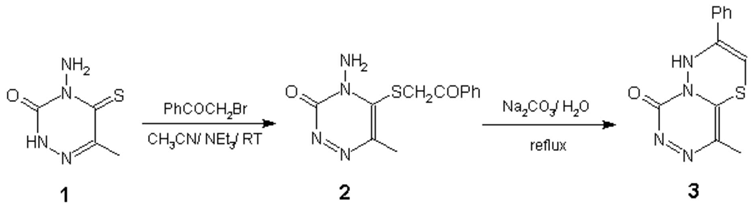
4-Amino-6-methyl-5-thio-1,2,4-triazin-3-one 1 was reacted with phenacyl bromide in the presence of triethylamine in acetonitrile to give the corresponding 5-phenacylthio derivative 2. The latter was refluxed in aqueous solution of sodium carbonate to afford a novel heterocyclic system 3 (Scheme). Compound 1 (0.316 g, 2 mmol) was dissolved in a solution of CH3CN (10 mL) and triethylamine (2 mL). To this solution phenacyl bromide (0.4 g, 2 mmol) was added. The reaction mixture was stirred at room temperature for 5 hrs. The solvent was evaporated to dryness and the residue was crystallized from methanol to afford 2. Compound 2 was refluxed in a solution of sodium carbonate (0.5 g in water (6 mL)) for 2 hrs. The solution was neutralized by addition of 2N HCl and extracted with CHCl3. The solvent was evaporated to dryness and the residue was directly subjected to column chromatography (CHCl3:MeOH; 98:2) to afford 3.
Selected Data for 2. Yield: 71%, mp.: 202-3 °C, 1HNMR (DMSO, d6) d, 2.3(s, 3H, Me), 5.6(s, 2H, NH2, exchangeable with D2O), 7.6 and 8.8 (m, 5H, Ph). IR, ṽ (KBr disc): 3400, 3350, 1690, 1580, 1400, 1210 cm−1, M.S., m/z, M+, 276(65), 275(8), 105(58.5), 104(100), 103(63), 89(70), 80(74), 77(63).
Selected Data for 3. Yield: 50%, mp.: 230-2 °C, 1HNMR (DMSO, d6) d, 2.3(s, 3H, Me), 5.3(s, 2H, NH, exchangeable with D2O), 5.4 (s, 1H, =CH), 7.2-8.3(m, 5H, Ph). IR, ṽ (KBr disc): 3490, 1690, 1410, 800 cm−1, M.S., m/z, M+, 258(3), 255(11), 103(100), 71(44), 42(41).
References
- Heravi, M.M.; Rajabzadeh, Gh.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M. P, S, Si and Related Elements. 2000. submitted. [Google Scholar]
Sample Availability: Available from the authors and from MDPI. |
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/.