Abstract
Parkinson’s disease (PD) is a complex neurodegenerative disorder with debilitating non-motor symptoms, including gastrointestinal dysfunction, cardiovascular abnormalities, mood and anxiety disorders, cognitive decline, sleep disturbances, respiratory dysfunction, and pain. Despite their significant impact on quality of life, these symptoms are often inadequately addressed. Propolis is a natural bee-derived product, rich in bioactive compounds with anti-inflammatory, antioxidant, immunomodulatory, and neuroprotective properties, which holds potential in PD due to its multitarget and multipathway actions, addressing various underlying mechanisms of non-motor symptom diseases. Preclinical and clinical studies suggest that propolis may influence key pathological mechanisms in PD’s non-motor symptoms. Evidence points to its potential benefits in improving cognition, mood disorders, gastrointestinal health, and alleviating cardiovascular and sleep-related issues. Although research on propolis in non-motor symptoms of PD remains scarce, findings from related conditions suggest its ability to influence mechanisms associated with these symptoms. This review underscores the underexplored therapeutic potential of propolis in non-motor symptoms of PD, drawing on existing evidence and advocating for further research to fully assess its role in addressing these symptoms and improving patient outcomes.
1. Introduction
Neurological disorders account for a substantial share of disability worldwide, with Parkinson’s disease (PD) standing out as the most rapidly increasing brain disorder, posing significant challenges to global public health systems [1]. Over the past 25 years, the prevalence of PD has doubled, a trend influenced by demographic shifts toward an aging population, improved life expectancy, and heightened exposure to environmental factors such as pesticides, industrial pollutants, and other chemical agents [1,2,3,4]. Genetic research has underscored the multifactorial nature of PD. While monogenic forms account for approximately 3–5% of cases, the majority are sporadic or idiopathic, arising from a complex interplay between genetic predispositions and environmental influences [5,6,7].
PD has long been understood as a neurodegenerative condition primarily defined by motor symptoms like bradykinesia, tremors, and rigidity. Although these hallmark motor features are widely acknowledged, non-motor symptoms—such as autonomic dysfunction (Figure 1), mood and anxiety disorders, gastrointestinal issues, pain, and sleep disturbances—are equally common and can profoundly affect patients’ quality of life [6,8,9,10]. Non-motor symptoms can manifest years before the onset of motor symptoms, and in some patients, they may remain the predominant features of the disease [11,12]. These prodromal non-motor symptoms can appear up to 20 years before a clinical diagnosis, which typically focuses on motor dysfunction [10,13]. Identifying biomarkers for the prodromal stage of the disease remains a critical challenge, as this would pave the way for interventions aimed at altering the course of the disease. Due to this complexity, PD is now recognized as a heterogeneous disorder, encompassing a spectrum of subtypes with unique etiologies, clinical presentations, and progression patterns [6,14]. Understanding these nuances is essential for advancing the development of more targeted and effective therapeutic strategies.
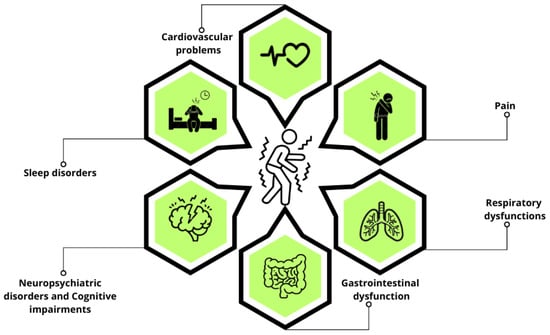
Figure 1.
Non-motor symptoms in PD. This schematic representation highlights the primary non-motor symptoms of PD, including sleep disorders, neuropsychiatric disorders, cognitive impairments, cardiovascular problems, pain, gastrointestinal dysfunction, and respiratory dysfunctions.
To date, there is no cure for PD, nor any treatment that can slow or halt its progression [15]. While levodopa remains the gold standard for the symptomatic management of motor dysfunction, the degree of response can vary widely. Some patients experience significant improvements in motor symptoms, while others have a limited or fluctuating response. Current therapies primarily address motor aspects, offering little relief for non-motor issues [16,17]. Despite their profound impact on patient outcomes, non-motor features remain an underrecognized and undertreated component of the disease, leaving a significant gap in patient care. The complexity and subtle nature of non-motor symptoms, combined with the lack of targeted treatments, contribute to the difficulty in effectively managing these aspects of PD.
The key pathological feature of PD is the loss of dopaminergic neurons in the substantia nigra, accompanied by the presence of Lewy bodies, which are abnormal aggregates of the alpha-synuclein protein. However, the degree and distribution of these pathological features can vary significantly among patients [18]. Despite this variability, common underlying mechanisms, such as oxidative stress, mitochondrial dysfunction, neuroinflammation, and protein aggregation, are observed across all PD patients [6,19,20]. This multifactorial nature of PD presents a significant challenge in developing an effective therapeutic approach.
In this context, propolis emerges as a promising yet underexplored therapeutic candidate. Its pleiotropic properties enable it to modulate multiple pathological pathways simultaneously, offering a potential advantage over conventional therapies that typically target only a single mechanism. By addressing key contributors to PD pathology, propolis holds the potential to provide a multitarget approach that could more effectively address the complex and interconnected nature of the disease.
Propolis, a resinous substance produced by honeybees, has attracted growing interest as a potential therapeutic agent for neurological diseases due to its diverse and potent biological properties [21,22,23,24,25,26]. Rich in flavonoids, phenolic acids, terpenoids, and other bioactive compounds, propolis exhibits antioxidant, anti-inflammatory, immunomodulatory, antiviral, antibacterial, and neuroprotective effects, making it a promising natural product for scientific investigation (Figure 2) [27,28,29,30,31,32]. Research from our lab, along with findings from other studies, reveals that propolis helps preserve dopaminergic neurons and increases dopamine levels in the basal ganglia of PD animals, resulting in improved motor function [33,34,35,36,37]. Despite these promising results, there remains a notable gap in the literature regarding the impact of propolis on non-motor symptoms of PD, including gastrointestinal dysfunction, cardiovascular problems, neuropsychiatric disorders, cognitive impairments, sleep disturbances, respiratory challenges, and pain. These manifestations are often inadequately addressed by current therapies, yet they contribute significantly to disability, compromised quality of life, and reduced life expectancy.

Figure 2.
Key mechanisms underlying the beneficial effects of propolis’s bioactive compounds. The biological activity of these natural compounds is driven by anti-inflammatory, antioxidant, immunomodulatory, antibacterial, and antiviral properties. Abbreviations: NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; COX-2, cyclooxygenase 2; PGE-2, prostaglandin E2; TGF-β, transforming growth factor beta; Nrf2, nuclear factor erythroid 2-related factor 2; IL-6, interleukin-6; IL-IB, interleukin-1beta; IL-8, interleukin-8; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon-gamma; TLR4, toll-like receptor 4; IKB-kinase, inhibitory kappa B kinase beta; MAPK, mitogen-active protein kinase; JAK-STAT, janus kinase/signal transducers and activators of transcription; NRF2/Keap1-ARE, nuclear factor erythroid 2-related factor, kelch-like ECH-associated protein 1, antioxidant response element; HO-1, heme oxygenase-1; NQO1, NAD(P)H quinone dehydrogenase 1; GPx, glutathione peroxidase; GR, glutathione reductase; CAT, catalase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; STAT3, signal transducer and activator of transcription 3; M1/M2, macrophages; HMGB1, high-mobility group box 1 protein; DC, dendritic cell; CD4+T, T-lymphocytes; Trag cell, regulatory T cells; FOXP3, forkhead box P3; TLR2/TLR4, toll-like receptor 2/4; MyD88, myeloid differentiation primary response 88; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; Th cells, helper T cells; NK cell, natural killer cell; B cells, B lymphocyte cells; NOD1/NOD2, nucleotide-binding oligomerization domain-containing protein 1/2; P13k, phosphatidylinositol 3-kinase; AKt, protein kinase B; mTOR, mammalian target of rapamycin; sre, sterol response element. The upward and downward arrows represent an increase or decrease in the specified components.
