl ether | methyl tert-pentyl ether | diethyl ether |
| 298.15 | 2.39 | 0.316 | −0.0164 | 1.19 | 1.53 | 1.21 |
| 308.15 | 2.27 | 0.325 | 0.0104 | 1.20 | 1.54 | 1.21 |
| 318.15 | 2.19 | 0.331 | 0.0335 | 1.21 | 1.54 | 1.21 |
| 328.15 | 2.10 | 0.337 | 0.0458 | 1.22 | 1.55 | 1.21 |
| 338.15 | 2.01 | 0.345 | 0.0626 | 1.23 | 1.55 | 1.21 |
| 348.15 | 1.92 | 0.348 | 0.0878 | 1.24 | 1.56 | 1.21 |
| 358.15 | 1.85 | 0.355 | 0.101 | 1.24 | 1.56 | 1.20 |
| T/K | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone | 3-pentanone | 2-hexanone |
| 298.15 | 2.24 | 3.06 | −0.445 | −0.0425 | −0.0790 | 0.210 |
| 308.15 | 2.21 | 2.99 | −0.421 | −0.0239 | −0.0528 | 0.225 |
| 318.15 | 2.18 | 2.94 | −0.398 | −0.0018 | −0.0208 | 0.238 |
| 328.15 | 2.14 | 2.87 | −0.379 | 0.0155 | 0.0047 | 0.247 |
| 338.15 | 2.12 | 2.83 | −0.358 | 0.0298 | 0.0266 | 0.261 |
| 348.15 | 2.09 | 2.78 | −0.344 | 0.0427 | 0.0464 | 0.272 |
| 358.15 | 2.06 | 2.73 | −0.325 | 0.0601 | 0.0678 | 0.283 |
| T/K | 3-hexanone | |||||
| 298.15 | 0.276 | |||||
| 308.15 | 0.294 | |||||
| 318.15 | 0.314 | |||||
| 328.15 | 0.330 | |||||
| 338.15 | 0.343 | |||||
| 348.15 | 0.355 | |||||
| 358.15 | 0.371 | |||||
| [dmim][TCB] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | 2,2,4-trimethylpentane | n-nonane |
| 328.15 | 1.98 | 2.11 | 2.27 | 2.44 | 2.35 | 2.62 |
| 338.15 | 1.94 | 2.07 | 2.23 | 2.39 | 2.30 | 2.57 |
| 348.15 | 1.90 | 2.03 | 2.18 | 2.34 | 2.25 | 2.52 |
| 358.15 | 1.85 | 1.99 | 2.13 | 2.30 | 2.21 | 2.47 |
| 368.15 | 1.81 | 1.94 | 2.09 | 2.25 | 2.17 | 2.42 |
| T/K | n-decane | cyclopentane | cyclohexane | methylcyclohe xane | cycloheptane | cyclooctane |
| 328.15 | 2.82 | 1.58 | 1.73 | 1.84 | 1.78 | 1.88 |
| 338.15 | 2.76 | 1.54 | 1.68 | 1.79 | 1.73 | 1.83 |
| 348.15 | 2.71 | 1.50 | 1.63 | 1.75 | 1.69 | 1.79 |
| 358.15 | 2.65 | 1.46 | 1.59 | 1.71 | 1.65 | 1.74 |
| 368.15 | 2.60 | 1.42 | 1.54 | 1.67 | 1.61 | 1.70 |
| T/K | 1-pentene | 1-hexene | cyclohexene | 1-heptene | 1-octene | 1-hexyne |
| 328.15 | 1.49 | 1.63 | 1.28 | 1.78 | 1.96 | 0.853 |
| 338.15 | 1.47 | 1.59 | 1.25 | 1.75 | 1.93 | 0.857 |
| 348.15 | 1.45 | 1.56 | 1.23 | 1.73 | 1.90 | 0.860 |
| 358.15 | 1.42 | 1.53 | 1.21 | 1.70 | 1.87 | 0.859 |
| 368.15 | 1.40 | 1.51 | 1.18 | 1.68 | 1.84 | 0.861 |
| T/K | 1-heptyne | 1-octyne | benzene | toluene | ethylbenzene | o-xylene |
| 328.15 | 0.983 | 1.14 | 0.0698 | 0.182 | 0.382 | 0.266 |
| 338.15 | 0.987 | 1.14 | 0.0826 | 0.201 | 0.396 | 0.283 |
| 348.15 | 0.990 | 1.14 | 0.0957 | 0.218 | 0.409 | 0.302 |
| 358.15 | 0.991 | 1.14 | 0.105 | 0.233 | 0.421 | 0.318 |
| 368.15 | 0.992 | 1.14 | 0.114 | 0.247 | 0.429 | 0.330 |
| T/K | m-xylene | p-xylene | methanol | ethanol | 1-propanol | 1-butanol |
| 328.15 | 0.361 | 0.343 | 0.997 | 0.835 | 0.682 | 0.635 |
| 338.15 | 0.381 | 0.366 | 0.929 | 0.759 | 0.613 | 0.565 |
| 348.15 | 0.402 | 0.386 | 0.870 | 0.693 | 0.555 | 0.507 |
| 358.15 | 0.416 | 0.401 | 0.803 | 0.625 | 0.497 | 0.452 |
| 368.15 | 0.437 | 0.422 | 0.752 | 0.566 | 0.441 | 0.392 |
