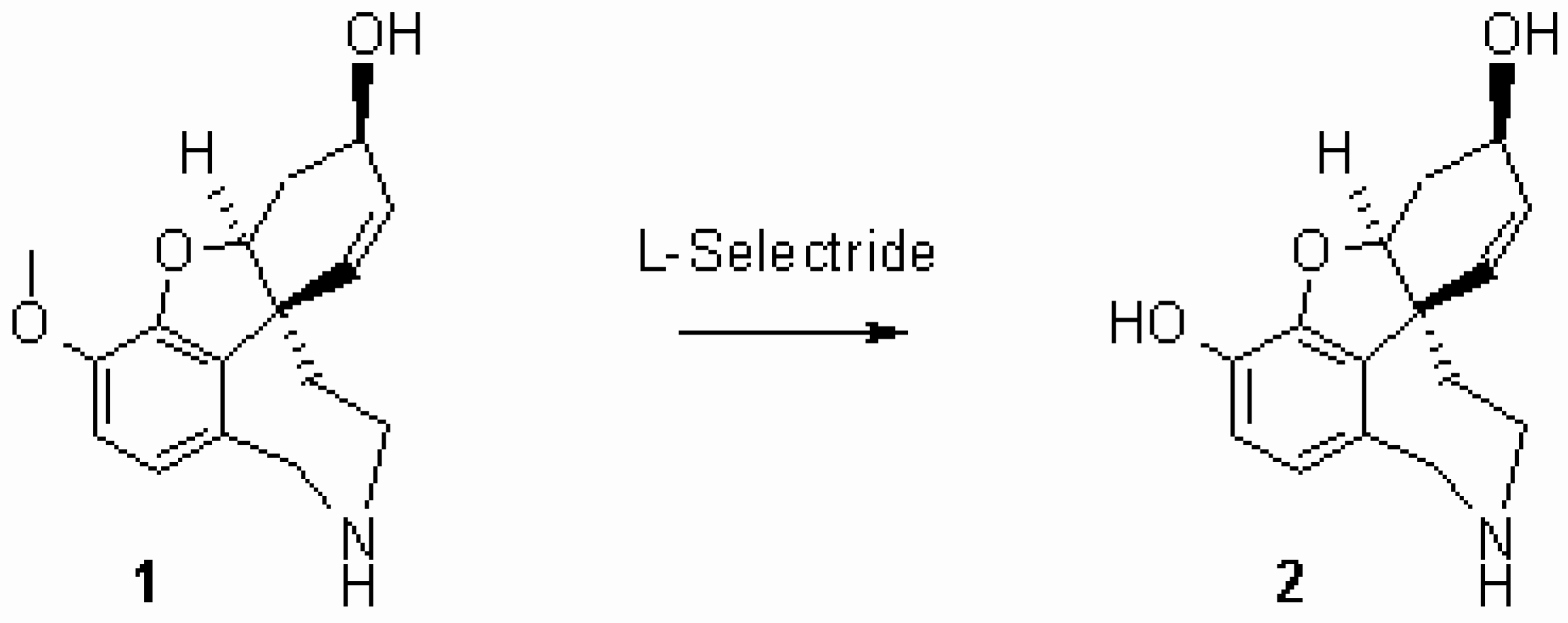
Norsanguinine (2) is an alkaloid isolated from the bulbs of L. sanguinea [1] and synthesized as racemate by Kita [2]. We report the first synthesis of chiral norsanguinine starting from N-norgalanthamine (1) [3] by O-demethylation using L-Selectride® [4]. To a solution of norgalanthamine (1.0 g, 3.66 mmol) in dry THF (40 mL) L-Selectride® (17.0 mL, 17.0 mmol, 1 M in THF) was added and refluxed for 24 h. The solution was cooled to ambient temperature, then EtOAc (20 mL) and distilled water (100 mL) were added. The layers were separated, and the organic layer was extracted with distilled water (4 x 20 mL). The combined aqueous layers were washed with EtOAc (2 x 20 mL) and concentrated in vacuo. The residue was purified by column chromatography (100 g SiO2, CHCl3 : MeOH : conc. NH3 = 90 : 9 : 1) and triturated with acetone (5 mL). Yield: colorless crystals (0.78 g, 82%).
Mp. 142 - 144 °C (lit [1] 141 – 142 °C).
TLC: CHCl3: MeOH : conc. NH3= 89 : 10 : 1, Rf = 0.15.
[α]D20 = -54° (c 0.1, MeOH) (lit [1] [α]D20 = -14° (c 0.1, CHCl3) ) [5].
1H NMR (DMSO-d6): δ 6.40 (d, J = 8.3 Hz, 1H), 6.49 (d, J = 8.3 Hz, 1H), 6.02 (d, J = 11.2 Hz, 1H), 5.79 (dd, J = 11.2 Hz, J = 5.4 Hz, 1H), 4.43 (s, 1H), 4.08 (s, 1H), 3.90 (d, J = 15.9 Hz, 1H), 3.71 (d, J = 15.9 Hz, 1H), 3.19 - 2.92 (m, 2H), 2.31 (d, J = 15.9 Hz, 1H), 2.12 - 1.94 (m, 1H), 1.79 - 1.56 (m, 2H).
13C NMR (DMSO-d6): δ 145.6 (s), 140.4 (s),133.0 (s),132.3 (s),127.7 (d), 127.6 (d), 119.6 (d), 114.8 (d), 86.5 (d), 60.1 (d), 53.1 (t), 48.3 (s), 46.6 (t), 40.2 (t), 30.8 (t).
Anal. Calcd for C15H17NO3: C, 69.48; H, 6.61; N, 5.40. Found: C, 69.26; H, 6.84; N, 5.38.
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes:
- Abdallah, O. M. Phytochemistry 1995, 39, 477–478.
- Kita, Y.; Arisawa, M.; Gyoten, M.; Nakajima, M.; Hamada, R.; Tohma, H.; Takada, T. J. Org. Chem. 1998, 63, 6625–6633.
- Thal, C.; Guillou, C.; Mary, A.; Renko, D.; Potier, P.; Christen, Y. Preparation and pharmaceutical compositions of galanthamine derivatives. WO 9703987, 1997. [Chem. Abstr.: 126:212284]. [Google Scholar]
- Coop, A.; Janetka, J. W.; Lewis, J. W.; Rice, K. C. J. Org. Chem. 1998, 63, 4392–4396.
- Pure 2 was found to be almost insoluble in CHCl3.
- Sample Availability: Available from the author and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/