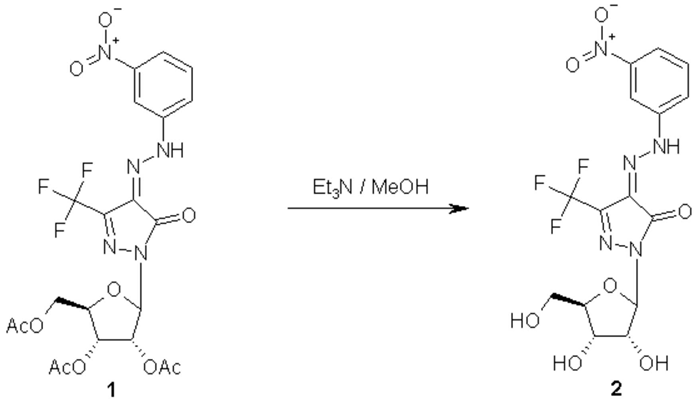
The desired compound 2 was obtained by complete deprotection of the acetylated nucleoside 1 [1] using triethylamine [2]. To a solution of 1 (0.9g, 1.4 mmol) in methanol (25 ml) was added triethylamine (2 ml). The mixture was stirred at room temperature and the reaction was followed by tlc. After complete deprotection (24 hours), the reaction mixture was evaporated and coevaporated with methanol (3 x 30 ml), then chromatographed over silica gel using CH2Cl2/MeOH (92:8 v/v) to give 0.56g (90%) of 2 as yellow powder.
Rf (CH2Cl2/MeOH, 94/6 v/v): 0.2.
UV (lmax, 95% ethanol): 255, 415.
MS (m/z): 433.
1H-NMR (250 MHz, DMSO-d6): 3.39-3.54(m, 2H, H-5`, H-5``); 3.82-3.87(m, 1H, H-4`); 4.07(t, 1H, H-3`); 4.36(t, 1H, H-2`); 5.63(d, 1H, H-1`, J1`,2`=4.95); 7.74-8.55(m, 4H, aromatic CH).
13C-NMR (75 MHz, DMSO-d6): 61.92(C-5`), 70.35(C-3`); 71.83(C-2`); 84.80(C-4`); 86.10(C-1`); 111.95, 117.51, 120.66, 121.12, 123.90, 131.04(6 aromatic carbons); 136.63(q, CF3); 142.71(C-4); 148.54(C-5); 156.06(C-3).
Supplementary Materials
References
Sample Availability: Available from the authors. |
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.