| T/K | water | acetic acid | butyric acid | thiophene | tetrahydrofuran | methyl tert-butyl ether |
| 328.15 | 2.78 | −0.332 | 0.118 | 0.0626 | −0.338 | 0.547 |
| 338.15 | 2.68 | −0.284 | 0.116 | 0.0761 | −0.307 | 0.565 |
| 348.15 | 2.57 | −0.238 | 0.114 | 0.0845 | −0.279 | 0.585 |
| 358.15 | 2.49 | −0.198 | 0.111 | 0.0968 | −0.255 | 0.605 |
| 368.15 | 2.42 | −0.164 | 0.109 | 0.107 | −0.230 | 0.619 |
| T/K | methyl tert-pentyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone |
| 328.15 | 0.711 | 0.626 | 1.15 | 1.49 | −0.450 | −0.462 |
| 338.15 | 0.728 | 0.635 | 1.14 | 1.48 | −0.428 | −0.431 |
| 348.15 | 0.746 | 0.641 | 1.13 | 1.47 | −0.409 | −0.404 |
| 358.15 | 0.760 | 0.646 | 1.13 | 1.46 | −0.394 | −0.380 |
| 368.15 | 0.772 | 0.649 | 1.12 | 1.45 | −0.378 | −0.353 |
| T/K | 3-pentanone | |||||
| 328.15 | −0.497 | |||||
| 338.15 | −0.460 | |||||
| 348.15 | −0.426 | |||||
| 358.15 | −0.395 | |||||
| 368.15 | −0.364 | |||||
| [bmPIP][SCN] | ||||||
| T/K | n-hexane | n-heptane | n-octane | n-nonane | n-decane | cyclopentane |
| 318.15 | 4.90 | 5.19 | 5.49 | 5.82 | 6.19 | 3.55 |
| 328.15 | 4.73 | 5.00 | 5.36 | 5.69 | 6.07 | 3.42 |
| 338.15 | 4.57 | 4.89 | 5.24 | 5.60 | 5.97 | 3.32 |
| 348.15 | 4.44 | 4.75 | 5.11 | 5.46 | 5.84 | 3.21 |
| 358.15 | 4.30 | 4.67 | 5.03 | 5.37 | 5.74 | 3.15 |
| T/K | cyclohexane | cycloheptane | cyclooctane | 1-hexene | 1-heptene | 1-octene |
| 318.15 | 3.85 | 3.91 | 4.17 | 3.82 | 4.16 | 4.54 |
| 328.15 | 3.74 | 3.84 | 4.07 | 3.71 | 4.08 | 4.46 |
| 338.15 | 3.64 | 3.74 | 3.97 | 3.64 | 4.00 | 4.38 |
| 348.15 | 3.54 | 3.65 | 3.88 | 3.56 | 3.92 | 4.30 |
| 358.15 | 3.46 | 3.60 | 3.81 | 3.49 | 3.87 | 4.24 |
| T/K | 1-hexyne | 1-heptyne | 1-octyne | benzene | toluene | ethylbenzene |
| 318.15 | 1.94 | 2.30 | 2.66 | 0.907 | 1.32 | 1.76 |
| 328.15 | 1.94 | 2.30 | 2.66 | 0.916 | 1.33 | 1.76 |
| 338.15 | 1.95 | 2.30 | 2.66 | 0.924 | 1.33 | 1.75 |
| 348.15 | 1.95 | 2.30 | 2.66 | 0.930 | 1.33 | 1.75 |
| 358.15 | 1.95 | 2.30 | 2.66 | 0.938 | 1.34 | 1.74 |
| T/K | o-xylene | m-xylene | p-xylene | methanol | ethanol | water |
| 318.15 | 1.54 | 1.77 | 1.72 | −0.187 | 0.103 | 0.413 |
| 328.15 | 1.55 | 1.77 | 1.73 | −0.190 | 0.0822 | 0.429 |
| 338.15 | 1.55 | 1.77 | 1.73 | −0.191 | 0.0600 | 0.445 |
| 348.15 | 1.56 | 1.77 | 1.73 | −0.196 | 0.0405 | 0.460 |
| 358.15 | 1.56 | 1.77 | 1.74 | −0.198 | 0.0241 | 0.476 |
| T/K | thiophene | tetrahydrofuran | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether |
| 318.15 | 0.434 | 1.14 | 2.70 | 2.67 | 3.63 | 4.39 |
| 328.15 | 0.459 | 1.15 | 2.66 | 2.62 | 3.56 | 4.31 |
| 338.15 | 0.486 | 1.16 | 2.63 | 2.57 | 3.50 | 4.24 |
| 348.15 | 0.504 | 1.16 | 2.59 | 2.54 | 3.44 | 4.17 |
| 358.15 | 0.525 | 1.17 | 2.57 | 2.50 | 3.39 | 4.12 |
| T/K | acetone | 2-pentanone | 3-pentanone | |||
| 318.15 | 0.795 | 1.29 | 1.30 | |||
| 328.15 | 0.794 | 1.29 | 1.30 | |||
| 338.15 | 0.792 | 1.29 | 1.30 | |||
| 348.15 | 0.790 | 1.29 | 1.30 | |||
| 358.15 | 0.789 | 1.29 | 1.30 | |||
