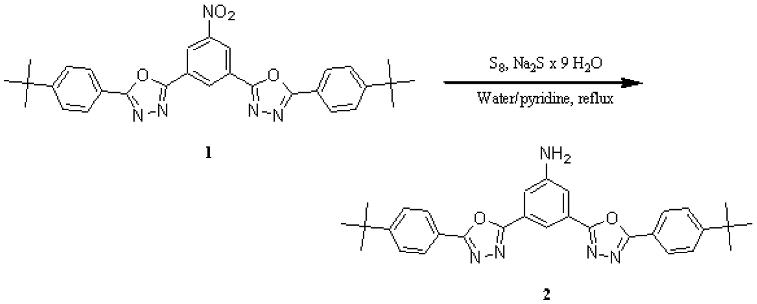
The reduction was carried out as described in reference [1] by using a mixture of sulfur (7.10 g, 0.22 mol), Na2S — 9 H2O (240 g, 0.22 mol), water (140 mL) and pyridine (20 mL). A solution of this reducing agent (60 mL) was added to a solution of 3,5-bis[5-(4-tert-butylphenyl)-1,3,4-oxadiazol-2-yl]nitrobenzene (1) (1.00 g, 1.9 mmol) in warm pyridine (10 mL). The reaction mixture was heated to reflux for 90 min, during which time a yellow precipitate separated. The mixture was filtered and the filtrate was added dropwise to water (200 mL). The precipitate was collected by suction filtration and dried. The combined solids were extracted with chloroform, the solution was filtered and concentrated in vacuum to yield 2 as a light yellow solid (260 mg, 28%). The yield was not optimised [2].
Mp. : 291-293°C.
IR (KBr): 3357s; 2964s; 1616m; 1543m; 1495s; 1464m; 1458m; 1114w; 1011w; 842w; 726w.
1H NMR (CDCl3:DMSO-d6 6:1, 300 MHz): 8.09 (t, J = 1.4 Hz, 1 H, C6H3); 8.09 (AA′XX′, 4 H, C6H4); 7.65 (d, J = 1.4 Hz, 2 H, C6H3); 7.59 (AA′XX′, 4 H, C6H4); 4.92 (br. s, 2 H, NH2); 1.39 (s, 18 H, CH3).
13C NMR (CDCl3:DMSO-d6 7:3, 125 MHz): 164.4; 163.8; 155.2; 149.4; 126.6; 126.04; 126.0; 125.2; 120.7; 114.8; 34.9; 31.0.
CI-MS (NH3) : 512 (35); 511 (100, M+NH4+); 495 (28); 494 (78, M+H+); 194 (36); 177 (28).
Reference and Notes
- Krasovitskii, B. M.; Matskevich, R. M.; Malt'seva, N. I. J. Gen. Chem. USSR 1961, 2107.
- The reduction procedure worked reasonably well and provided the only successful way to the amine. Other reduction conditions [formic acid, NEt3, Pd/C; hydrazine hydrate, Raney-Ni; carbon monoxide, SnCl4, PtCl2(PPh3)2, dioxane] failed completely. However, considerable amounts of H2S evolved during the reaction; because of the stench involved, we decided in the end not to repeat the procedure a second time.
Sample availability: available from the authors. |
© 1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/