Although most studies investigating this natural compound have not specifically focused on PD, substantial evidence supporting the beneficial properties of propolis in related conditions provides a compelling basis for exploring its potential role in managing the non-motor manifestations of PD. Given the scarcity of specific research on the direct use of propolis in addressing these symptoms, it becomes crucial to examine the state of the art of existing scientific evidence on its application in other clinical conditions and experimental models with similar signs and symptoms. By identifying and synthesizing such evidence, this literature review aims to enhance the understanding of the therapeutic potential of propolis in the non-motor symptoms of PD, highlight gaps in the current knowledge, and indicate directions for future research. Ultimately, by addressing this under-researched area, we seek to underscore the promise of propolis and its bioactive constituents, encouraging further studies that could lead to significant advancements in the treatment and management of this debilitating neurodegenerative disorder.
2. Results
The search carried out in the PubMed and Embase databases resulted in the identification of 1626 studies. After excluding duplicates and applying the inclusion and exclusion criteria, 775 records were screened. Of these, 851 records were considered eligible after excluding repetitions and applying filters. After reading all the texts, 445 articles were selected for detailed analysis. Of these, 280 studies met the inclusion criteria and were included in the final review, as illustrated in Figure 3 (PRISMA flowchart of the study selection process). The included studies were organized and synthesized according to the pathophysiological mechanisms of Parkinson’s disease (PD) and the potential therapeutic effects of propolis on non-motor symptoms. The relevant bioactive compounds and their mechanisms of action were identified and classified. In addition, three tables were drawn up: Table 1, which lists the non-motor symptoms of PD, the related diseases, the observed effects of propolis, and the bioactive compounds identified; Table 2, which presents the bioactive compounds of propolis, their mechanisms of action, the non-motor symptoms that potentially benefited, and the main associated references; Table 3, which highlights the key mechanisms underlying the benefits of propolis, relating them to the compounds involved and their respective references.
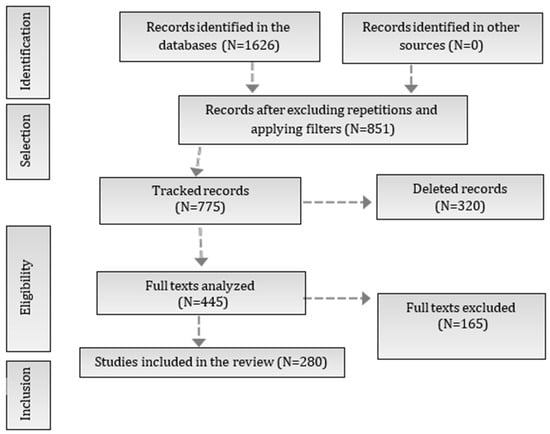
Figure 3.
PRISMA flowchart of the study selection process. The flowchart illustrates the stages of the study selection process in the systematic review, following the PRISMA model. Initially, 1626 records were identified in the databases, of which 851 remained after removing duplicates and applying filters. From these, 775 records were tracked, leading to a full-text analysis of 445 studies. Finally, 280 studies were included in the review after excluding 165 full texts that did not meet the eligibility criteria.

Table 1.
Relationship between non-motor symptoms of PD, related diseases investigated, effects of propolis, and active compounds identified.

Table 2.
Propolis bioactive compounds, mechanisms of action, potentially beneficial non-motor symptoms, and main associated references.

Table 3.
Key mechanisms underlying the beneficial effects of propolis with references.
3. Methods
This literature review was conducted to synthesize the available evidence on the potential therapeutic effects of propolis on the non-motor symptoms of Parkinson’s disease (PD), and on different clinical conditions, with the aim of exploring its applicability in PD, particularly in its non-motor manifestations. Given the scarcity of specific studies on the use of propolis in these PD symptoms, this review sought to identify research investigating the action of propolis on pathophysiological mechanisms underlying the disease, such as neuroinflammation, oxidative stress, mitochondrial dysfunction, and the dysregulation of the neuroimmune axis, which are also involved in the manifestation of non-motor symptoms.
3.1. Data Sources and Search Strategy
The search was carried out in the PubMed and Embase databases, considering articles published up to 2025, in English, Portuguese, and Spanish.
To ensure the traceability and reproducibility of the search, the terms used were carefully selected and documented. The search strategy was formulated based on a combination of keywords and Boolean operators in order to identify relevant studies. The search syntax included the following terms:
3.1.1. PUBMED
(Propolis OR Bee resin OR “Bee propolis”) AND (Neuroinflammation OR Oxidative stress OR “Mitochondrial dysfunction” OR “Neuroprotection” OR “Cognitive impairment” OR “Anxiety” OR “Depression” OR “Autonomic dysfunction” OR “Gastrointestinal dysfunction” OR “Pain” OR “Cardiovascular dysfunction” OR “Sleep disorder” OR “respiratory dysfunction”).
3.1.2. Embase
(’propolis’/exp OR ’propolis’:ti,ab,kw OR ’bee propolis’:ti,ab,kw) AND (’nervous system inflammation’/exp OR ’neuroinflammatory diseases’:ti,ab,kw OR ’neuroinflammation’:ti,ab,kw OR ’oxidative stress’/exp OR ’oxidative stress’:ti,ab,kw OR ’mitochondrial dysfunction’:ti,ab,kw OR ’neuroprotection’:ti,ab,kw OR ’cognitive impairment’ OR ’anxiety’ OR ’depression’ OR ’autonomic dysfunction’ OR ’gastrointestinal dysfunction’ OR ’pain’ OR ’cardiovascular dysfunction’ OR ’sleep disorder’ OR ’respiratory dysfunction’).
In addition, reference articles from relevant publications were reviewed to identify additional studies not captured by the initial search.
Inclusion:
- Original articles investigating the effects of propolis in in vitro models, animals, or clinical studies.
- Studies addressing the biological mechanisms of propolis that may be related to the non-motor symptoms of PD.
Exclusion:
- Studies irrelevant to the scope of this review, such as those focused only on beekeeping aspects or diseases unrelated to PD mechanisms.
3.2. Study Selection Process
The selection of studies was carried out in three stages, starting with a preliminary screening based on titles and abstracts, conducted independently by two researchers. Next, the full texts of the pre-selected articles were evaluated in detail, applying the previously defined inclusion and exclusion criteria. Finally, data were extracted and analyzed from the selected publications, with special emphasis on the mechanisms of action of propolis. Agreement between the reviewers was duly assessed, and any disagreements were resolved by consensus.
3.3. Summary of Data
The data were qualitatively synthesized, with the evidence organized into tables highlighting the bioactive compounds in propolis and their mechanisms of action relevant to PD, as well as their effects on different clinical conditions related to the non-motor symptoms of the disease, in addition to the clinical and preclinical studies relevant to the topic. In addition, as recommended by the reviewers, a PRISMA flowchart was drawn up to clearly illustrate the article selection process.
4. Discussion
4.1. Neuropsychiatric Disorders
Anxiety and depression are common non-motor manifestations of PD and often coexist, although they can also occur independently [38,39]. Apathy is also part of the non-motor triad of the disease [40]. Studies suggest that during the prodromal phase of PD, dopaminergic depletion and serotonergic degeneration contribute to these conditions [38]. Research shows a high prevalence of mood disorders in PD patients, with depression affecting 20–50% [41,42,43,44] and anxiety disorders impacting 20–60% of patients [44,45,46], though both are often underdiagnosed and overlooked in clinical practice [10]. Anxiety and depression are associated with disruptions in the limbic-cortico-striato-thalamo circuit, which are driven by the degeneration of dopaminergic, noradrenergic, and serotonergic neurons [40]. Dysfunctions in the GABAergic and cholinergic systems also contribute to these neuropsychiatric symptoms of PD [47,48].