| [pmPIP][NTf2] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | n-nonane | n-decane |
| 308.15 | 3.31 | 3.40 | 3.58 | 3.81 | 4.08 | 4.38 |
| 318.15 | 3.02 | 3.21 | 3.44 | 3.70 | 3.98 | 4.30 |
| 328.15 | 2.98 | 3.14 | 3.35 | 3.60 | 3.88 | 4.18 |
| 338.15 | 2.85 | 3.06 | 3.29 | 3.53 | 3.80 | 4.09 |
| 348.15 | 2.75 | 2.95 | 3.19 | 3.43 | 3.70 | 3.99 |
| 358.15 | 2.72 | 2.93 | 3.14 | 3.37 | 3.63 | 3.91 |
| T/K | cyclopentane | cyclohexane | cycloheptane | cyclooctane | 1-pentene | 1-hexene |
| 308.15 | 2.72 | 2.93 | 3.05 | 3.23 | 2.48 | 2.64 |
| 318.15 | 2.52 | 2.77 | 2.94 | 3.15 | 2.30 | 2.52 |
| 328.15 | 2.47 | 2.71 | 2.86 | 3.04 | 2.27 | 2.48 |
| 338.15 | 2.38 | 2.62 | 2.78 | 2.98 | 2.32 | 2.41 |
| 348.15 | 2.29 | 2.51 | 2.70 | 2.89 | 2.13 | 2.34 |
| 358.15 | 2.26 | 2.48 | 2.64 | 2.82 | 2.13 | 2.31 |
| T/K | 1-heptene | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 308.15 | 2.84 | 3.11 | 1.47 | 1.71 | 1.98 | 0.418 |
| 318.15 | 2.76 | 3.04 | 1.46 | 1.70 | 1.98 | 0.427 |
| 328.15 | 2.71 | 2.96 | 1.45 | 1.68 | 1.95 | 0.430 |
| 338.15 | 2.65 | 2.92 | 1.46 | 1.69 | 1.94 | 0.433 |
| 348.15 | 2.59 | 2.85 | 1.44 | 1.67 | 1.95 | 0.444 |
| 358.15 | 2.55 | 2.81 | 1.44 | 1.67 | 1.91 | 0.456 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 308.15 | 0.615 | 0.913 | 0.752 | 0.822 | 0.812 | 1.62 |
| 318.15 | 0.619 | 0.915 | 0.772 | 0.851 | 0.845 | 1.54 |
| 328.15 | 0.635 | 0.932 | 0.775 | 0.849 | 0.849 | 1.44 |
| 338.15 | 0.642 | 0.930 | 0.787 | 0.865 | 0.864 | 1.36 |
| 348.15 | 0.655 | 0.936 | 0.795 | 0.875 | 0.876 | 1.28 |
| 358.15 | 0.668 | 0.942 | 0.811 | 0.892 | 0.894 | 1.20 |
| T/K | ethanol | 1-propanol | 1-butanol | water | thiophene | tetrahydrofuran |
| 308.15 | 1.54 | 1.55 | 1.64 | 3.36 | 0.377 | 0.355 |
| 318.15 | 1.45 | 1.46 | 1.56 | 3.27 | 0.386 | 0.363 |
| 328.15 | 1.35 | 1.35 | 1.42 | 3.14 | 0.389 | 0.370 |
| 338.15 | 1.26 | 1.26 | 1.32 | 3.04 | 0.401 | 0.385 |
| 348.15 | 1.18 | 1.17 | 1.23 | 2.90 | 0.404 | 0.384 |
| 358.15 | 1.10 | 1.10 | 1.15 | 2.78 | 0.416 | 0.398 |
| T/K | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone |
| 308.15 | 1.38 | 1.47 | 2.16 | 2.78 | −0.0329 | 0.181 |
| 318.15 | 1.37 | 1.41 | 2.12 | 2.72 | −0.0217 | 0.200 |
| 328.15 | 1.36 | 1.41 | 2.10 | 2.68 | −0.0151 | 0.209 |
| 338.15 | 1.35 | 1.39 | 2.07 | 2.63 | 0.0007 | 0.225 |
| 348.15 | 1.34 | 1.36 | 2.02 | 2.57 | −0.0029 | 0.229 |
| 358.15 | 1.33 | 1.36 | 2.01 | 2.53 | 0.0071 | 0.244 |
| T/K | 3-pentanone | |||||
| 308.15 | 0.161 | |||||
| 318.15 | 0.178 | |||||
| 328.15 | 0.208 | |||||
| 338.15 | 0.225 | |||||
| 348.15 | 0.235 | |||||
| 358.15 | 0.256 | |||||
| [bmPIP][NTf2] | ||||||
| T/K | n-pentane | n-hexane | 3-methylpentane | 2,2-dimethylbutane | n-heptane | n-octane |
| 308.15 | 2.62 | 2.85 | 2.74 | 2.66 | 3.13 | 3.42 |
| 318.15 | 2.51 | 2.72 | 2.62 | 2.54 | 3.00 | 3.27 |
| 328.15 | 2.39 | 2.62 | 2.50 | 2.42 | 2.89 | 3.16 |
| 338.15 | 2.34 | 2.56 | 2.45 | 2.37 | 2.82 | 3.09 |
