Abstract
The synthesis of some isoxazolic compounds from carbohydrate derivatives is described. These products are obtained by 1,3-dipolar cycloaddition reaction and their func-tionalization leads to derivatives with potential biological activities.
Introduction
The isoxazoles derivatives are a family of interesting compounds due to their biological activities. Some of these are used as muscle relaxants [1] and for the treatment of hypercholesteremia, arterio- sclerosis, and hyperlipidemia [2].
In previous papers we performed the synthesis of 3-glycosyl-5-substituted-2-isoxazoles by 1,3-dipolar cycloaddition, where the N-oxide came from protected carbohydrate derivatives [3]. In this work we describe the deprotection and functionalization of the polihydrated moiety as synthetic pre- cursors of new di-heterocyclic compound.
Experimental part
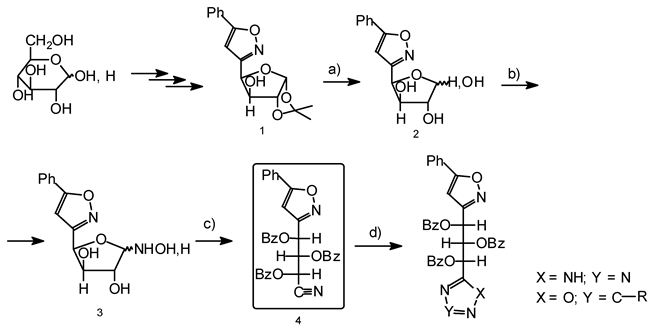
The following synthetic route is applied.
Results and discussion
The 3-(1’,2’-O-isopropylidene-α-D-xilofuranos-4’-il)-5-phenyl-2-isoxazol (1) was obtained by 1,3-dipolar cycloaddition, where the N-oxide was a glucose derivative and the dipolarophile was phen- ylacetylene. The treatment of compound 1 with acetic acid (10%) yielded compound 2. The reaction of 2 with hydroxylamine gave the oxime (3). The benzoylation of the oxime allowed us to obtain the ni- trile 4, which is the suitable synthetic intermediate to prepare different heterocyclic compound with biological interest.
All the compounds were characterized for 1H-MNR, 13C y mass spectrometry.
Acknowlegments
The authors thank to UBA and CONICET for financial support research and to UMYMFOR for mass spectra.
References and Notes
- Mitsui Toatsu Chemicals; Mitsui Seiyaku Kogyoo, K.K.; Toyama Chemical Co. Ltd. Jpn. Kokai 1994, 06, 116–146.
- Marquis, E. T.; Sanderson, J. R. US Pat. 52833356 (1994); (Chem. Abstr., 1994, 120, 217 649),
- Fascio, M. L.; Montesano, V. J.; D’Accorso, N. B. J. Carbohydrate Chem. accepted.