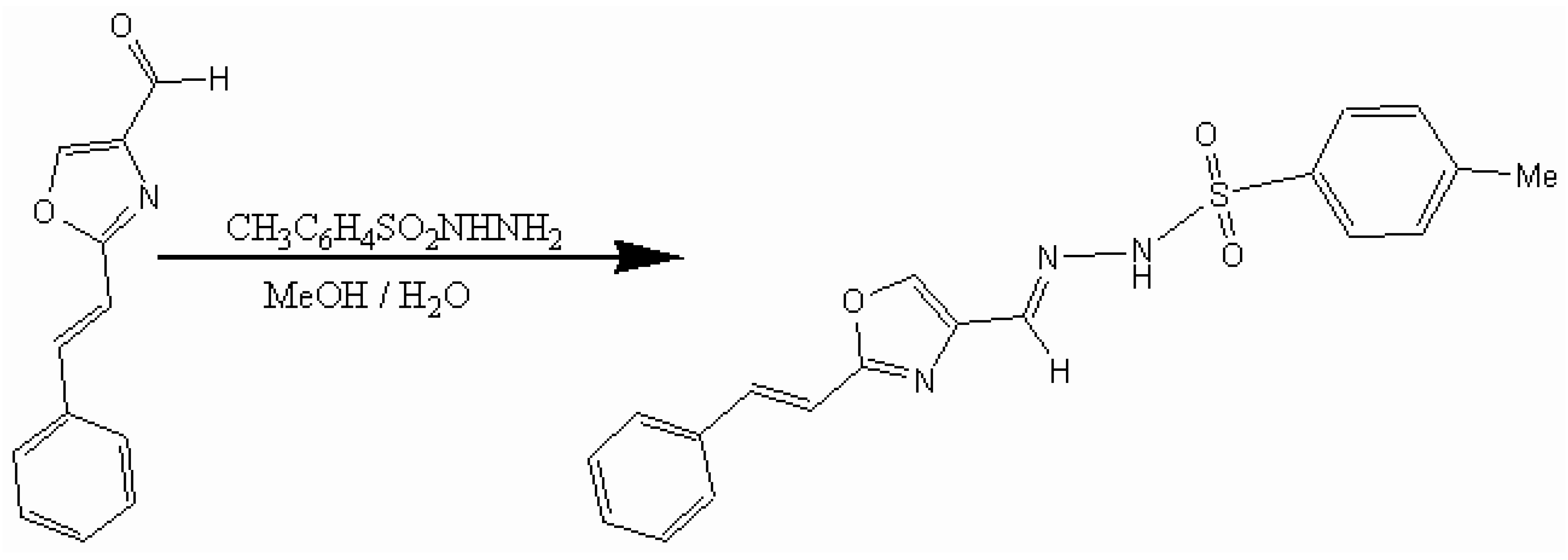
To a stirred solution of p-toluenesulfonhydrazide (0.61g, 3.3 mmol) in methanol/water (6:4, 20 ml) at 60°C was added 2-cinnamyl-1,3-oxazole-4-carboxaldehyde [1](0.50 g, 2.5 mmol). The solution was cooled to room temperature and stored in the refrigerator overnight. The precipitate which formed was collected by filtration and washed with methanol/water mixture (6:4, 10 mL) to give 2-cinnamyl-1,3-oxazole-4-carboxaldehyde p-toluenesulfonyl hydrazone as a colorless solid (0.66 g, 72%).
M.p. 177-179 °C.
1H NMR (CDCl3): 12.04 (s, 1H); 7.93-7.82 (m, 3H); 7.64-7.62 (m, 3H); 7.46-7.43 (m, 3H); 7.42-7.34 (m, 3H); 6.90 (d, J=16.7, 1H); 2.42 (s, 3H).
13C NMR (CDCl3): 163.0, 144.7, 139.0, 138.9, 137.3, 136.0, 135.3, 129.9, 129.8, 129.5, 128.9, 127.8, 127.4, 114.4, 21.4.
IR (neat): νmax 3149, 2934, 2919, 1716, 1648, 1060, 962, 805cm-1.
Anal.calc. for C19H17N3O3S (367.43): C 62.11, H 4.66, N 11.44; found: C 62.15, H 4.73, N 11.26.
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgment
The Financial support of the National Institute of Health (GM-42641) and the Marquette University Graduate School is acknowledged. The author would like to thank Dr. William A. Donaldson (E-mail: william.donaldson@marquette.edu) for his encouragement.
References
- Evans, D.A.; Fitch, D.M.; Smith, T.E.; Cee, V.J. Application of Complex Aldol Reactions to the total Synthesis of Phorboxazole B. J. Am. Chem. Soc. 2000, 122, 10033–10046. [Google Scholar] [CrossRef]
- Sample Availability: Available from the author and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/