Inflammation is a crucial element in the pathophysiology of PD, beginning as early as the prodromal phase and contributing to the disease’s progression [31,49]. Evidence points to increased inflammatory activation as a factor in triggering depression. Research indicates that increased pro-inflammatory cytokines TNFα, IL-1β, and IL-6 levels are noted in PD [50]; their presence in peripheral blood correlates with the severity and progression of the disease. Specifically, high concentrations of IL-6 in plasma have been identified as a significant indicator of future risk for developing the disease, highlighting the crucial role of inflammation in PD pathogenesis [49,51]. Individuals with PD exhibiting more pronounced symptoms of depression, fatigue, and cognitive decline showed elevated serum concentrations of TNFα [52]. Evidence from the literature suggests that anti-inflammatory strategies may contribute to reducing the risk of neuropsychiatric disorders. For instance, a meta-analysis found that pro-inflammatory diets increase the risk of depression by 45% and anxiety by 66% compared to anti-inflammatory diets [53]. In this context, propolis emerges as a promising adjunctive therapy due to its potent anti-inflammatory properties that may help re-establish balance in the neuroimmune system.
Results from a randomized, placebo-controlled clinical trial demonstrated that adjunctive propolis therapy significantly reduces depressive symptoms in PD patients with depression [54]. A growing body of research suggests that propolis significantly alleviates depression-like and anxiety-like behaviors in PD animal models [35,55,56,57]. Propolis has been identified as a potent inhibitor of monoamine oxidase (MAO), the enzyme responsible for the breakdown of key neurotransmitters such as serotonin, dopamine, and norepinephrine in the brain [58]. These findings highlight its potential as a therapeutic option for managing PD and alleviating neuropsychiatric symptoms. Moreover, in rats subjected to a chronic unpredictable stress model of depression, propolis has been found to exhibit antidepressant-like actions by reducing oxidative stress and inflammation [59].
The medicinal properties of propolis-derived flavonoids, such as quercetin, apigenin, and chrysin, are gaining attention for their potential to alleviate neuropsychiatric disorders [60,61,62]. A study found that quercetin administration decreases brain concentrations of inflammatory factors, including IL-1β, IL-6, and NF-kB, and mitigates anxiety-like behaviors in rats with lipopolysaccharide-induced chronic neuroinflammation [63]. Preclinical studies in mice have demonstrated the antidepressant potential of apigenin, as evidenced by improved outcomes in the forced swim and tail suspension tests, possibly mediated through the modulation of the dopaminergic system [63]. Another study found that the antidepressant actions of chrysin in rodents exposed to chronic mild unpredictable stress are associated with an upregulation of brain levels of the brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), along with its antioxidant activity [64].
The flavonoid isochlorogenic acid B in propolis helps to reduce lead-induced anxiety-like and depressive-like states in mice by reducing oxidative stress and brain inflammation while modulating the BDNF and PI3K/AKT signaling pathways [65]. A study using human neuroblastoma SH-SY5Y cells, an in vitro model for studying PD, revealed that treatment with propolis led to a significant increase in BDNF expression, mediated by the PI3K signaling pathway [66]. Research also indicates that isochlorogenic acid A reduces anxiety-like behavior in mice resulting from lead exposure by targeting neuroinflammation driven by ferroptosis through the BDNF/Nrf2/GPX4 pathways [67].
4.2. Cognitive Impairments
Cognition refers to the set of mental processes involved in the acquisition, processing, storage, and use of information. These processes are essential for the individual’s interaction with the environment, allowing the performance of daily tasks, problem solving, decision-making, and memory formation. Cognitive impairments in individuals with PD can manifest as difficulties in attention, memory, executive functioning, and visuospatial skills [68]. These deficits can present with significant variations in onset, characteristics, and progression rates [69]. As a major cause of disability for patients and a challenge for caregivers, cognitive impairment is observed to be up to six times more prevalent among individuals with PD than in the general population [68]. It is noteworthy that PD patients with cognitive impairment may have a significantly higher risk of developing dementia [69].
Furthermore, neuropsychiatric conditions commonly impair cognitive abilities, leading to deficits that can be both severe and complex, thereby negatively impacting patients’ quality of life. There is considerable overlap in the neurobiological mechanisms that contribute to both neuropsychiatric symptoms and cognitive impairments. Changes in neurotransmitter concentrations, such as those of dopamine, serotonin, norepinephrine, and acetylcholine, can influence mood and cognitive processes [70,71,72,73,74]. Additionally, these two aspects are affected by inflammation, oxidative stress, and neurodegenerative processes. Due to its bioactive compounds with anti-inflammatory and antioxidant properties, propolis has promising potential to counteract the biological processes that contribute to mood disorders and cognitive impairment.
A placebo-controlled, double-blind study conducted over 24 weeks in elderly Japanese participants evaluated the effects of a propolis-based dietary supplement on cognitive function [75]. The results indicated significant improvements across key cognitive domains, such as memory, attention, and information processing, highlighting propolis’s promise as an effective strategy for preserving cognitive function in the elderly [75]. In the study conducted by Zhu et al., the effects of Brazilian green propolis on cognitive decline and systemic inflammation were investigated in elderly individuals living at high altitudes (2260 m) [76]. This 24-month double-blind study involving 60 participants showed that those receiving propolis experienced significant improvements in cognitive scores (MMSE) and reductions in inflammatory cytokines, including IL-1β and IL-6, compared to the placebo group [76]. Propolis also increased levels of TGFβ1, which correlated with protection against cognitive decline compared to the placebo group, highlighting its anti-inflammatory and neuroprotective effects.
Non-pharmacological approaches, such as physical activity and nutritional interventions, combined with pharmacological treatments, have demonstrated significant benefits in alleviating both motor and non-motor symptoms of PD. These methods, being less invasive and more accessible, can effectively modulate neural activity and glial function while also reducing oxidative stress and inflammatory responses, contributing to a more balanced and comprehensive therapeutic effect [74]. In this context, propolis has emerged as a promising agent for enhancing cognitive function through multiple mechanisms, including acetylcholinesterase inhibition, the upregulation of BDNF, and a reduction in oxidative stress. The effects of propolis also lead to increased levels of brain catecholamines (norepinephrine, serotonin, and dopamine), the activation of the acetylcholine pathway, reduced neuronal loss, and the stimulation of neurogenesis [23,77].
BDNF is essential for neuron development, survival, and synaptic plasticity, playing a crucial role in the neural plasticity associated with learning and memory processes [78]. Recent studies highlight that propolis has the potential to regulate BDNF concentrations. In experiments with the SH-SY5Y cell line, a widely used model for studying PD, propolis treatment was observed to increase BDNF expression in a dose- and time-dependent manner [66]. Exhibiting adrenergic and dopaminergic traits, these cells offer a platform for studying molecular and cellular mechanisms underlying neuronal disorders, pointing to the possibility that propolis may upregulate BDNF expression and improve synaptic efficacy in human neurons [66,79].
Propolis treatment has been shown to decrease homocysteine-induced reactive oxygen species (ROS) and cell death in assays using the SH-SY5Y cell model of PD [80]. Results also revealed a reduced accumulation of cerebral protein aggregates due to hyperhomocysteinemia and improved cognitive abilities in mice on a propolis-supplemented diet [80].
The findings from Zulhendri et al. highlighted that propolis decreases neuroinflammatory and oxidative stress markers, limits leukocyte migration to inflamed areas, and reduces ROS and pro-inflammatory cytokine expression, such as IL-1β, IL-6, and TNF-α [81]. This modulation of endocrine and biochemical markers contributed to the reduction in depressive behaviors and cognitive deficits in animals. Research conducted by Zhang and colleagues investigated quercetin’s neuroprotective effects in a mouse model of memory deficits induced by paradoxical sleep deprivation (PSD). Results showed that quercetin improved memory impairment by affecting microglial activation and TLR4/MyD88/NF-κB signaling in the hippocampus [82]. As the prevalence of cognitive impairment and sleep disorders increases, these findings enhance our understanding of the underlying biological processes and open avenues for targeted interventions in conditions associated with neuroinflammation and cognitive dysfunction.
Further research into the interplay between cognitive function, mental health in PD, and the potential benefits of dietary supplements is essential to develop more effective and holistic treatment strategies and, ultimately, improve the quality of life of PD patients.
