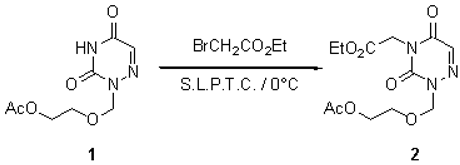
The product 2 was prepared via a direct condensation under solid-liquid phase transfer catalysis (S.L.P.T.C.) [1] conditions. To a solution of 0.02 mmole of tetraglyme in 4 ml of anhydrous THF, 0.11 mmole of potassium tert-butoxide is added. Then 0.1 mmole of the acyclonucleoside 1 [2] is added, the reaction mixture is stirred at room temperature for 15 min. The reaction mixture is cooled to 0°C and 0.11 mmole of alkylating agent in 2 ml of dry THF is added dropwise with stirring. When the addition is finished, the reaction mixture is stirred at 0°C for 30 min. The reaction mixture is then filtered and the filtrate is evaporated in vacuo to dryness. The residue is then chromatographed on a silica gel column and the expected acyclonucleoside 2 was isolated. Yield: 90 % (viscous and colourless).
Rf: 0.64 (CHCl3 / MeOH, 9/1, V/V).
1H NMR (DMSO-d6): 1.20 (t, 3H, CH3CH2); 2.00 (s, 3H, COOCH3); 3.75 (m, 2H, OCH2CH2O); 4.10 (m,
2H, OCH2CH2O); 4.20 (q, 2H, CH3CH2); 4.75 (s, 2H, NCH2); 5.25 (s, 2H, OCH2N); 7.70 (s, 1H, H5).
UV (l max (nm), H2O): 265.
MS (FAB, m/z): 316 [MH]+
Anal. calc. for C12H17N3O7: C 45.71, H 5.43, N 13.33; Found: C 45.69, H 5.40, N 13.40.
Supplementary Materials
References
- Lazrek, H. B.; Taourirte, M.; Barascut, J. L.; Imbach, J. L. Nucleosides & Nucleotides 1991, 10, 1285–1293.
- Purkayastha, S.; Lazrek, H. B.; Panzica, R. P.; Naguib, F. N. M.; El-Kouni, M. H. Nucleosides & Nucleotides 1989, 8, 349–356.
Acknowledgement: This work was supported by the C.N.R. (Marocco), the Deutsche Forschungsgemeinschaft and the University of Konstanz (Germany). Sample Availability: Available from the authors. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.