Abstract
Carbohydrate containing dimeric surfactants were synthesized starting from Dglucose. Three different spacers were used to link the sugar moieties. The critical micelle concentration (CMC) for these new compounds was determined.
Introduction
Alkyl glucosides are found in nature as glycolipids, and are biosynthesized by micro-organisms using rhamnose, sophorose and trehalose as carbohydrate sources. Industrially they are prepared from fatty alcohols and carbohydrates. These compounds have surfactant properties when the alkyl chain contains at least 4 carbon atoms. Alkyl glucosides are replacing usual non ionic surfactants due to their biodegradability and to the absence of toxic effects.
Recently, a new class (type) of surfactants, named dimeric [1] or gemini [2] have been prepared. They have 2 hydrophobic chains, 2 hydrophilic groups, and a spacer (flexible or rigid) keeping away the two polar groups.
In this communication, we report on the synthesis of dimmer surfactants from butyl α-D-glucopyranoside, and we analyze their interfacial properties.
Experimental
Gemini surfactants were prepared by condensation between suitable protected butyl α-D-glucopyranoside and acyl dichlorides [3,4] (Fig. 1). The products were characterized by spectroscopic/methods (NKr, IR., MS). Molecular formula were confirmed by elemental analysis. Critical micelle concentrations (CMK) of two compounds was determined by the maximum bubble pressure methods [5].
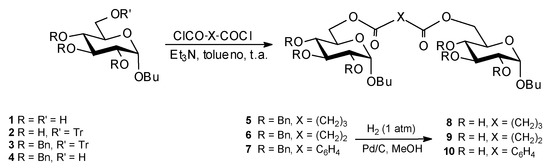
Figure 1.
Results and Discussion
CMC data showed an important (one order of magnitude) diminution of CMC of dimeric surfactants when compared to their monomeric counterpart.
On the order hand, differences in interfacial properties were observed varying the nature of the spacer and the position of linking, that can be explained from the conformation adopted by the surfactant molecule (Fig. 2).
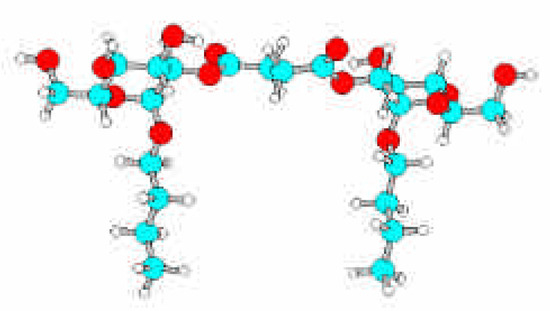
Figure 2.
Optimized structure of 1,4-bis-[2-O-(n-butyl-α-D-glucopyranosid] succinate calculated by AM1 method.
Acknowledgements
This work have been performed with financial support of Universidad de Buenos Aires (UBA) and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
References and Notes
- Zana, R.; Benrraou, M.; Rueff, R. Langmuir 1991, 7, 1072.
- Menger, F. M.; Littau, C. A. J. Am. Chem. Soc. 1991, 113, 1451. [CrossRef]
- Castro, M. J. L.; Kovensky, J.; Fernández Cirelli, A. Tetrahedron Lett. 1997, 38, 3995.
- Castro, M. J. L.; Kovensky, J.; Fernández Cirelli, A. Tetrahedron 1999, 55, 12711.
- Möbius, D.; Miller, R. Drops and Bubbles in Interfacial Research; Elsevier Science, 1998; pp. 279–326. [Google Scholar]