4.3. Sleep Disorders
The reduction in dopamine availability may be a determining factor in alterations in the circadian cycle, impacting the sleep–wake dynamics of PD patients [83]. This can result in symptoms related to sleep disturbances, a condition reported by over 60–90% of individuals diagnosed with the disease [84,85], encompassing a variety of conditions such as insomnia, circadian rhythm disorders, parasomnias, excessive daytime sleepiness, sleep behavioral disorder, rapid eye movement (REM) sleep behavior disorder, restless legs syndrome (RLS), and sleep-related breathing disorders (e.g., sleep-apnea syndrome (SAS)) [86,87,88]. Recent studies have highlighted a high prevalence of sleep-related breathing disorders in this group, including obstructive SAS, central sleep apnea, and sleep-related hypoventilation. These conditions significantly affect respiratory and circulatory functions, potentially causing a variety of systemic impacts [89].
SAS is a common condition in PD, occurring in up to 70% of patients, significantly impacting their quality of life and disease progression [89]. This frequently underrecognized condition induces intermittent hypoxia and subsequent neuroinflammation. Clinically, SAS may manifest before the motor symptoms of PD, causing recurrent upper airway obstruction, periodic breathing pauses during sleep, and sleep disruption [89,90]. A study in a mouse model of sleep apnea revealed that pinocembrin mitigates neuroinflammation by triggering BNIP3-dependent mitophagy via the JNK-ERK signaling cascade [91]. Repeated exposure to intermittent hypoxia leads to oxidative stress and inflammatory responses in brain areas associated with neurodegeneration, contributing to dopaminergic neuron loss [92]. Dopaminergic neurons in the substantia nigra are particularly sensitive to oxygen deprivation due to their higher rates of energy metabolism, basal respiration, and glycolysis, as well as increased mitochondrial populations, vulnerability to cytotoxins, and extensive axonal branching [93]. Hypoxia induces microglial overactivation, leading to the secretion of pro-inflammatory cytokines, exacerbating neurodegeneration in PD [92,94,95].
The presence of SAS increases morbidity and impairs quality of life. The pathogenesis of SAS is complex, and the therapeutic options are limited. Chronic inflammatory processes, like in PD, represent a prolonged and dysregulated immune system response to persistent stimuli, resulting in a series of negative effects on the body, including the disruption of the internal biological clock. This clock is crucial for coordinating a wide range of physiological functions throughout the day and night, encompassing sleep, wakefulness, body temperature, and hormonal regulation [96].
Chronic inflammation can negatively impact the internal biological clock in various ways. It can disrupt the production and release of neurotransmitters (e.g., serotonin and dopamine), which are essential for regulating sleep and wakefulness [97,98]. Moreover, it is associated with changes in gene expression in brain areas critical for the biological clock. These changes can prevent the synchronization of circadian rhythms with environmental light–dark cycles, resulting in irregular sleep–wake cycles and inconsistent sleep patterns [99,100].
Inflammation can also impair glial cells, which support and protect the central nervous system. The compromised function of these cells can disrupt neuronal signal transmission in the suprachiasmatic nucleus and other regions involved in the regulation of the circadian cycle [101,102,103]. Substances with anti-inflammatory, immunomodulatory, and antioxidant properties, like propolis and its constituents, may help in regulating the sleep–wake cycle.
The study conducted by Siddique and Jyoti found that transgenic flies with human alpha-synuclein aggregates in the brain, a model of PD, had a longer lifespan and higher dopamine concentrations, along with decreased oxidative stress and apoptosis, when supplemented with apigenin in their diet [104]. These findings suggest a compelling link between apigenin supplementation and enhanced dopamine levels in the Drosophila model of PD, which could have implications for sleep modulation. Dopamine concentrations are known to play a crucial role in regulating sleep–wake cycles. Additionally, the reduction in oxidative stress and apoptosis may contribute to healthier neuronal function, further supporting sleep patterns. The use of apigenin in rodents has been linked to enhanced sedation and decreased locomotor activity, both of which are key determinants of sleep latency [105,106].
Studies have highlighted apigenin as a promising candidate in modulating sleep processes and addressing sleep disturbances [107]. Apigenin, a flavonoid found in propolis, has gained interest for its anti-inflammatory, neuroprotective, and anxiolytic properties while improving neurovascular activity and concentrations of molecules such as BDNF, TrkB, and cAMP Response Element Binding (CREB), suggesting it may have a direct role in promoting sleep [107]. The potential of apigenin to improve sleep seems to be associated with its interaction with central neurotransmitter systems. Apigenin’s GABAergic activity in rats appears to work independently of GABA–benzodiazepine receptors, possibly through the GABA-A receptor mechanism [106]. This interaction is thought to facilitate an increase in inhibitory signaling, leading to sedative and calming effects that can improve sleep onset and maintenance. Evidence also suggests that apigenin amplifies sleep behaviors triggered by pentobarbital by activating chloride ion channels [108]. Moreover, the dual role of apigenin in promoting sleep and potentially attenuating age-related cognitive decline positions it as a unique molecule at the intersection of sleep and aging [107].
A study conducted by Kambe and colleagues suggests that quercetin may influence sleep architecture, partly through the activation of GABA-A receptors. The findings revealed that quercetin alters the sleep–wake cycle in rats by reducing REM sleep and increasing non-REM sleep during dark periods [109].
A study suggests that Suanzaoren Decoction, a traditional Chinese herbal remedy, may help treat sleep disorders in Parkinson’s disease. The combination of key active compounds, quercetin and kaempferol, targets important molecules such as AKT1, IL6, MAPK1, and VEGFA, potentially contributing to its therapeutic effects [110].
Apigenin, when administered in combination with a GABA-A receptor agonist, has been shown to prolong sleep duration and increase sleep rate, as well as improve sleep onset in animal models [107]. Hydroxy-cinnamic acid derivatives have been identified as agonists for GABA receptors and act synergistically with 5-hydroxytryptophan (5-HTP), both of which play a role in sleep quality [111]. These compounds can provide sedative effects, extend sleep duration, and reduce sleep latency.
Cinnamic acid (CA), a phenolic compound in propolis [112], has been explored for its antioxidant properties [113]. Its effects on circadian rhythms were assessed by monitoring wheel-running activity in constant darkness. To reduce daily fluctuations impacting the circadian clock, CA was administered continuously via intraperitoneal injection using mini pumps, addressing its short half-life. The data indicate that CA and its derivatives, like ferulic acid, may significantly influence free-running rhythms, potentially through metabolic pathways. These compounds have potential for assisting in the prevention or treatment of sleep disorders induced by environmental factors such as jet lag or shift work [114]. The inclusion of CA, along with apigenin and quercetin, in sleep disorder research could create new opportunities for developing therapeutic strategies based on natural compounds.
4.4. Gastrointestinal Dysfunction
Gastrointestinal dysfunction is acknowledged as a prevalent and challenging non-motor feature of PD, affecting up to 80% of patients [115,116]. Mounting evidence has highlighted the aggregation of α-syn in the enteric nervous system as a critical element in the onset and progression of PD, hinting at a potential association between gut dysfunction and the disease [117]. Braak and colleagues proposed an ’ascending anatomical theory’ after observing the dissemination pattern of α-syn aggregates, suggesting that PD advances from the GI system to the brain [118,119]. In turn, the body-first and brain-first hypotheses of PD were introduced by Borghammer and collaborators, reshaping our understanding of PD [120]. According to the body-first subtype, the disease is suggested to start in the intestine or peripheral autonomic nervous system, which could offer a more comprehensive rationale for the heterogeneity of clinical presentations observed across PD. An emerging theory underscores chronic gut pro-inflammatory processes as central, albeit silent, contributors to PD pathogenesis [121].