| 348.15 | 2.29 | 2.51 | 2.40 | 2.33 | 2.76 | 3.02 |
| 358.15 | 2.23 | 2.47 | 2.35 | 2.28 | 2.69 | 2.95 |
| T/K | 2,2,4-trimethylpentane | n-nonane | n-decane | cyclopentane | cyclohexane | methylcyclohe xane |
| 308.15 | 3.03 | 3.71 | 4.01 | 2.22 | 2.48 | 2.67 |
| 318.15 | 2.92 | 3.55 | 3.84 | 2.11 | 2.36 | 2.54 |
| 328.15 | 2.81 | 3.42 | 3.71 | 2.02 | 2.26 | 2.44 |
| 338.15 | 2.74 | 3.35 | 3.63 | 1.96 | 2.20 | 2.38 |
| 348.15 | 2.69 | 3.28 | 3.54 | 1.90 | 2.12 | 2.31 |
| 358.15 | 2.63 | 3.20 | 3.47 | 1.87 | 2.09 | 2.27 |
| T/K | cycloheptane | cyclooctane | 1-pentene | 1-hexene | cyclohexene | 1-heptene |
| 308.15 | 2.70 | 2.91 | 2.00 | 2.26 | 1.95 | 2.51 |
| 318.15 | 2.57 | 2.78 | 1.91 | 2.16 | 1.87 | 2.41 |
| 328.15 | 2.47 | 2.67 | 1.83 | 2.08 | 1.79 | 2.33 |
| 338.15 | 2.40 | 2.60 | 1.81 | 2.03 | 1.75 | 2.29 |
| 348.15 | 2.32 | 2.51 | 1.76 | 1.97 | 1.70 | 2.23 |
| 358.15 | 2.28 | 2.47 | 1.73 | 1.94 | 1.64 | 2.21 |
| T/K | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene | toluene |
| 308.15 | 2.80 | 1.28 | 1.52 | 1.79 | 0.304 | 0.486 |
| 318.15 | 2.70 | 1.24 | 1.47 | 1.72 | 0.315 | 0.498 |
| 328.15 | 2.60 | 1.20 | 1.43 | 1.67 | 0.317 | 0.508 |
| 338.15 | 2.55 | 1.20 | 1.42 | 1.66 | 0.324 | 0.518 |
| 348.15 | 2.49 | 1.18 | 1.40 | 1.65 | 0.338 | 0.534 |
| 358.15 | 2.46 | 1.20 | 1.40 | 1.63 | 0.354 | 0.550 |
| T/K | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol | ethanol |
| 308.15 | 0.793 | 0.632 | 0.702 | 0.698 | 1.60 | 1.49 |
| 318.15 | 0.800 | 0.646 | 0.723 | 0.716 | 1.52 | 1.40 |
| 328.15 | 0.795 | 0.644 | 0.718 | 0.719 | 1.42 | 1.31 |
| 338.15 | 0.797 | 0.650 | 0.729 | 0.733 | 1.34 | 1.21 |
| 348.15 | 0.807 | 0.667 | 0.748 | 0.747 | 1.25 | 1.12 |
| 358.15 | 0.816 | 0.671 | 0.759 | 0.758 | 1.16 | 1.04 |
| T/K | 1-propanol | 1-butanol | water | thiophene | tetrahydrofuran | methyl tert-butyl ether |
| 308.15 | 1.47 | 1.54 | 3.49 | 0.299 | 0.206 | 1.06 |
| 318.15 | 1.38 | 1.42 | 3.34 | 0.302 | 0.215 | 1.06 |
| 328.15 | 1.28 | 1.33 | 3.21 | 0.301 | 0.205 | 1.05 |
| 338.15 | 1.18 | 1.22 | 3.07 | 0.306 | 0.207 | 1.04 |
| 348.15 | 1.09 | 1.12 | 2.94 | 0.308 | 0.207 | 1.04 |
| 358.15 | 1.01 | 1.04 | 2.81 | 0.323 | 0.219 | 1.04 |
| T/K | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone | 2-pentanone | 3-pentanone |
| 308.15 | 1.19 | 1.86 | 2.46 | −0.0841 | 0.0558 | 0.0284 |
| 318.15 | 1.18 | 1.85 | 2.43 | −0.0782 | 0.0667 | 0.0492 |
| 328.15 | 1.11 | 1.75 | 2.33 | −0.0764 | 0.0885 | 0.0770 |
| 338.15 | 1.12 | 1.75 | 2.29 | −0.0739 | 0.0943 | 0.0842 |
| 348.15 | 1.11 | 1.73 | 2.25 | −0.0732 | 0.106 | 0.114 |
| 358.15 | 1.10 | 1.70 | 2.21 | −0.0706 | 0.115 | 0.124 |
| [OQuin][NTf2] | ||||||
| T/K | n-pentane | n-hexane | n-heptane | n-octane | n-nonane | n-decane |
| 328.15 | 1.90 | 2.00 | 2.17 | 2.30 | 2.46 | 2.65 |
| 338.15 | 1.86 | 1.97 | 2.12 | 2.26 | 2.42 | 2.60 |
| 348.15 | 1.82 | 1.93 | 2.07 | 2.21 | 2.37 | 2.54 |
| 358.15 | 1.79 | 1.90 | 2.03 | 2.18 | 2.33 | 2.50 |
| 368.15 | 1.75 | 1.87 | 1.99 | 2.14 | 2.29 | 2.45 |
