Abstract
The structure of methyl 4-amino-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-α-l- talopyranoside was established by X-ray analysis confirming a talo configuration at C-4 and suggesting a 1C4 conformation of the pyranose ring. The values of relevant torsion angles and calculated puckering parameters revealed a distortion into the direction of 5E con- formation, thus indicating a flattening at C-2.
Introduction
The amino sugars represent a very important class of organic compounds primarily because of their biological role in living organisms. Their presence in bacteria and all tissues and fluids of pluricellular organisms as well as their association with many proteins and lipids suggests a great medicinal importance. The rapid increase of knowledge in the field of relationship between structure of amino sugar-containing compounds and biological activity requires availability many of suitable synthetically prepared model compounds with well established structures.
Within our research on synthesis of new amino sugar derivatives, we have prepared [1] two sugar amino nitriles – methyl 4-amino-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-α-l-talopyranoside (1) and methyl 4-amino-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-β-d-allopyranoside (2) which are structurally related to naturally occurring biologically important Perosamine (3) (Figure 1). Because of the difficulties in unambiguous establishing the configuration at C-4 position of the pyranose ring (talo versus manno for 1 and allo versus gulo for 2) by NMR methods, X-ray analysis of corresponding N- acetylated derivative 4 was presented [1] and recently, we have also published [2] the crystal structure of N-acetylated derivative 5.
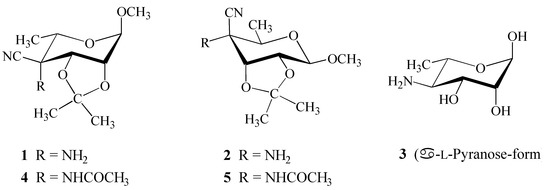
Figure 1.
Finally, we were successful in generating suitable crystals of the N-unprotected amino nitrile 1 and thus complete this structural study by presentation of the corresponding X-ray analysis data.
Results and Discussion
Structure Elucidation
The title compound 1 was fully characterized by 1H and 13C NMR, EIMS, CIMS, [α]d, TLC, mp and elemental analysis data [1]. The coupling constants J1,2 of 0 Hz and J2,3 of 6.5 Hz suggest a 1C4 conformation with an axial glycosidic methoxyl group, H-3 and H-5, an equatorial H-1 and H-2 and favoured 2,3-cis stereochemistry for the isopropylidene group. Because the data obtained from NMR measurements were unsufficient, X-ray analysis was used to determine unambiguously correct actual configuration at C-4 and simultaneously, conformation of the pyranose ring.
X-ray Analysis
The relevant crystallographic data and structure refinement are given in Table 1. The bond lengths and bond angles are listed in Table 2. A list of selected torsion angles is given in Table 3. The final positional parameters are summarized in Table 4. Perspective view and the numbering of the atoms is depicted in Figure 2. The hydrogen atoms were refined isotropically in idealized positions riding on the atom to which they are attached.

Table 1.
Crystal and experimental data for compound 1a.

Table 2.
Selected bond lengths [in Å] and bond angles [in °] for compound 1a.

Table 3.
Selected torsion angles [in °] for compound 1a.