GI dysfunction preceding motor symptoms by as much as 20 years is well documented, with significant implications for the quality of life of individuals [122]. Within this context, constipation stands out as a common prodromal manifestation of PD. Kakino et al.’s study delineated the laxative attributes of utilizing water extract obtained from Brazilian green propolis [123]. This was demonstrated by a significant elevation in fecal mass and the restoration of stool frequency in an animal model of constipation induced by clonidine. Additionally, this intervention induced a notable augmentation in the contractile force of the isolated ileum in rodents. This enhancement was blocked by atropine, an antagonist of acetylcholine receptors, indicating that this effect is, to some extent, mediated through the activation of acetylcholine receptors [123].
Irritable bowel syndrome (IBS) without diarrhea is also recognized as a potential risk factor of PD [124,125,126]. Recent research indicated that propolis might offer utility as a supplement for individuals with IBS with constipation [127]. In this study, patients treated with propolis for a period of six weeks experienced a marked reduction in both the severity and frequency of abdominal pain in comparison to those on placebo. The effects of propolis have been attributed to its ability to aid in supporting the immune system of the GI tract and fostering beneficial gut bacteria [128]. Moreover, propolis exhibits potent antioxidant properties and can also assist in decreasing inflammation and improving nutrient absorption. Administering pinocembrin, a bioactive component found in propolis, demonstrated effectiveness against colitis by inhibiting the aberrant activation of toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) signaling in mice. Furthermore, this intervention reversed disruptions in gut microbiota and enhanced the integrity of the intestinal barrier in mice with ulcerative colitis [129].
Increasing evidence suggests that disruptions in the gut microbiome significantly contribute to the initiation and progression of PD pathology [130]. Disturbances in the symbiotic relationship between hosts and microbes result in alterations in the composition of bacteria that produce short-chain fatty acids (SCFAs) and a rise in potentially harmful intestinal pathogens [131]. As a consequence, intestinal permeability becomes compromised, facilitating the passage of microbiome-derived metabolites into the bloodstream, inducing inflammation, oxidative stress, and systemic immune reactions [132,133]. Preclinical studies have pointed out that the use of propolis can contribute to the restoration of damaged intestinal mucosa, positively impact the flora community, and boost the production of metabolites such as SCFAs [134]. SCFAs are hypothesized to have a significant role in regulating neuroimmunoendocrine functions [135]. For instance, manipulating the microbiota is posited as a potential strategy for managing PD. The expanding body of evidence proposes that propolis has the potential to enhance gut health and warrants consideration for studies investigating its utility as a prebiotic agent [136]. Propolis supplementation extends beyond the increase in SCFA content, showcasing the potential to enhance richness and diversity in gut flora, along with a reduction in both endotoxemia and inflammatory biomarkers [137]. An increasing body of research suggests that dietary propolis has favorable impacts on addressing diverse diseases by virtue of its ability to influence gut microbiota regulation [134,136,137,138,139,140].
The research undertaken by Palacios et al. yielded significant insights into the involvement of the gut microbiome in PD, particularly in its prodromal stage, involving participants with newly diagnosed PD, prodromal PD manifestations, constipation controls, and healthy individuals [141]. The study revealed a depletion in various gut butyrate-producing bacteria, known for their anti-inflammatory properties, including Anaerostipes, in both PD patients and those displaying prodromal PD features. These findings suggest that alterations in the microbiome could potentially serve as new indicators for the initial stages of PD [141]. Other studies have found evidence of a link between Anaerostipes and the risk of developing PD [142]. There is evidence indicating that fermenting propolis with the gut microbiota of obese children resulted in the enhancement of particular genera of strict anaerobes, including Anaerostipes [136]. Another investigation revealed that quercetin, a predominant flavonoid compound in propolis, reduces gut inflammation and enhances intestinal function by bolstering the proportion of SCFA-producing bacteria in lipopolysaccharide-exposed laying hens [143].
According to the latest findings, polyphenols, including those contained in propolis, are considered prebiotics as they are selectively metabolized by the host’s intestinal microorganisms and contribute to health benefits [144,145]. Propolis polyphenols may assist in fostering a favorable intestinal microbiota environment by restraining the growth of harmful bacteria and hindering the adherence of intestinal pathogens to human intestinal cells [144]. Wang and colleagues demonstrated that administering polyphenol-rich propolis resulted in positive impacts on human intestinal epithelial Caco-2 cells, a commonly utilized in vitro model for assessing intestinal barrier function [146]. The treatment resulted in a significant improvement in tight junction integrity, as evidenced by the rise in transepithelial electrical resistance and the concurrent reduction in flux rates. These regulatory effects on intestinal barrier function were partly facilitated by AMP-activated protein kinase (AMPK) and Extracellular Signal-Regulated Kinases (ERK1/2) activation and were negatively regulated by p38 MAPK signaling. AMPK acts as a key regulator of energy metabolism, and its activation strengthens epithelial barrier function and facilitates tight junction assembly [147]. ERK1/2, a member of the mitogen-activated protein kinase (MAPK) family, participates in signal transduction pathways. In turn, the p38 MAPK pathway is recognized for its involvement in relaying stress signals from the external environment [148].
In addition, Wang et al. documented that rodents fed a diet enriched with polyphenol-rich propolis showed an increased expression of the cytoplasmic scaffolding protein zonula occludens (ZO-1), a protein associated with tight junctions, in the colonic epithelium [146]. These outcomes suggest promising avenues for leveraging polyphenol-rich propolis to improve human gut health. Other investigations have also shown that polyphenolic compounds such as quercetin and kaempferol present in propolis possess the capability to enhance gut barrier function by affecting the cytoskeletal association and expression of tight junction integral membrane proteins in an intestinal epithelial barrier model [149].
The gastric pathogen Helicobacter pylori is implicated in chronic gastritis, ulcers, and gastric cancer, while releasing a pro-inflammatory enzyme known as urease. It has also been suggested as a potential initiator of neurodegenerative diseases [150,151,152]. Moreover, epidemiological research has described that patients with PD are at a higher risk of Helicobacter pylori infection [152]. Additionally, data reveal that these patients are typically older and exhibit more severe motor dysfunction, hinting at a possible association between Helicobacter pylori infection and the progression of PD [152]. Baltas et al. conducted research highlighting the protective properties of ethanolic propolis extracts against Gram-negative Helicobacter pylori. Their study revealed a notable suppression in both the growth and urease production of Helicobacter pylori upon propolis treatment [153]. Another study’s findings illustrated that Korean propolis possesses the capability to hinder Helicobacter pylori proliferation while diminishing the occurrence of its virulence elements, encompassing the cytotoxin-associated gene A responsible for encoding the urease A subunit, the surface antigen gene, and neutrophil-activating protein A [154]. Korean propolis treatment also resulted in a significant reduction in the release of pro-inflammatory cytokines and nitric oxide (NO) levels. More precisely, the findings revealed that the treatment inhibited the phosphorylation of IκBα and NF-κB p65 subunits, leading to the downregulation of their downstream target genes [154]. A mounting body of research underscores the potential of propolis supplementation as a natural means to counter Helicobacter pylori infection [153,154,155,156,157,158].
Investigations have pointed to a high prevalence of small intestinal bacterial overgrowth (SIBO) within the PD population [159]. Reports indicate that approximately half of PD patients exhibit SIBO, whereas its occurrence in individuals without PD stands at 8%. It raises concerns that SIBO may interfere with the absorption of medications in PD individuals [159]. Moreover, SIBO is a mechanism implicated in promoting bacterial translocation. In their research, Sabuncuoglu et al. provided evidence suggesting that propolis administration lowers bacterial translocation. This is achieved by diminishing bacterial overgrowth, improving mucosal barrier function, while reinforcing immune responses in an experimental model that affects multiple layers of gut barrier defense [160].
Despite the well-documented effects of propolis as a potent functional food capable of regulating intestinal microbiota, inflammation, metabolism, and immune response in different scenarios, its direct impact on GI disruptions in PD remains largely unexplored in scientific research. Nonetheless, given the potential implications of GI-related disturbances in PD, it is an area of interest for future research.