| T/K | cyclopentane | cyclohexane | cycloheptane | cyclooctane | 1-pentene | 1-hexene |
| 328.15 | 1.57 | 1.70 | 1.76 | 1.85 | 1.50 | 1.60 |
| 338.15 | 1.54 | 1.66 | 1.72 | 1.81 | 1.48 | 1.58 |
| 348.15 | 1.50 | 1.61 | 1.68 | 1.76 | 1.46 | 1.55 |
| 358.15 | 1.47 | 1.58 | 1.64 | 1.72 | 1.43 | 1.53 |
| 368.15 | 1.43 | 1.54 | 1.61 | 1.68 | 1.41 | 1.51 |
| T/K | 1-heptene | 1-octene | 1-hexyne | 1-heptyne | 1-octyne | benzene |
| 328.15 | 1.74 | 1.89 | 0.977 | 1.09 | 1.22 | 0.188 |
| 338.15 | 1.72 | 1.87 | 0.982 | 1.09 | 1.22 | 0.194 |
| 348.15 | 1.70 | 1.84 | 0.981 | 1.09 | 1.21 | 0.206 |
| 358.15 | 1.68 | 1.82 | 0.983 | 1.09 | 1.22 | 0.216 |
| 368.15 | 1.66 | 1.80 | 0.987 | 1.08 | 1.22 | 0.224 |
| T/K | toluene | ethylbenzene | o-xylene | m-xylene | p-xylene | methanol |
| 328.15 | 0.247 | 0.471 | 0.255 | 0.386 | 0.399 | 1.62 |
| 338.15 | 0.267 | 0.485 | 0.282 | 0.402 | 0.416 | 1.54 |
| 348.15 | 0.285 | 0.495 | 0.303 | 0.419 | 0.424 | 1.43 |
| 358.15 | 0.302 | 0.505 | 0.322 | 0.430 | 0.437 | 1.35 |
| 368.15 | 0.320 | 0.517 | 0.340 | 0.445 | 0.447 | 1.27 |
| T/K | ethanol | 1-propanol | 1-butanol | 1-pentanol | water | thiophene |
| 328.15 | 1.43 | 1.29 | 1.24 | 1.15 | 3.53 | 0.215 |
| 338.15 | 1.32 | 1.20 | 1.14 | 1.07 | 3.40 | 0.223 |
| 348.15 | 1.22 | 1.10 | 1.02 | 0.979 | 3.24 | 0.227 |
| 358.15 | 1.14 | 1.02 | 0.943 | 0.896 | 3.12 | 0.231 |
| 368.15 | 1.06 | 0.942 | 0.865 | 0.827 | 2.98 | 0.236 |
| T/K | tetrahydrofuran | methyl tert-butyl ether | diethyl ether | di-n-propyl ether | di-n-butyl ether | acetone |
| 328.15 | 0.0326 | 0.778 | 0.879 | 1.33 | 1.65 | −0.0485 |
| 338.15 | 0.0506 | 0.787 | 0.880 | 1.31 | 1.63 | −0.0465 |
| 348.15 | 0.0653 | 0.793 | 0.881 | 1.30 | 1.60 | −0.0456 |
| 358.15 | 0.0742 | 0.796 | 0.879 | 1.28 | 1.57 | −0.0458 |
| 368.15 | 0.0853 | 0.803 | 0.877 | 1.26 | 1.54 | −0.0465 |
| T/K | 2-pentanone | 3-pentanone | ||||
| 328.15 | −0.0778 | −0.0596 | ||||
| 338.15 | −0.0694 | −0.0483 | ||||
| 348.15 | −0.0621 | −0.0372 | ||||
| 358.15 | −0.0521 | −0.0285 | ||||
| 368.15 | −0.0461 | −0.0216 | ||||
Example of Calculation of the Solubility Parameter
Experimental data for n-octane + [emim][TCB] system at T = 298.15 K:
- T = 298.15 K
- pi = 137423 Pa
- po = 97423 Pa
- Tf = 297.15 K
- U = 41.2 mL·min−1
- tR − tG = 270.66 s
- m2 = 2.1053 g
- Pw (at Tf) = 2986.2 Pa (from [30])
- Uo = 6.679·10−7 m3·s−1 (from Equation 6)
- J23 = 1.217 (from Equation 5)
- VN = 1.485·10−4 m3 (from Equation 4)
- P1* = 1871.0 Pa (from [30])
- M1 = 114.2285 g·mol−1 (from [30])
- B11 = −4.496·10−3 m3·mol−1 (from [30])
- V1* = 1.6256·10−4 m3·mol−1 (from [30])
- V2* = 2.1818·10−4 m3·mol−1 (calculated from density from [19])
- ρ1 = 0.70268 g·cm−3 (from [30])
- ρ2 = 1.03627 g·cm−3 (from [19])
- χ12∞ = 4.463 (from Equation 2)
- δ1 = 15486 (J·m3)0.5 (from [30])
Analogous calculations were made for the rest of solutes. The results are presented in the Table 1S. Based on these values the Equation 7 can be plotted (see Figure S1).
