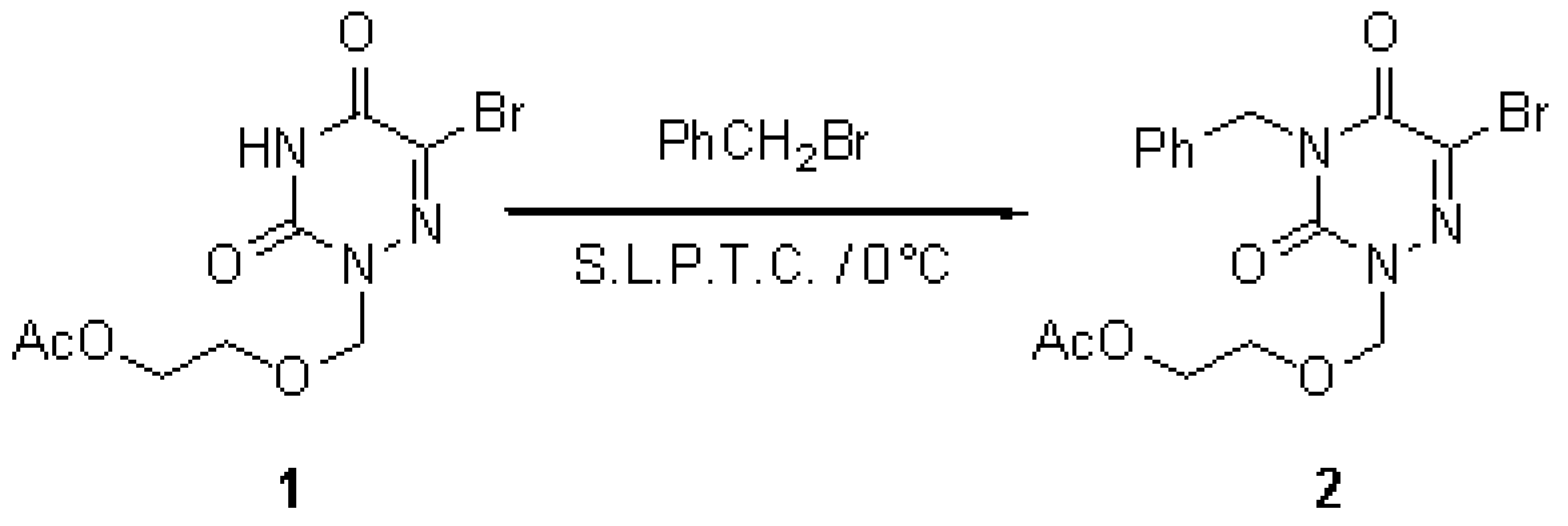
The product 2 was prepared via a direct condensation under solid-liquid phase transfer catalysis (S.L.P.T.C.) [1] conditions. To a solution of 0.02 mmole of tetraglyme in 4 ml of anhydrous THF, 0.11 mmole of potassium tert-butoxide is added. Then 0.1 mmole of the acyclonucleoside 1 [2] is added, the reaction mixture is stirred at room temperature for 15 min. The reaction mixture is cooled to 0°C and 0.11 mmole of alkylating agent in 2 ml of dry THF is added dropwise with stirring. When the addition is finished, the reaction mixture is stirred at 0°C for 30 min. The reaction mixture is then filtered and the filtrate is evaporated in vacuo to dryness. The residue is then chromatographed on a silica gel column and the expected acyclonucleoside 2 was isolated. Yield: 75 % (viscous and colourless).
Rf: 0.76 (CHCl3 / MeOH, 9/1, V/V).
1H NMR (DMSO-d6): 2.00 (s, 3H, COOCH3); 3.70 (m, 2H, OCH2CH2O); 4.10 (m, 2H, OCH2CH2O); 5.10 (s, 2H, CH2Ph); 5.25 (s, 2H, OCH2N); 7.14 (sl, 5H, C6H5).
UV (λ max (nm), H2O): 288, 263sh.
MS (m/z): 397 [M (79Br)]+, 399 [M (81Br)]+
Anal. calc. for C15H16BrN3O5: C: 45.24, H: 4.05, N: 10.55; Found: C: 45.67, H: 4.29, N: 10.18.
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgement
This work was supported by the C.N.R. (Marocco), the Deutsche Forschungsgemeinschaft and the University of Konstanz (Germany).
References
- Lazrek, H. B.; Taourirte, M.; Barascut, J. L.; Imbach, J. L. Nucleosides & Nucleotides, 1991; 10, 1285–1293.
- Purkayastha, S.; Lazrek, H. B.; Panzica, R. P.; Naguib, F. N. M.; El-Kouni, M. H. Nucleosides & Nucleotides, 1989; 8, 349–356.
- Sample Availability: Available from the authors.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/