4.5. Cardiovascular Problems
Cardiac dysfunctions have been detected in approximately 80% of PD patients and can cause considerable disability and negatively impact quality of life, contributing to reduced life expectancy [56,161,162,163]. Autonomic nervous system dysfunction often occurs in PD, with damage to both the sympathetic and parasympathetic systems contributing to irregular blood pressure regulation and decreased heart rate variability (HRV), increasing the risk of cardiovascular complications [164]. Cardiac dysautonomia in PD includes symptoms such as orthostatic and postprandial hypotension, and both supine and postural hypertension [165,166]. PD patients are also more prone to ischemic heart disease, heart failure, and arrhythmias [166]. Moreover, non-dipping is highly prevalent among PD individuals, with a prevalence of up to 90% [167]. In healthy individuals, blood pressure typically decreases at night, a phenomenon known as dipping, whereas a failure to experience this nocturnal drop is referred to as non-dipping.
Additionally, sudden unexpected death in Parkinson’s disease (SUDPAR) is a critical aspect that cannot be overlooked, as it highlights the complex interplay of neurodegenerative progression, autonomic dysfunction, and potential cardiac complications associated with the disease [161,168,169,170,171]. SUDPAR is defined as an unexpected death in PD patients where no definitive cause can be determined through autopsy.
PD patients have a higher prevalence of type 2 diabetes, which increases cardiovascular disease risk [172].
Conversely, research indicates that there is an increased risk of PD in individuals with type 2 diabetes, and the potential benefit of antidiabetic medication in influencing the course of PD has been suggested [173,174,175,176,177]. While research has revealed a complex link between PD, cardiovascular diseases, and type 2 diabetes, the underlying mechanisms remain unclear; however, these may involve common factors such as disrupted lipid and energy metabolism, inflammation, and oxidative stress [178]. There is significant evidence that propolis and its bioactive constituents can counteract the primary mechanisms involved in the pathophysiology of these diseases [25,32,35,55,179,180,181].
The bioactive compounds of propolis target inflammatory pathways by downregulating pro-inflammatory cytokines via the inhibition of the NF-κB pathway and mitigating oxidative stress through the activation of the Nrf2 pathway, enhancing the activity of endogenous antioxidant enzymes [144,182,183]. The compounds in propolis also modulate critical metabolic pathways involved in glucose, insulin, and lipid metabolism [185,186,187,188]. They activate AMPK, enhancing glucose uptake and improving insulin sensitivity, and influence peroxisome proliferator-activated receptors (PPARs), promoting lipid oxidation and reducing lipid accumulation [189,190,191,192,193,194]. Propolis compounds can improve endothelial function, reduce atherosclerotic plaque formation, and attenuate myocardial oxidative damage [59,180,195,196,197,198,199,200,201,202]. Propolis and its constituents influence key signaling molecules like NO and endothelial NO synthase (eNOS), promoting vasodilation and enhancing vascular health [203,204,205,206]. Therefore, the comprehensive actions of propolis and its compounds underscore its therapeutic potential in alleviating cardiovascular complications in PD by bolstering mitochondrial function, balancing energy and lipid metabolism, reducing inflammation, and counteracting oxidative stress.
Ji and collaborators demonstrated a significant improvement in carotid obstruction, the amelioration of vascular lesions, a reduction in serum lipid profiles, and improved antioxidant activities in hypercholesterolemic rabbits following propolis administration. This effect is attributed to the downregulation or inhibition of pro-inflammatory cytokine expression, leading to the regulation of blood lipid levels and the mitigation of oxidative stress and inflammation [207]. El-Hakam and collaborators used a combination of propolis, royal jelly, and bee venom to treat hypertensive rats and noted a significant reduction in blood pressure. The flavonoids and enzymes inherent in propolis exhibit the capability to eliminate free radicals and inhibit the formation of lipid peroxides [208].
Another study found that caffeic acid phenethyl ester (CAPE), when administered to aged rats, increased the expression of antioxidant enzymes and significantly mitigated age-related ultrastructural changes in the heart and aorta [209]. Moreover, ultrastructural images of the heart and aorta showed that aged rats treated with CAPE displayed characteristics nearly identical to those observed in young rats [209]. Research findings reveal that galangin can effectively lower arterial pressure, blood glucose, insulin, and cholesterol levels in rats with metabolic syndrome [205]. Additionally, the galangin-treated group showed an increased aortic eNOS protein, elevated levels of circulating NO, and improvements in aortic endothelial dysfunction and hypertrophy. Galangin also played a role in reducing inflammation and suppressing the angiotensin II/g II/AT1R/TGF-β signaling pathway in the metabolic syndrome group [205]. Studies suggest that CAPE may serve as a promising cardioprotective agent with anti-inflammatory, antioxidant, and antiapoptotic effects, and antiarrhythmic activity [206,207,208,209,210,248,249,250,251]. However, there are significant gaps in the literature regarding the effects of propolis and its compounds on cardiovascular dysfunction in PD.
A study conducted by our research group, utilizing positron emission tomography imaging, demonstrated that rats treated with propolis exhibited increased glucose metabolism in the left ventricle compared to controls [252]. Enhanced glucose metabolism in cardiac tissue often suggests improved energy availability, which is crucial for maintaining heart function. Additionally, an untargeted metabolomics approach was employed to investigate the impact of propolis on cardiac metabolism in rats with PD, revealing its role in pathways related to oxidative stress, energy metabolism, protein and fatty acid biosynthesis, and the urea cycle [252]. Another investigation by our group showed that propolis treatment offered cardioprotective effects by countering the reductions in heart rate and HRV observed in rats with PD [35]. The examination of HRV is a non-invasive and sensitive indicator of autonomic changes. HRV reflects the capacity of the autonomic system to adapt to both external and internal stimuli, with lower HRV suggesting impaired autonomic function. Research has demonstrated that PD patients have cardiovascular changes, including decreased heart rate and HRV measurements [253,254]. Moreover, a prospective study suggested a link between decreased HRV in the non-PD population and a higher risk of developing the disease [255]. Therefore, propolis’s capacity to increase HRV suggests its potential to improve autonomic system adaptability, positioning it as a promising therapeutic agent for addressing autonomic dysfunction in PD.
4.6. Pain
Chronic pain is one of the most debilitating non-motor symptoms of PD. It is often underrecognized and poorly managed, affecting up to 85% of patients [256,257,258,259]. Despite its high prevalence, the mechanisms underlying pain in PD are difficult to diagnose and manage effectively. Pain in PD results from a complex interplay of central and peripheral factors, with primary causes including dopamine circuit dysfunction, altered pain pathways, increased musculoskeletal issues, disrupted inflammatory signals, and elevated oxidative stress [211,259].
Traditionally, pain in PD has been categorized based on its location and the temporal relationship to motor symptoms. However, recent advances in the understanding of pain mechanisms have led to the development of a new classification framework that emphasizes the underlying pathophysiological processes [212]. This new classification categorizes pain into three distinct types: nociceptive, neuropathic, and nociplastic, which applies when pain is neither nociceptive nor neuropathic [212,213,214,215]. This approach is in alignment with the International Classification of Diseases-11 (ICD-11), which recognizes the potential for chronic secondary musculoskeletal or nociceptive pain due to central nervous system disease. By incorporating mechanistic descriptors into the classification of pain, this framework offers a more nuanced understanding of the different pain types that may occur in PD, thus facilitating more targeted and effective treatment strategies.
Common pain types experienced by people with PD are dystonic, musculoskeletal, neuropathic, and central pain [216,217]. Given the complexity of pain and the global challenge of managing it effectively due to the adverse effects of conventional therapies like Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), opioids, and opiates, there is growing interest in natural substances like propolis and its bioactive constituents, which offer analgesic, anti-inflammatory, and antioxidant properties with minimal or no side effects [218].
Propolis has been extensively studied for its analgesic and antinociceptive effects, with a substantial body of literature supporting its efficacy in alleviating pain [219,220,221]. Research consistently indicates that propolis supplementation may provide therapeutic benefits for rheumatoid arthritis patients by dampening inflammatory cascades via NF-kB pathway inhibition, decreasing ROS, malondialdehyde, and pro-inflammatory cytokines, while also enhancing antioxidants and relieving pain [222]. According to a systematic review and meta-analysis of controlled clinical trials, propolis supplementation is safe and can improve levels of glutathione (GSH), glutathione peroxidase (GPX), and total antioxidant capacity (TAC), pointing to its usefulness as an adjunct therapy in conditions associated with oxidative stress [223].
