Synthesis and Crystal Structure of Hexakis(imidazole) nickel (II) O,O′-diphenyldithiophosphate [Ni(Im)6](Ph2O2PS2)2
Abstract
:Introduction
Results and Discussion
X-ray Crystal Structure of the Title Compound
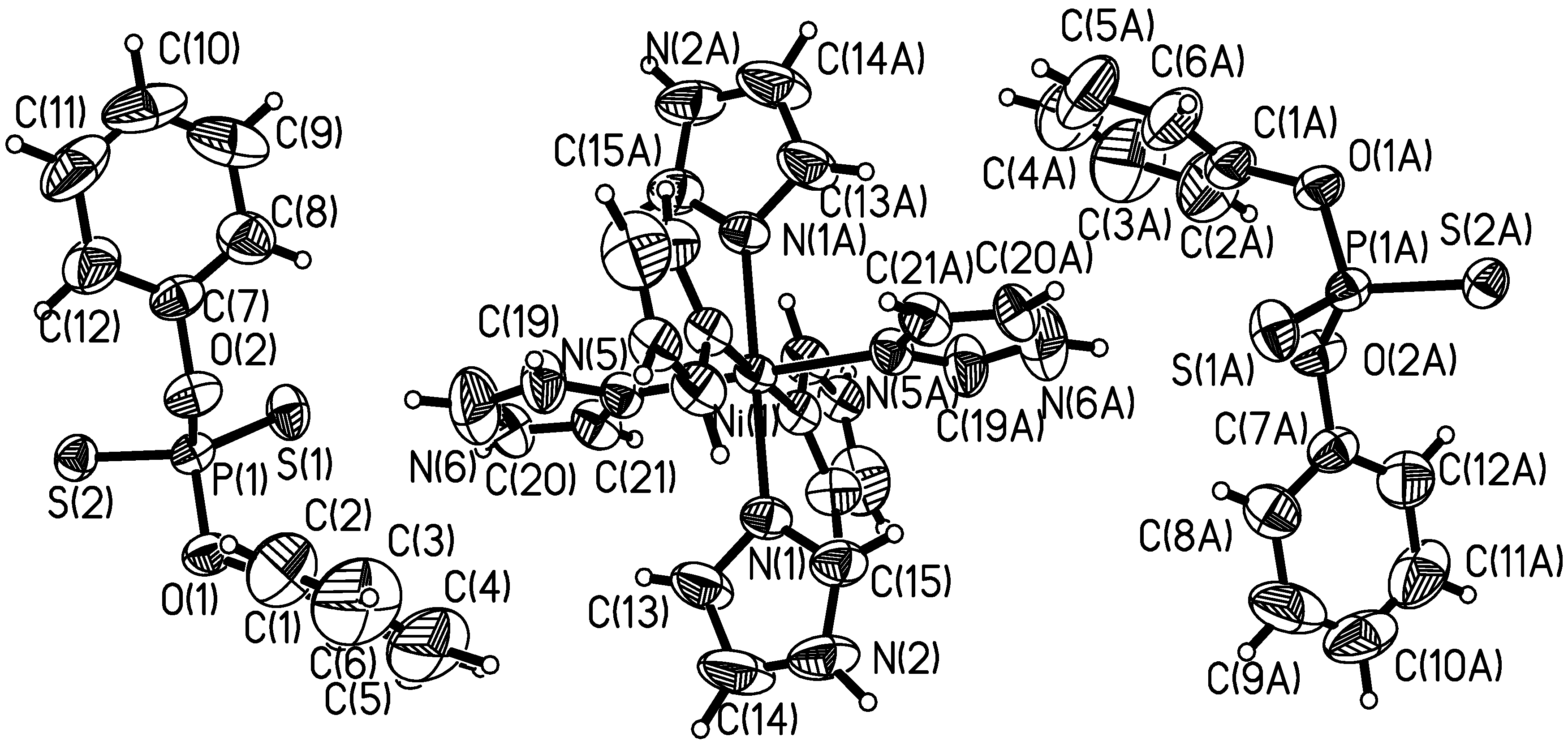
IR Spectroscopy
| Atom | x | y | z | Ueq |
| Ni1 | 0.50000 | 0.00000 | 0.50000 | 0.03408 |
| S1 | -0.02504 | 0.11075 | 0.11362 | 0.05583 |
| S2 | 0.23540 | 0.33035 | 0.36706 | 0.04651 |
| P1 | 0.11983 | 0.28764 | 0.20898 | 0.04016 |
| O1 | 0.25663 | 0.34717 | 0.16152 | 0.05387 |
| O2 | 0.02372 | 0.37457 | 0.19719 | 0.05210 |
| N1 | 0.70819 | 0.07677 | 0.45909 | 0.04516 |
| N2 | 0.86587 | 0.12438 | 0.36401 | 0.07807 |
| N3 | 0.49052 | 0.17820 | 0.55780 | 0.04184 |
| N4 | 0.44719 | 0.34158 | 0.64827 | 0.09284 |
| C1 | 0.22540 | 0.34804 | 0.05361 | 0.06202 |
| C2 | 0.20081 | 0.44556 | 0.03942 | 0.09705 |
| C3 | 0.17904 | 0.45204 | -0.06446 | 0.14530 |
| C4 | 0.18613 | 0.36170 | -0.15079 | 0.15496 |
| C5 | 0.20809 | 0.25992 | -0.13735 | 0.13347 |
| C6 | 0.22857 | 0.25275 | -0.03206 | 0.09396 |
| C7 | -0.10835 | 0.36540 | 0.23377 | 0.05025 |
| C8 | -0.25918 | 0.27493 | 0.16484 | 0.07322 |
| Ni1-N1 | 2.133(3) | S1-P1 | 1.955 (2) |
| Ni1- N3 | 2.132(3) | S2-P1 | 1.959 (2) |
| Ni1-N5 | 2.125(3) | P1-O1 | 1.609(2) |
| C1-C2 | 1.355(7) | P1-O2 | 1.632(2) |
| C1-C6 | 1.376(7) | O1-C1 | 1.405(4) |
| O1-P1-O2 | 97.47(13) | C1-C2-C3 | 119.7(6) |
| O1-P1-S2 | 105.29(11) | C4-C3-C2 | 119.6(7) |
| S1-P1-S2 | 117.31(7) | N5-Ni1-N3 | 90.40(11) |
| N5-Ni1-N1 | 90.81(11) | N3-Ni1-N1 | 91.05(11) |
| C14-C13-N1 | 109.2(4) | N1-C15-N2 | 111.6(4) |
| D | H | A | symm | D-H | H…A | D…A | D-H…A Bond angle |
| N(2) | H(8A) (2a) | S(1) | 0.9287 | 2.7403 | 3.6547 | 168.35 | |
| C(17) | H(4A) | S(2) | 2766 | 0.9224 | 2.3836 | 3.3052 | 177.03 |
| C(11) | H(11A) | S(2) | 2766 | 0.9467 | 2.8717 | 3.7945 | 165.15 |
| C(20) | H(20A) | S(1) | 2756 | 0.9170 | 2.5156 | 3.3807 | 157.42 |
Experimental
General
Synthesis of the complex [Ni(Im)6](dtp)2
Crystallographic Data and Structure Determination [9]
| Formula | C42H44N12O4P2S4Ni1 |
| Formula weight | 1029.78 |
| Color/shape | Blue/prism |
| Crystal system | Triclinic |
| Space group | Pī |
| a / Å | 9.375(2) |
| b/ Å | 12.324(3) |
| c/ Å | 13.285(3) |
| α/° | 107.86(3) |
| β/° | 102.28(3) |
| γ/° | 109.24(3) |
| V/ Å3 | 1292(5) |
| Z value | 1 |
| D(calcd.)/g.cm-3 | 1.307 |
| μ/ mm-1 | 0.65 |
| F (000) | 534 |