Table 4.
Atomic coordinates (x 104) and equivalent isotropic displacement parameters (Å2 x 103) for compound 1a.
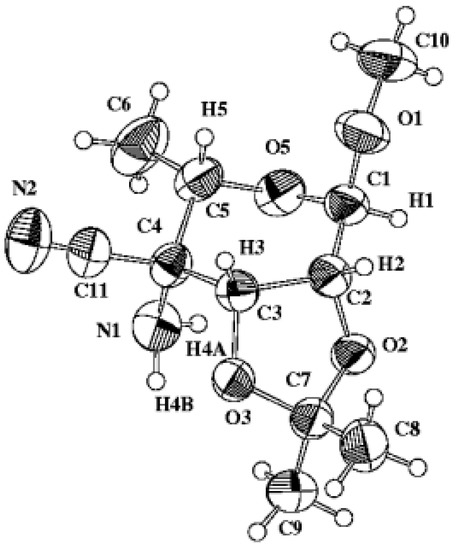
Figure 2.
ZORTEP plot and atomic numbering of compound 1.
The analysis of ring conformation by calculating puckering parameters [Q = 0.544(2) Å, θ = 151.3 (2)°, φ = 126.1(4)°] according to Cremer and Pople [3] has shown that pyranose ring in 1 adopt a 1C4 conformation which is slightly distorted into the direction of 5E [4,5], thus indicating a considerable flattening at C-2.
The values of selected relevant torsion angles [O3–C3–C4–C11 = 85.07(18)°, C3–C4–C5–C6 = –179.59(19)°] clearly demonstrate a talo configuration respecting the above mentioned conformation of the pyranose ring. On the other hand, torsion angle O1–C1–C2–O2 = –152.71(14)° indicates an α-l- anomeric linkage. Additionally, the values of torsion angles H1–C1–C2–H2 = 83.2° and H2–C2–C3– H3 = 29.4° obtained from X-ray analysis are in good agreement with those obtained from 1H NMR measurements. According to Karplus curve [6], observed vicinal coupling constants J1,2 = 0 Hz and J2,3 = 6.5 Hz correlate with dihedral angles of about 90° and 28°, respectively.
Analysis of the molecular packing in the unit cell revealed a weak intermolecular interaction [C(8) – H(8B)…O(5) = 3.534(3)Å, C(8) – H(8B) = 0.96Å, H(8B)…O(5) = 2.585Å, C(8) – H(8B)…O(5) = 170°, symmetry code = 2-x+y,1-x,1/3+z] which probably stabilizes the crystal structure.
Experimental
General
The synthesis and relevant data of analytical methods as well as instruments used for the preparation and characterization of the title amino nitrile 1 have already been published [1]. An analytical sample of 1 was used for generation of suitable crystals. These were obtained by slow crystallization from a mixture of ethyl acetate–hexane (1:2, v/v) at room temperature.
X-ray Analysis
Crystal and experimental data for compound 1 are given in Table 1. The structure was solved by direct methods and refined by anisotropic full-matrix least-squares technique. The choice of space group and hence the absolute configuration of the compound (1-R, 2-R, 3-S, 4-R, 5-S) was based on the fact that configuration on positions 1, 2, 3 and 5 of pyranose ring is known and could not change. The crystallographic computations were performed with Bruker SHELXTL [7]. The ZORTEP program [8] was used for the molecular graphics drawing.
Crystallographic data for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre. The corresponding deposition number is CCDC 143616. Copies of the data can be obtained free of charge on request to The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Tel.: +44-1223-336408, Fax: +44-1223 336-033).
Acknowledgements
Financial support of this work by the Scientific Grant Agency (VEGA, Slovak Academy of Sciences and Ministry of Education, Bratislava, projects No. 2/4144/99, 2/7144/20 and 2/7204/20) is gratefully appreciated.
References and Notes
- Steiner, B.; Koóš, M.; Langer, V.; Gyepesová, D.; Smrčok, Ľ. 4-Amino-4-cyano-4,6-dideoxy Sugar Derivatives from Methyl 6-deoxy-2,3-O-isopropylidene-α-l-lyxo-hexopyranosid-4-ulose via Strecker-type Reaction. Carbohydr. Res. 1998, 311, 1–9. [Google Scholar] [CrossRef]
- Koóš, M.; Steiner, B.; Gajdoš, J.; Langer, V.; Gyepesová, D.; Smrčok, Ľ; Ďurík, M. Crystal Structure of Methyl 4-acetamido-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-β-d-allopyranoside. Molecules 2000, 5, 219–226. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J. A. A General Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Köll, P.; Saak, W.; Pohl, S.; Steiner, B.; Koóš, M. Preparation and crystal and molecular structure of 6-O-[(2S)-2,3-epoxypropyl]-1,2:3,4-di-O-isopropylidene-α-d-galactopyranose. Pyranoid ring conformation in 1,2:3,4-di-O-isopropylidene-galactopyranose and related systems. Carbohydr. Res. 1994, 265, 237–248. [Google Scholar] [CrossRef]
- Boeyens, J.C.A. The conformation of six-membered rings. J. Cryst. Mol. Struct. 1978, 8, 317–320. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spectroscopic Methods in Organic Chemistry; Enders, D., Noyori, R., Trost, B.M., Eds.; Georg Thieme Verlag Stuttgart: New York, 1997; p. 108. [Google Scholar]
- Bruker AXS Inc. SHELXTL Version 5.10, Madison, Wisconsin: USA, 1997.
- Zsolnai, L.; Huttner, G. Program ZORTEP; University of Heidelberg: Germany, 1994. [Google Scholar]
- Sample Availability: The title compound is available from the corresponding author.
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.