The resurgence of interest in Boron Neutron Capture Therapy (BNCT) as a treatment for malignant lesions has resulted in the synthesis of numerous boron compounds as candidates for clinical use. BNCT is a selective radiotherapy using boron-10 which absorbs thermal neutrons and releases high Linear Energy Transfer (LET) alpha particles by 10 B (n, α) 7 Li reaction. The alpha radiation kills cells in the range of 5-9 μm from the site of the α generation. Therefore, it is theoretically possible to kill tumor cells without affecting adjacent healthy tissues, if 10 B-compounds could be selectively delivered. Boron analogues of amino acids constitute a topic of major importance, and also peptides, antibodies, nucleosides and nucleotides [1], etc. In spite of the promising results with p-boronophenylalanine (BPA) and B12H11SH2-(Na+) (BSH), which presently attract considerable clinical interest, they display far from optimal selectivity for cancer cells.
The anatomical distribution of [3H]Harmalas binding sites was determined by quantitative autoradiography in rat brain slices [2]. They have a well know brain distribution, so these compounds, labeled with 10B are potential agents for BNCT. A general synthetic method has been developed for the rapid and efficient production of boronated Harmine.
Results And Discussion
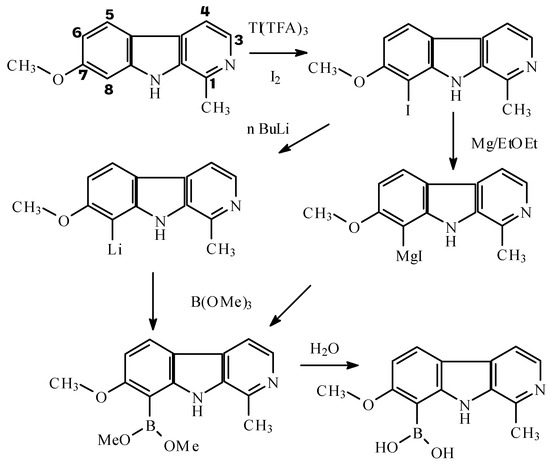
Iodination
The methods for iodination have been used previously for indolealkylamine [4] and phenethyla- mines [4] using thallium trifluoroacetate as specific oxidizing agent of molecular iodine for iodination of aromatic compounds [5].
Boronation
We used the condensation of the Grignard reagent from 8-I-Harmine with trimethylborate. This method was previously used [6] for preparation of phenol from phenylbromide and trimethylborate with formation of phenylboronic acid as intermediate and for preparation of boronic analogue of choline [7]. An alternative synthesis previously used for preparation of boronic analogues of nucleosides and nucleic bases [8,9,10,11] consist in treating the halogenated substrate with n-buthyllithium in THF followed by addition of trimethylborate at –86°C. Trimethylborate was prepared by standard procedures [12] from 95% 10B-enriched or natural isotope abundance boric acid and methanol and recovered from the formed azeotrope.
Summary and Conclusions
We have described a method for preparation of [10B]-enriched-8-dihydroxyborylharmine (III) and characterized it by their spectral properties (MS, IR and NMR). This compound is a potential BNCT agent.
Acknowledgments
Thanks are due to CONICET and Universidad de Buenos Aires (UBA; Argentina) for financial support. One of us, (J.A.S), also thank CONICET for research fellowship.
References and Notes
- Morin, C. Tetrahedron 1994, 50, 12521. [CrossRef]
- Pawlik, M.; Kaulen, P.; Baumgarten, H.G.; Rommelspacher, H. Journal of Neuroanathomy 1990, 3, 19.
- Sintas, J.A.; Vitale, A.A. J. Lab Comp. Radioparm. 1997, 39, 676.
- Sintas, J.A.; Vitale, A.A. J. Lab Comp. Radioparm. 1998, 41, 53. [CrossRef]
- McKillop, A.; Hunt, J.D.; Zelesko, M.J. J. Am. Chem Soc. 1971, 93, 4841. [CrossRef]
- Hawthorne, M.F. J. Org. Chem. 1957, 22, 1001. [CrossRef]
- Koehler, K.A.; Hess, G.P. Biochemistry 1974, 13, 5345. [CrossRef] [PubMed]
- Liao, T.K.; Prodrebarac, E.G.; Cheng, C.C. J. Am. Chem. Soc. 1964, 86, 1869. [CrossRef]
- Schinazi, R.F.; Prusoff, W.H. Tetrahedron Lett. 1978, 4981.
- Schinazi, R.F.; Prusoff, W.H. J. Org. Chem. 1985, 50, 841. [CrossRef]
- Tjarks, W.; Gabel, D.J. J. Med. Chem. 1991, 34, 315. [CrossRef] [PubMed]
- Shlesinguer, H.I.; Browz, H.C.; Mayfield, D.L. J. Am. Chem. Soc. 1953, 71, 213.
- Hall, L.W.; Odon, J.D.; Ellis, P.D. J. Am. Chem. Soc. 1975, 97, 4527. [CrossRef]