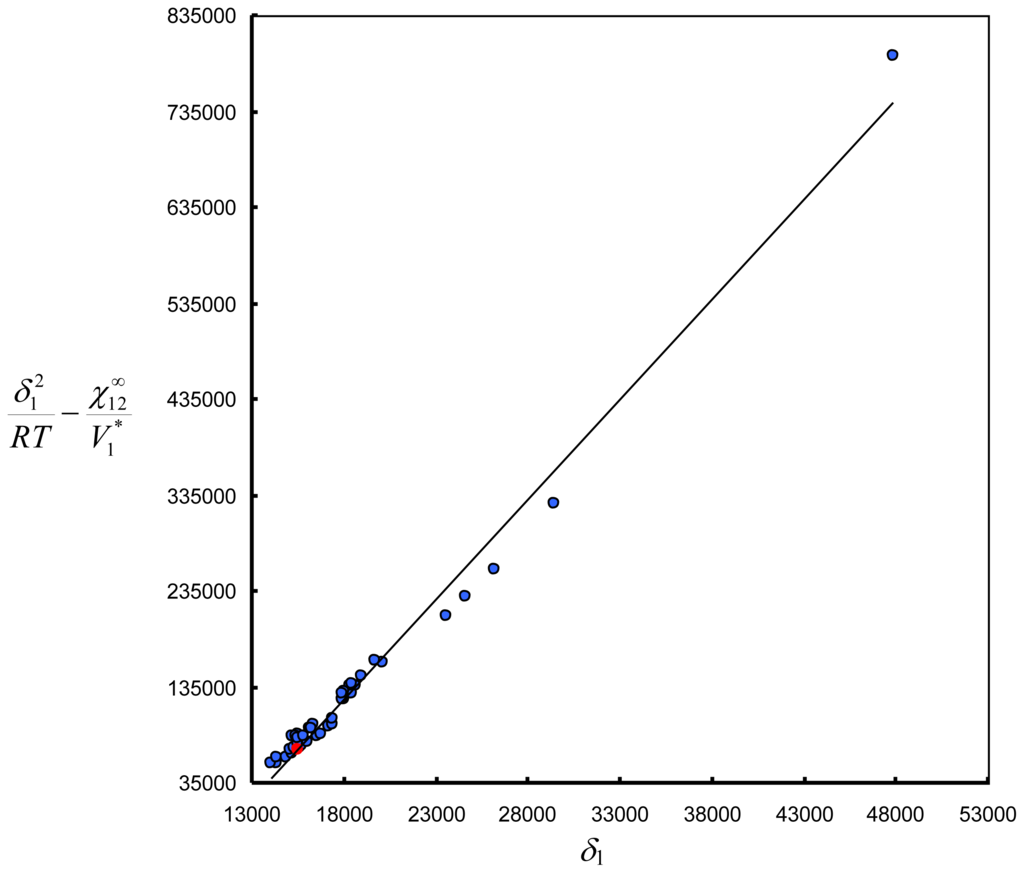
Figure S1.
An example of the determination of solubility parameter δ2. Plot of
versus δ1 according to the Equation 7 for ionic liquid [emim][TCB] at T = 298.15 K. (
 ) n-octane, (
) n-octane, (
 ) rest of solutes.
) rest of solutes.
 ) n-octane, (
) n-octane, (
 ) rest of solutes.
) rest of solutes.
From the slope (2δ2/RT) the value of 20.874 is obtained. From this value the δ2 is calculated giving value 25.9 MPa0.5 (see Table 2).
Calculation of the Kv Constant from Equation 2
Using data presented in the Table S2 and the Kv value of 7.8 the solubility parameters were determined using Equation 2. Then the Kv value was optimized using the objective function
using MS Excel Solver. Densities and viscosities were taken from the ILThermo database available at http://ilthermo.boulder.nist.gov/ILThermo/. Solubility parameters were calculated from enthalpies of vaporization [25–29].

Table S2.
