Abstract
New substituted thieno[2,3-b]pyridines which contain 4-nitropehnyl and 5- nitro-, carboxy-, methoxycarbonyl-2-furyl groups in the 2 position have been obtained.
Introduction
The alkylation of substituted 3-cyano-2(1H)-pyridinethiones and Thorpe-Ziegler cyclization of the latter in alkali medium to give 3-aminothieno[2,3-b]pyridines have been extensively studied [1,2,3]. However there is no literature data on the use of 2-halomethyl furan derivatives and 2-furoic acid as alkylating agents or on the synthesis of substituted 3-amino-2-furyl- thieno[2,3-b]pyridines.
Results and Discussion
To further develop our studies on the alkylation of 6-methyl-4-methoxymethyl-3-cyano-2(1H)- pyridinethione (1a) and its structural isomer 1b [4,5,6], the pyridinethiones 1a,b were reacted with 5- nitro-furylmethyl- and 4-nitrobenzyl bromides and the 5-chloromethyl derivative of methyl 2 furoate (Scheme 1).
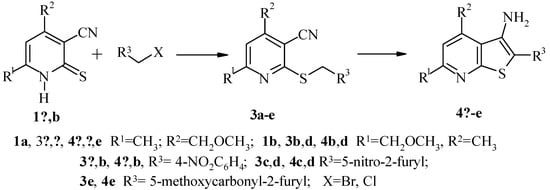
Scheme 1.
The reactions of pyridinethiones 1a,b with 4-nitrobenzyl bromide and methyl 5-chloromethyl-2-furoate were run in dimethylformamide (DMF) in the presence of KOH, and for 5-nitro-2-furylmethyl bromide, in ethanol in the presence of K2CO3. The ratio of compounds 1a,b to alkylating agent to base was 1:1:1. Alkylation was assumed to be regioselective relative to the more nucleophilic centre − the sulphur atom − to give 2-thiopyridines 3 (yields 81-92%) (Table 1). Their structures were confirmed by IR, UV and NMR data (Table 2, Table 3). The presence in the structure of compounds 3 of furan and benzene rings containing an electron-withdrawing substituent enhances the acidity of the CH2 group, thus making Thorpe-Ziegler isomerization of these compounds possible to afford thieno[2,3-b]pyridines 4a-e, possibly under the action of the base (KOH was used for the syntheses of 4a,b,e and K2CO3 for that of 4c,d). The acid 4f was obtained by alkaline hydrolysis of the methyl ester 4e in aqueous DMF, followed by acidification of the reaction mixture. Aminothieno[2,3- b]pyridines 4a-g are crystalline substances ranging in colour from yellow to dark red. Their IR spectra show no νC≡N absorption for the nitrile group at 2230-2205 cm-1, as is found in the spectra of the alkylation products, and they also display a broadening of the νNH bands of the NH2 group at 3480-3220 cm-1 (Table 2), which is characteristic of the 3-amino-thienopyridines [2,3,4,7].

