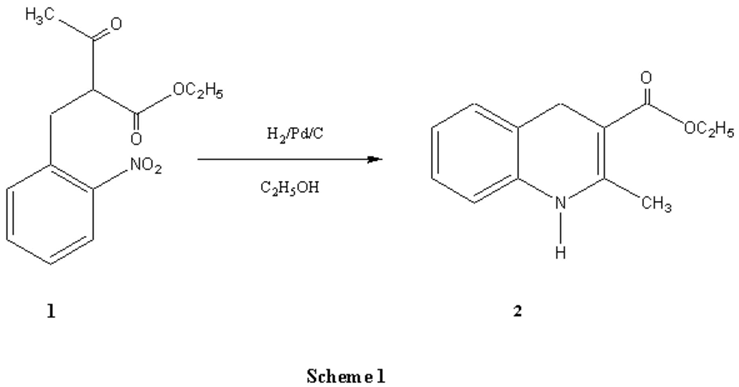
Compound 1 [1] (1.23 g, 4.94 mmol ) and 0.1 g of Pd/C (10%) in 50 ml of ethanol are introduced into an hydrogenation reactor. The mixture is first degassed under reduced pressure and then left at normal pressure. When the reaction is finished (at end of hydrogen consumption), the solution is filtered under vacuum and then concentrated under reduced pressure. The residue is purified by column chromatography on silica gel. (hexane / ethyl acetate 60/40). Compound 2 was obtained in 60% yield.
M.P.130-132 °C.
TLC ( Hexane/EtOAc 6:4 ): Rf 0.60.
IR ( KBr, cm–1) : 3395, 1725, 1608.
1H-NMR ( CDCl3 ) : 6.43 – 7.04 (m ,4H); 4 (q, 2H. J= 7.2Hz ); 4.02 (broad, NH); 3.04 (q, 2H, JAB= 17.1Hz); 1.56 (s, 3H); 1.1 (t, 3H, J= 7.2Hz).
NMR : 4.02 (broad, NH).
MS (EI): m/z : 217; 216; 188; 144; 106 (100); 77.
References
- Gordon, E. H.; Hughes, D.; Rae, D.; Rhodes, A.P. Tetrahedron Letters 1967, 3, 241–246.
Sample Availability: Available from the authors and from MDPI. |
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/.