Data used in calculation of Kv constant.
| Ionic Liquid | ρ/g·cm−3 | M/g·mol−1 | μ/mPa·s | υ/cm3·mol−1 | δ2/MPa0.5 |
|---|---|---|---|---|---|
| [emim][NTf2] | 1.5192 | 391.32 | 34.29 | 257.6 | 21.3 |
| 1.5192 | 391.32 | 34.29 | 257.6 | 22.6 | |
| 1.5192 | 391.32 | 34.29 | 257.6 | 22.7 | |
| [bmim][NTf2] | 1.4366 | 419.37 | 50.70 | 291.9 | 21.2 |
| 1.4366 | 419.37 | 50.70 | 291.9 | 19.8 | |
| 1.4366 | 419.37 | 50.70 | 291.9 | 22.9 | |
| [hmim][NTf2] | 1.3706 | 447.42 | 70.96 | 326.5 | 20.5 |
| 1.3706 | 447.42 | 70.96 | 326.5 | 19.0 | |
| 1.3706 | 447.42 | 70.96 | 326.5 | 22.9 | |
| [omim][NTf2] | 1.3206 | 475.48 | 92.51 | 360.1 | 20.2 |
| 1.3206 | 475.48 | 92.51 | 360.1 | 20.2 | |
| 1.3206 | 475.48 | 92.51 | 360.1 | 23.0 | |
| 1.3206 | 475.48 | 92.51 | 360.1 | 18.9 | |
| [dmim][NTf2] | 1.2780 | 499.50 | 108.20 | 390.8 | 17.8 |
| [bmPYR][NTf2] | 1.3940 | 422.41 | 76.92 | 303.0 | 22.2 |

Table S3.
Densities and viscosities for [N-C3OHPY][NTf2], [pmPIP][NTf2] and [bmPIP][NTf2] ionic liquids.
| Ionic Liquid | T/K | ρ/g·cm−3a | μ/m Pa·s b |
|---|---|---|---|
| [N-C3OHPY][NTf2] | 308.15 | 1.5451 | 67.03 |
| 318.15 | 1.5357 | 43.85 | |
| 328.15 | 1.5266 | 30.56 | |
| 338.15 | 1.5175 | 22.21 | |
| 348.15 | 1.5085 | 16.90 | |
| [pmPIP][NTf2] | 308.15 | 1.4010 | 86.70 |
| 318.15 | 1.3923 | 55.24 | |
| 328.15 | 1.3837 | 37.75 | |
| 338.15 | 1.3751 | 27.17 | |
| 348.15 | 1.3666 | 20.32 | |
| [bmPIP][NTf2] | 308.15 | 1.3706 | 97.76 |
| 318.15 | 1.3621 | 61.05 | |
| 328.15 | 1.3536 | 40.92 | |
| 338.15 | 1.3452 | 29.01 | |
| 348.15 | 1.3369 | 21.28 | |
adetermined using Anton Paar DMA 4500 densitometer;
bdetermined using Anton Paar AMVn viscometer.
References
- Voelkel, A; Strzemiecka, B; Adamska, K; Milczewska, K. Inverse gas chromatography as a source of physiochemical data. J. Chromatogr. A 2009, 1216, 1551–1566. [Google Scholar]
- Marciniak, A. The solubility parameters of ionic liquids. Int. J. Mol. Sci 2010, 11, 1973–1990. [Google Scholar]
- Revelli, A-L; Mutelet, F; Jaubert, J-N. Partition coefficients of organic compounds in new imidazolium based ionic liquids using inverse gas chromatography. J. Chromatogr. A 2009, 1216, 4775–4786. [Google Scholar]
- Mutelet, F; Jaubert, J-N. Measurement of activity coefficients at infinite dilution in 1-hexadecyl- 3-methylimidazolium tetrafluoroborate ionic liquid. J. Chem. Thermodyn 2007, 39, 1144–1150. [Google Scholar]
- Mutelet, F; Butet, V; Jaubert, J-N. Application of inverse gas chromatography and regular solution theory for characterization of ionic liquids. Ind. Eng. Chem. Res 2005, 44, 4120–4127. [Google Scholar]
- Foco, GM; Bottini, SB; Quezada, N; de la Fuente, JC; Peters, CJ. Activity coefficients at infinite dilution in 1-alkyl-3-methylimidazolium tetrafluoroborate ionic liquids. J. Chem. Eng. Data 2006, 51, 1088–1091. [Google Scholar]
- Barton, AFM. Solubility parameters. Chem. Rev 1975, 75, 731–753. [Google Scholar]
- Hansen, CM. Hansen Solubility Parameters: A User’s Handbook, 2nd ed; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Camper, D; Scovazzo, P; Koval, C; Noble, R. Gas Solubilities in room-temperature ionic liquids. Ind. Eng. Chem. Res 2004, 43, 3049–3054. [Google Scholar]
- Lee, SH; Lee, SB. The Hildebrand solubility parameters, cohesive energy densities and internal energies of 1-alkyl-3-methylimidazolium-based room temperature ionic liquids. Chem Commun 2005, 3469–3471. [Google Scholar]
- Kilaru, PK; Scovazzo, P. Correlations of low-pressure carbon dioxide and hydrocarbon solubilities in imidazolium-, phosphonium-, and ammonium-based room-temperature ionic liquids. Part 2. Using activation energy of viscosity. Ind. Eng. Chem. Res 2008, 47, 910–919. [Google Scholar]
- Moganty, SS; Baltus, RE. Regular solution theory for low pressure carbon dioxide solubility in room temperature ionic liquids: Ionic liquid solubility parameter from activation energy of viscosity. Ind. Eng. Chem. Res 2010, 49, 5846–5853. [Google Scholar]