For instance, research investigating the effects of Brazilian green propolis in different mouse models of pain and inflammation revealed significant analgesic and anti-inflammatory actions, with the latter linked to the inhibition of the iNOS gene [224]. The ability of propolis to inhibit cyclooxygenase-2 (COX-2) is also crucial in its modulation of inflammatory processes, with the potential to alleviate pain and prevent the progression of inflammation-related tissue damage [225,226,227,228,229]. COX-2 is an enzyme that catalyzes the conversion of arachidonic acid into prostaglandins, which are lipid compounds that play a key role in promoting inflammation, fever, and pain. In many pathological conditions, including neurodegenerative diseases, chronic inflammation, and pain, COX-2 is upregulated, leading to the excessive production of pro-inflammatory prostaglandins.
Studies have also reported the anti-hyperalgesic effects of the ethanolic extract of propolis in chemical nociception models in rats and mice [230]. The extract of propolis has been shown to reduce neurogenic and inflammatory pain induced by the intraplantar administration of formalin, decrease paw edema, significantly inhibit capsaicin-induced pain, and reverse bradykinin-induced hyperalgesia in rodents [230]. The study by Azevedo et al. suggested that propolis flavonoids rutin and quercetin can counteract the peripheral neuropathy induced by oxaliplatin, a colorectal cancer chemotherapy agent whose use is often limited by its painful neuropathic effects. Their research found that these propolis constituents prevented neuropathy in rodents and protected dorsal horn neurons from oxidative stress, lipid peroxidation, and protein nitrosylation [231].
Evidence also indicates that CAPE, a phenolic ester compound of propolis, mitigates neuropathic pain, with its effects mainly attributed to the suppression of p38 MAPK phosphorylation, the inhibition of NF-κB translocation, and the downregulation of pro-inflammatory cytokines [232]. Research has found that CAPE relieves neuropathic pain in paclitaxel-treated rats through the inhibition of β-catenin and inflammation, along with an increase in the levels of the endogenous antioxidant Nrf2 [233].
In individuals with PD, musculoskeletal pain is linked to rigidity, reduced movement, and arthritis. CAPE reduces osteoarthritis progression through the activation of the NRF2/HO-1 pathway and suppression of the NF-κB pathway, two key regulators of oxidative stress and inflammation [234]. In response to oxidative stress or other cellular stressors, NRF2 is released and translocates to the nucleus, where it binds to antioxidant response elements (AREs) in the promoters of its target genes, such as HO-1 [235,236]. Evidence suggests that HO-1 treatment amplifies the local antinociceptive effects of opioid receptor agonists during chronic inflammatory pain in mice, involving the activation of the carbon monoxide (CO)/NO-cGMP-PKG-KATP channel’s signaling pathway [237,238,239].
Chrysin is another compound found in propolis that shows robust analgesic and antinociceptive effects. It exhibits antinociceptive actions in models of formalin-induced pain and diabetic neuropathy, with its action likely mediated by spinal opioid receptors and the CREB protein [240]. In rats with knee osteoarthritis, chrysin can alleviate neuropathic pain and reduce peripheral sensitization by inhibiting both the HMGB1-mediated activation of the RAGE/PI3K/AKT pathway and the NLRP3 inflammasome, leading to a reduction in synovitis [241]. Chrysin was found to significantly reduce heat-induced hyperalgesia and mechanical allodynia in a mouse model of experimental mononeuropathy through the involvement of serotonin 5-HT1A receptors [242]. Moreover, GABA-A receptors play a crucial role in mediating the hyperalgesic effects of chrysin observed in the tail-immersion test in mice [243].
A comprehensive examination of the analgesic and anti-inflammatory effects of propolis flavonoids such as quercetin, apigenin, luteolin, kaempferol, myricetin, hesperetin, and naringenin is documented in excellent reviews [244,245,246]. Although research on the effects of propolis and its compounds on pain in PD is still limited, current evidence highlights significant potential and growing interest in this promising field.
4.7. Respiratory Dysfunctions
Respiratory dysfunctions are major determinants of morbidity and mortality in PD [260]. They include a range of symptoms: respiratory dysrhythmias, tachypnea, dyspnea, sleep-related breathing disorders, and others, resulting in a complex clinical presentation that drastically reduces patient’s quality of life [261,262]. Aspiration pneumonia is cited as the leading cause of death in PD patients, primarily due to oropharyngeal dysphagia and impaired cough reflex [263].
PD-related respiratory changes are considered peripheral when associated with upper airway or breathing muscle dysfunction and central when driven by alterations in the brainstem’s respiratory control centers [260,264]. For instance, studies have shown that the neurodegeneration of medullary respiratory neurons results in respiratory impairment in PD animal models, with oxidative stress identified as a key underlying mechanism [265,266]. Given the antioxidant and anti-inflammatory properties of propolis, it could theoretically help reduce oxidative damage and protect neuronal cells within central respiratory nuclei. However, it is important to note that, despite the promising neuroprotective effects already observed in dopaminergic systems, there is currently a lack of studies directly investigating the role of propolis in the respiratory centers of the brainstem of PD patients. This points to a critical gap in the literature and could serve as a key focus for future research.
Studies showed that genistein, a phytoestrogen found in propolis, protects the genioglossus, a pharyngeal muscle essential for maintaining an open upper airway, from hypoxia-induced oxidative stress and apoptosis by engaging the PI3K-Akt and ERK1/2 MAPK signaling pathways [267,268,269]. Hypoxia is suggested as a key inducer of neuroinflammation by stimulating microglia to release pro-inflammatory cytokines [270]. Evidence points to the neuroprotective properties of Brazilian green propolis against hypoxia-driven neuroinflammation through the inhibition of microglial NF-κB activation [271]. The administration of quercetin prior to hypoxia exposure in rats reinstated the normal levels of Nrf2, HO-1, and pulmonary surfactant proteins, effectively countering the increase in inflammation, oxidative stress, and plasma protein leakage [247].
Numerous studies have highlighted the benefits of propolis and its biologically active compounds in treating respiratory tract infections [184,272,273,274]. Current research efforts have examined the anti-SARS-CoV-2 activity of propolis and its constituents [179,275,276,277,278,279]. For example, a clinical trial involving 124 patients hospitalized with COVID-19 found that those who consumed Brazilian green propolis had a shorter hospital stay [280]. Additionally, propolis may offer potential benefits by modulating the expression of transmembrane serine protease 2 (TMPRSS2), which plays a role in SARS-CoV-2 infection, and preventing the virus from binding to angiotensin-converting enzyme 2 (ACE-2) receptors, thus reducing its ability to enter cells. Propolis also shows promise in reducing inflammation through PAK1 inhibition [280].
5. Conclusions and Future Perspectives
While propolis and its constituents appear promising for addressing the non-motor symptoms of PD, this field of research is still underexplored. Comprehensive research is urgently needed to reveal their mechanisms of action, safety, and efficacy, which may offer new therapeutic insights and options for PD patients.
The study of natural products like propolis faces significant challenges that must be addressed to fully realize their therapeutic potential. One major limitation is the variability in propolis composition, which can result in inconsistent findings across different studies. Achieving standardization is essential to ensure consistency and reproducibility. This involves defining and controlling parameters such as the specific bioactive compounds, concentration, and extraction methods of propolis.
Another critical issue is the limited understanding of the pharmacokinetics and pharmacodynamics of propolis and its constituents. The complex mixture of bioactive compounds in propolis interacts with multiple biological targets, complicating the identification of specific mechanisms of action. Additionally, the poorly understood interactions with other nutritional supplements and conventional medications add layers of complexity to the research.
To advance the field, it is crucial to expand research involving human participants, as most studies on propolis have relied predominantly on animal models and in vitro experiments. Furthermore, the older adult population is often excluded from research, which limits the applicability of findings to this age group. Given that older adults may have different physiological responses and comorbidities, future studies should prioritize aged animals and elderly human participants to improve relevance and applicability.
