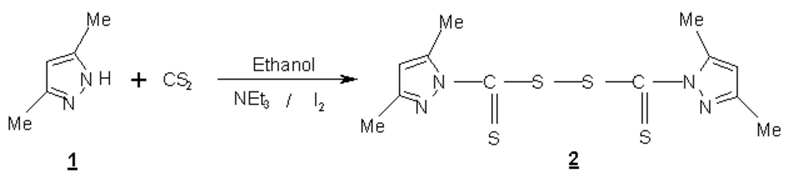
This experiment is performed according to literature method [1,2,3,4]. 3,5-Dimethyl pyrazole 1 (2,3 g; 0.024 mole) in ethanol solution and triethylamine (6.65 g, 0.048 mole) were cooled to 5°C under stirring, then carbon disulfide (3.65 g, 0.048 mole) was added to the solution. After 1 hour of stirring, solid iodine (2,8 g, 0.022 mol) was added in portions and stirred until the colour disappeared completely. Then a methanolic solution of iodine was added dropwise until a faint colour persists. Excess of iodine was neutralised with Na2S2O3solution. The product was extracted with diethyl ether, washed thrice with water, dried over Na2SO4, filtered, and diethyl ether was evaporated at room temperature to give compound 2 as a white solid. Yield: 93%.
Mp.: 87-89°C (diethyl ether/hexane: 8/2).
1H-NMR (CDCl3) d (ppm): 2,43 (s, 12H, CH3); 6,00 (s, 2H, Hpyrazole).
13C-NMR(CDCl3) d :193 ppm (-C=S), 150 (C3), 148 (C5), 110 (C4), 12 (CH3).
IR (KBr , cm-1) : 3000 (-S-S-); 1290 (C=S).
MS (m/z): 342 [M]+.
U.V.: lmax = 285 nm (-C=S).
References
- Jones, R.G.; Hanret, M.J.; Lauglin, K.M. J. Org. Chem. 1954, 19, 1428.
- Haque, S.A.; Clouet, G. Makromol. Chem. Phys. 1994, 195, 315–327.
- Reiser, A. Photoreactive Polymer. The Science and Technology of Resist; Wiley: New York, 1986; p. 26. [Google Scholar]
- El Idrissi, A.; Tebbji, K.; Radi, S. Molecules. 2001, 6, M232. [Google Scholar]
Sample Availability: Available from the authors and from MDPI. |
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/.