- Jin, H; O’Hare, B; Dong, J; Arzhantsev, S; Baker, GA; Wishart, JF; Benesi, AJ; Maroncelli, M. Physical properties of ionic liquids consisting of the 1-butyl-3-methylimidazolium cation with various anions and the bis(trifluoromethylsulfonyl)imide anion with various cations. J. Phys. Chem. B 2008, 112, 81–92. [Google Scholar]
- Swiderski, K; McLean, A; Gordon, CM; Vaughan, DH. Estimates of internal energies of vaporisation of some room temperature ionic liquids. Chem Commun 2004, 2178–2179. [Google Scholar]
- Paduszyński, K; Chiyen, J; Ramjugernath, D; Letcher, TM; Domańska, U. Liquid-liquid phase equilibrium of (piperidinium-based ionic liquid + an alcohol) binary systems and modelling with NRHB and PCP-SAFT. Fluid Phase Equilib 2011, 305, 43–52. [Google Scholar]
- Camper, D; Becker, C; Koval, C; Noble, R. Low pressure hydrocarbon solubility in room temperature ionic liquids containing imidazolium rings interpreted using regular solution theory. Ind. Eng. Chem. Res 2005, 44, 1928–1933. [Google Scholar]
- Marciniak, A; Wlazło, M. Activity Coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-(3-hydroxypropyl)pyridinium trifluorotris(perfluoroethyl) phosphate. J. Phys. Chem. B 2010, 114, 6990–6994. [Google Scholar]
- Marciniak, A. Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(3-hydroxypropyl)pyridinium bis(trifluoromethylsulfonyl)- amide. J Chem Thermodyn 2011. [Google Scholar] [CrossRef]
- Domańska, U; Królikowska, M; William, EA; Baker, GA. Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-ethyl-3- methylimidazolium tetracyanoborate. J. Chem. Thermodyn 2011, 43, 1050–1057. [Google Scholar]
- Domańska, U; Marciniak, A. Physicochemical properties and activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-decyl-3-methylimidazolium tetracyanoborate. J. Phys. Chem. B 2010, 114, 16542–16547. [Google Scholar]
- Domańska, U; Królikowska, M. Measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-butyl-1-methylpiperidinium thiocyanate. J. Chem. Eng. Data 2011, 56, 124–129. [Google Scholar]
- Domańska, U; Paduszyński, K. Measurements of activity coefficients at infinite dilution of organic solutes and water in 1-propyl-1-methylpiperidinium bis{(trifluoromethyl)sulfonyl}imide ionic liquid using g.l.c. J. Chem. Thermodyn 2010, 42, 1361–1366. [Google Scholar]
- Paduszyński, K; Domańska, U. Limiting activity coefficients and gas-liquid partition coefficients of various solutes in piperidinium ionic liquids–measurements and LSER calculations. J Phys Chem B 2011. in submit. [Google Scholar]
- Domańska, U; Zawadzki, M; Królikowska, M; Tshibangu, MM; Ramjugernath, D; Letcher, TM. Measurements of activity coefficients at infinite dilution of organic compounds and water in isoquinolinium-based ionic liquid [C8iQuin][NTf2] using GLC. J. Chem. Thermodyn 2011, 43, 499–504. [Google Scholar]
- Luo, H; Baker, GA; Dai, S. Isothermogravimetric determination of the enthalpies of vaporization of 1-alkyl-3-methylimidazolium ionic liquids. J. Phys. Chem. B 2008, 112, 10077–10081. [Google Scholar]
- Armstrong, JP; Hurst, C; Jones, RG; Licence, P; Lovelock, KRJ; Satterley, CJ; Villar-Garcia, IJ. Vapourisation of ionic liquids. Phys. Chem. Chem. Phys 2007, 9, 982–990. [Google Scholar]
- Santos, LMNBF; Lopes, JNC; Coutinho, JAP; Esperança, JMSS; Gomes, LR; Marrucho, IM; Rebelo, LPN. Ionic liquids: First direct determination of their cohesive energy. J. Am. Chem. Soc 2007, 129, 284–285. [Google Scholar]
- Zaitsau, DH; Kabo, GJ; Strechan, AA; Paulechka, YU; Tschersich, A; Verevkin, SP; Heintz, A. Experimental vapor pressures of 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. J. Phys. Chem. A 2006, 110, 7303–7306. [Google Scholar]
- Deyko, A; Lovelock, KRJ; Corfield, J-A; Taylor, AW; Gooden, PN; Villar-Garcia, IJ; Licence, P; Jones, RG; Krasovskiy, VG; Chernikova, EA; et al. Measuring and predicting ΔvapH298 values of ionic liquids. Phys. Chem. Chem. Phys 2009, 11, 8544–8555. [Google Scholar]
- DIPPR Project 801-Full Version; Design Institute for Physical Property Data/AIChE: New York, NY, USA, 2010.
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).