| Crystal size/mm | 0.20×0.24×0.20 |
| Temp. /K | 293(2) |
| θ ranges/° | 0.995-25.04 |
| h/k/l | -10,11/-14,0/-15,15 |
| Reflections collected | 4551 |
| Independent reflections | 3843 |
| Absorption correction | Empirical |
| No. restrains/ No. parameters | 0/295 |
| GOF | 1.085 |
| Final R indices[I>2σ(I)] | R1=0.0473 wR2=0.1246 |
| R indices(all data) | R1=0.0604 wR2=0.1388 |
| Largest peak and hole/e·nm-3 | 660 and -490 |
Acknowledgements
References and Notes
- Brooks, P.; Davidson, N. J. Amer. Chem. Soc. 1960, 82, 2118.
- Davis, W. J.; Smith, J. J. Chem. Soc. (A) 1971, 317.
- Inova, M.; Kishita, M.; Kabo, M. Bull. Chem. Soc. Jpn. 1969, 39, 1352.
- Eillbeck, W.J.; Holmes, F.; Underhill, A.E. J. Chem. Soc. (A) 1967, 757.
- Xu, L. Z.; Zhao, P. S.; Zhang, S. S. Chin. J. Chem. 2001, 19, 436.
- Bernarducci, E. E.; Bharadwij, P. K. Inorg. Chem. 1983, 22, 3911.
- Tamura, H.; Imai, H.; Kuwahra, J.; Sugiura, Y. J. Amer. Chem. Soc. 1987, 109, 6870.
- Jian, F. F.; Wang, Z.X.; Bai, Z. P.; You, X. Z. J. Chem. Cryst. 1999, 29, 359.
- CCDC 212159 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
- Sheldrick, G. M. SHELXTL V5 Reference Manual, Siemens Analytical X-ray Systems; Inc., Madison: Wisconsin (USA), 1996. [Google Scholar]
- Wilson, A.J. International Table for X-Ray Crystallography; Vol C, Kluwer Academic Publishers: Dordrecht, 1992. [Google Scholar]
- Samples Availability: Available from the corresponding author.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhang, S.; Wang, S.; Wen, Y.; Jiao, K. Synthesis and Crystal Structure of Hexakis(imidazole) nickel (II) O,O′-diphenyldithiophosphate [Ni(Im)6](Ph2O2PS2)2. Molecules 2003, 8, 866-872. https://doi.org/10.3390/81200866
Zhang S, Wang S, Wen Y, Jiao K. Synthesis and Crystal Structure of Hexakis(imidazole) nickel (II) O,O′-diphenyldithiophosphate [Ni(Im)6](Ph2O2PS2)2. Molecules. 2003; 8(12):866-872. https://doi.org/10.3390/81200866
Chicago/Turabian StyleZhang, Shusheng, Shiying Wang, Yonghong Wen, and Kui Jiao. 2003. "Synthesis and Crystal Structure of Hexakis(imidazole) nickel (II) O,O′-diphenyldithiophosphate [Ni(Im)6](Ph2O2PS2)2" Molecules 8, no. 12: 866-872. https://doi.org/10.3390/81200866
APA StyleZhang, S., Wang, S., Wen, Y., & Jiao, K. (2003). Synthesis and Crystal Structure of Hexakis(imidazole) nickel (II) O,O′-diphenyldithiophosphate [Ni(Im)6](Ph2O2PS2)2. Molecules, 8(12), 866-872. https://doi.org/10.3390/81200866