Table 1.
Characteristics of the compounds obtained

Table 2.
IR and UV-VIS spectra of synthesised compounds

Table 3.
1H NMR – spectral data.
The reaction of amine 4c and acetic anhydride results in the mono- and diacyl derivatives 5a and 5b, whose formation depended on the reaction conditions (Scheme 2). Acvetylation of compound 4c at room temperature leads to amide 5a. Heating of amine 4c in acetic anhydride gives a mixture of acyl derivatives 5a and 5b, where imide 5b is predominant. Imide 5b was isolated by column chromatography.
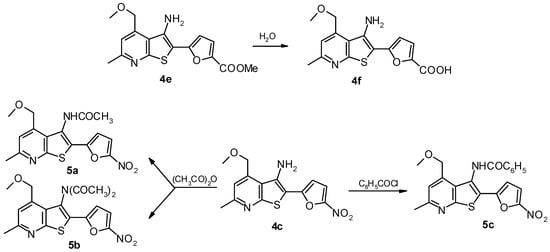
Scheme 2.
Experimental
General
1H-NMR spectra were recorded on Bruker WM-250 and Tesla BS-487A spectrometers using DMSO-d6 or CDCl3 as solvents. Chemical shifts (δ) are given in ppm relative to TMS. IR spectra (vaseline oil suspensions) have been measured on a Specord 75-IR spectrophotometer. UV-VIS spectra were recorded on a Specord M-40 spectrophotometer using ethanol as solvent. Thin layer chromatography (TLC) was performed on Silufol UV-254 silica gel plates using hexane-acetone (1-2:1) as the solvent system; plates were visualized with iodine vapour or after spraying with KMnO4 solution. Physical properties and spectral data of the compounds prepared are given in Table 1, Table 2 and Table 3.
6-Methyl-4-methoxymethyl-3-cyano-2-(4-nitrobenzyl)thiopyridine (3a).
A mixture of pyridinethione 1a [5] (1.94 g, 10 mmol) in DMF (20-25 mL), 4-nitrobenzyl bromide (2.16g 10 mmol) and a 10% aqueous solution of KOH (5.6 mL, 10 mmol) was kept at r.t for 2h, then diluted with water (10 mL). The precipitate formed was filtered off, washed with water, dried and recrystallized from ethanol to give 3.03 g (92%) of 3a. Compounds 3b-e were similarly obtained.
3-Amino-2-(4-nitrophenyl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (4a).
A suspension of thiopyridine 3a (3.29 g, 10 mmol) in DMF (30 ml) and a 10% aqueous solution of KOH (5.6 mL, 10 mmol) was mixed for 2 h at 45-50°C, then diluted with a two-fold volume of water. The precipitate formed was separated and recrystallized from ethanol to give 3.06 g (93%) of 4a. Compounds 4b,e were obtained in the same manner.
3-Amino-2-(4-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (4c).
A mixture of thiopyridine 3c (3.19 g, 10 mmol) in ethanol (20 mL) and 10% aqueous K2CO3 solution (6.9 mL, 5 mmol) was refluxed for 5 hours, then diluted with a two-fold volume of water. The precipitate formed was separated and recrystallized from ethanol to give 2.39 g (75%) of 4c. Compound 4d was obtained in the same manner.
3-Amino-2-(5-carboxylfuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (4f).
A mixture of thienopyridine 4e (3.32 g, 10 mmol) in DMF (20-25 mL) and a 10% aqueous solution of KOH (5.6 mL, 10 mmol) was brought to the boiling point. Then the reaction mixture was diluted with water to twice the volume and acidified with 10% aqueous hydrochloric acid until a precipitate formed. The precipitate was eparated, washed with water, dried and recrystallized from ethanol to yield 1.94g (61%) of 4f.
3-N-Acetylamino-2-(5-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]-pyridine (5a).
A solution of thienopyridine 4c (3.19 g, 10 mmol) in acetic anhydride (20 mL) was left to stand at r.t. for 2 h. Then the reaction mixture was diluted with a two-fold volume of water and neutralized with 10% aqueous solution of Na2CO3. The solid formed was collected by filtration, washed with water, dried in air and recrystallized from DMF to yield 2.94 g (73%) of 5a.
3-N-Acetylamino- and 3-N,N-diacetylamino-2-(5-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno- [2,3-b]-pyridine (5a) and (5b).
A solution of thienopyridine 4c (3.19 g, 10 mmol) in acetic anhydride (20 mL) was refluxed for 40 minutes. The reaction mixture was then concentrated under vacuum and the residue was washed with 5% aqueous solution of NaHCO3 followed by water, dried in the air and recrystallized from ethanol. The acylation products were separated by column chromatography using hexane-acetone mixture (1:2) as eluent. The yield of 5a was 0.43 g (12%) and of 5b, 2.70 g (67%).
3-N-Benzoylamino-2-(5-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (5c).
A mixture of thienopyridine 4c (3.19 g, 10 mmol) in chloroform (20 mL) and benzoyl chloride (1.16 mL, 10 mmol) was refluxed for 60 minutes. The solvent was evaporated under vacuum and the residue was washed with 2.5% aqueous solution of NaHCO3 and water, dried in the air and recrystallized from DMF to give 3.89 g (92%) of 5c.
References
- Litvinov, V. P.; Krivokolysko, S. G.; Dyachenko, V. D. Khim. Geterotsikl. Soedin. 1999, 35, 579.
- Gewald, K.; Hentschel, M.; Illgen, U. J. Prakt. Chem. 1974, 316, 1030.
- Sharanin, Yu.A.; Shestopalov, A.M.; Promonenkov, V.K. Zh. Org. Khim. 1984, 20, 2012.
- Furukawa, N.; Kawai, T.; Oae, S.; Iwasaki, F. Synthesis 1984, 746.
- Kaigorodova, E. A.; Konyushkin, L. D.; Niyazymbetov, M. E.; Kvak, S.N.; Zaplishny, V.N.; Litvinov, V. P. Russ. Chem. Bull. 1994, 43, 2095.
- Kaigorodova, E.A.; Konyushkin, L.D.; Mikhailichenko, S.N.; Vasilin, V.K.; Kulnevich, V.G. Khim. Geterotsikl. Soedin. 1996, 32, 1432.
- Rodinovskaya, L.A.; Sharanin, Yu.A.; Litvinov, V.P.; Shestopalov, A.M.; Promonenkov, V.K.; Zolotarev, B.M.; Mortikov, V.Yu. Zh. Org. Khim. 1985, 21, 2439.
- Sample Availability: Samples are available from the authors.
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes