Abstract
The acid -promoted [3+2] cycloaddition of alkenes with benzhydrylic alcohols af- ford products in good yield and with remarkable stereoselectivity.
Introduction
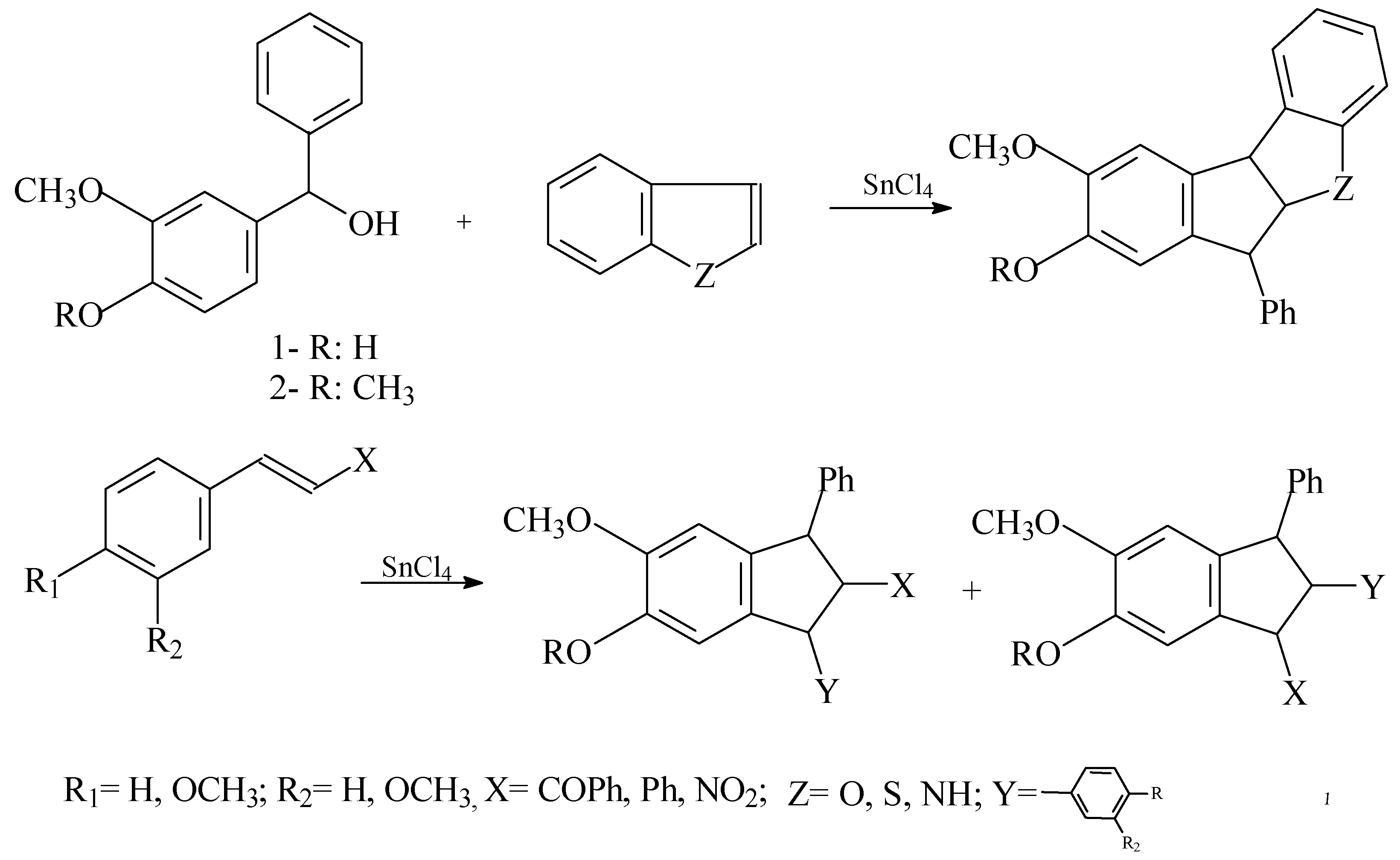
The formal [3+2] cycloaddtion, promoted by a Lewis acid, of alkenes with benzylic cations afford dihydroindenes in good yield and remarkable stereoselectivity [3]. These dihydroindene skeleton forms part of many natural products and of synthetic compounds that possess a significant biological activity [2]. These benzylic cations affords good yield of cycloadducts when the styrene appropriate provided, as long as there was a phenol para to the alcohol and at least one meta alkoxy or alkyl group. Using the diarylcarbinols 1 and 2 like the precursors of the cation and several alkenes of a varied elec- tronics wealth, we observed that was not necessary the presence of a hydroxil group para to the alcohol and that this could be replaced by metoxi group. As for the proven alkenes, those conjugated to a car- bonil group doesn't give the cycloaddition but if those gives it that they are also conjugated to a group electron-rich [3]. Following with this study we proved the cycloaddition using like alkenes: a) double bond conjugated to an aromatic ring and to a withdraw electron group and b) aromatic heterocyclics compounds condensed at benzenic ring.
Experimental
The alkene and the SnCl4 are sequentially added to a solution of alcohol in Cl2CH2. The resulting solution is stirred for a time at 0° and then poured into a solution of NaHCO3 5% Aqueous workup (NaHCO3, CH2Cl2 ) . Dry the organic extract over Na2SO4 and remove the solvent under reduced pres- sure. The purification of product is carried out in thin layer chromatography.
Results and Discussion
The stereochemical assignment for the adducts was followed directly from 1H-RMN coupling con-stants. The results obtained with the group a) show an trans-trans orientation in the products, con-firmed by the coupling constants J[H(1)- H(2)] and J[H(2)-H(3)] (8.46 to 9.9 Hz). These values dem- onstrate that the trans orientation of the alkene is retained in the product.
With regard to b) group the benzotiophene and the indol doesn't give the cycloaddition but rather the products of the electrophilic substitution in position 2 and 3. Three products are obtained with the benzofurane: one of them is resulted of electrophilic substitution by the cation in position 2 and the other two are cycloadducts. These are obtained in greater proportion.
References and Notes
- Angle, S. R.; Amaiz, D. O. J. Org. Chem. 1992, 57, 5937–5947.
- Kunstmann, R; Lerch, U.; Gerhards; et al. J. Med. Chem. 1984, 27, 432.
- Lantaño, B.; Finkielsztein, L. M.; Alesso, E. N.; Aguirre, J. M.; Moltrasio, G. Y. 8 th Brazilian Meeting on Organic Synthesis, September 1998; “Synthesis of Dihydro-1H-indenes using a formal [3+2] Cycloaddition”. 159.