Addressing the research gaps is not only important for advancing scientific knowledge but also crucial for developing new, effective therapeutic strategies for managing the non-motor symptoms of PD. As these symptoms significantly impact patients’ quality of life and are less responsive to conventional treatments, investigating the potential of propolis and its components offers an exciting opportunity for novel interventions in PD.
Author Contributions
Conceptualization, C.A.S.; writing—original draft preparation, C.A.S., K.V.M., and V.d.C.G.; writing—review and editing, C.A.S., K.V.M.; V.d.C.G., J.F., F.A.S., and R.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the São Paulo Research Foundation (FAPESP), grant number 2020/12791-6, Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant number 304268/2020-8, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PD | Parkinson’s disease |
| BDNF | Brain-derived neurotrophic factor |
| REM | Rapid Eye Movement |
| SAS | Sleep-Apnea Syndrome |
| CREB | cAMP Response Element Binding |
| GABA | Gamma-Aminobutyric Acid |
| CA | Cinnamic Acid |
| IBS | Irritable Bowel Syndrome |
| SCFA | Short-Chain Fatty Acids |
| AMPK | Active Adenosine Monophosphate-Activated Protein Kinase |
| cGMP | Cyclic Guanosine Monophosphate |
| PKG | Protein Kinase G |
| KATP | Adenosine Triphosphate-Sensitive Potassium |
| ERK 1/2 | Extracellular Signal-Regulated Kinases |
| IκBα | NF-kB Inhibitor Alpha |
| SIBO | Small Intestinal Bacterial Overgrowth |
| SUDPAR | Sudden Unexpected Death in Parkinson’s Disease |
| NO | Nitric Oxide |
| CAPE | Caffeic Acid Phenethyl Ester |
| SARS-Cov-2 | Severe Acute Respiratory Coronavirus 2 |
| COVID-19 | Coronavirus 19 |
| NF-kB | Nuclear Factor Kappa |
| COX-2 | Cyclooxygenase 2 |
| PGE-2 | Prostaglandin E2 |
| TGF-β | Transforming Growth Factor Beta |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| IL-6 | Interleukin-6 |
| IL-IB | Interleukin-1Beta |
| IL-8 | Interleukin-8 |
| TNF- α | Tumor Necrosis Factor Alpha |
| IFN- γ | Interferon-Gamma |
| IKB-kinase | Inhibitory Kappa B Kinase Beta |
| MAPK | Mitogen-Active Protein Kinase |
| JAK-STAT | Janus Kinase-Signal Transducers and Activators of Transcription |
| NRF2/Keap1-ARE | Nuclear Factor Erythroid 2-Related Factor/Kelch-Like ECH-Associated Protein 1-Antioxidant Response Element |
| HO-1 | Heme Oxygenase-1 |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| GPx | Glutathione Peroxidase |
| GR | Glutathione Reductase |
| CAT | Catalase |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| M1/M2 | Macrophages |
| HMGB1 | High-Mobility Group Box 1 Protein |
| DC | Dendritic Cell |
| CD4+T | T-Lymphocytes |
| Trag cell | Regulatory T Cells |
| FOXP3 | Forkhead Box P3 |
| TLR2/TRL4 | Toll-Like Receptor 2/4 |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NLRP3 | Nucleotide-Binding Domain Leucine-Rich-Containing Family Pyrin Domain-Containing-3 |
| Th cell | Helper T Cells |
| NK cell | Natural Killer Cell |
| B cells | B Lymphocyte Cells |
| AKt | Protein Kinase B |
| mTOR | Mammalian Target of Rapamycin |
| sre | Sterol Response Element |
References
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar]
- Dorsey, E.R.; Bloem, B.R. Parkinson’s Disease Is Predominantly an Environmental Disease. J. Park. Dis. 2024, 14, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.v.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar]
- World Health Organization. Parkinson Disease. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (accessed on 1 August 2024).
- Blauwendraat, C.; Nalls, M.A.; Singleton, A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020, 19, 170–178. [Google Scholar] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [PubMed]
- Tsalenchuk, M.; Gentleman, S.M.; Marzi, S.J. Linking environmental risk factors with epigenetic mechanisms in Parkinson’s disease. npj Park. Dis. 2023, 9, 123. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22, S119–S222. [Google Scholar] [CrossRef]
- Rana, A.Q.; Ahmed, U.S.; Chaudry, Z.M.; Vasan, S. Parkinson’s disease: A review of non-motor symptoms. Expert. Rev. Neurother. 2015, 15, 549–562. [Google Scholar]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar]
- Poewe, W. Parkinson disease Primer—A true team effort. Nat. Rev. Dis. Prim. 2020, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.-M.; Yao, C.; Pelletier, A.; Montplaisir, J.Y.; Gagnon, J.-F.; Postuma, R.B. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 2019, 142, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- Schaeffer, E.; Berg, D. Dopaminergic Therapies for Non-motor Symptoms in Parkinson’s Disease. CNS Drugs 2017, 31, 551–570. [Google Scholar] [CrossRef]
- Wüllner, U.; Borghammer, P.; Choe, C.-U.; Csoti, I.; Falkenburger, B.; Gasser, T.; Lingor, P. The heterogeneity of Parkinson’s disease. J. Neural Transm. 2023, 130, 827–838. [Google Scholar] [CrossRef]
- Rehman, M.U.; Sehar, N.; Dar, N.J.; Khan, A.; Arafah, A.; Rashid, S.; Rashid, S.M.; Ganaie, M.A. Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: An update on current advances and impediments. Neurosci. Biobehav. Rev. 2023, 144, 104961. [Google Scholar] [CrossRef]
- Stoker, T.B.; Greenland, J.C. (Eds.) Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Singapore, 2018. [Google Scholar]
- Balaha, M.; de Filippis, B.; Cataldi, A.; di Giacomo, V. CAPE and Neuroprotection: A Review. Biomolecules 2021, 11, 176. [Google Scholar] [CrossRef]
- Farooqui, T. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci. 2012, E4, 418. [Google Scholar] [CrossRef]
- da Silveira, C.C.S.M.; Luz, D.A.; da Silva, C.C.S.; Prediger, R.D.S.; Martins, M.D.; Martins, M.A.T.; Fontes-Júnior, E.A.; Maia, C.S.F. Propolis: A useful agent on psychiatric and neurological disorders? A focus on CAPE and pinocembrin components. Med. Res. Rev. 2021, 41, 1195–1215. [Google Scholar]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Scorza, C.; Goncalves, V.; Finsterer, J.; Scorza, F.; Fonseca, F. Exploring the Prospective Role of Propolis in Modifying Aging Hallmarks. Cells 2024, 13, 390. [Google Scholar] [CrossRef]
- Zulhendri, F.; Ravalia, M.; Kripal, K.; Chandrasekaran, K.; Fearnley, J.; Perera, C.O. Propolis in Metabolic Syndrome and Its Associated Chronic Diseases: A Narrative Review. Antioxidants 2021, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Biomedical Properties of Propolis on Diverse Chronic Diseases and Its Potential Applications and Health Benefits. Nutrients 2020, 13, 78. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Galvéz-Ruíz, J.C.; Ikner, L.A.; Umsza-Guez, M.A.; Castro, T.L.d.P.; Gerba, C.P. In vitro antiviral effect of Mexican and Brazilian propolis and phenolic compounds against human coronavirus 229E. Int. J. Environ. Health Res. 2023, 33, 1591–1603. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef]
- Ali, A.A.; Kamal, M.M.; Khalil, M.G.; Ali, S.A.; Elariny, H.A.; Bekhit, A.; Wahid, A. Behavioral, Biochemical and Histopathological effects of Standardised Pomegranate extract with Vinpocetine, Propolis or Cocoa in a rat model of Parkinson’s disease. Exp. Aging Res. 2022, 48, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Pyrgelis, E.-S.; Piperi, C. Neuroprotective potential of chrysin in Parkinson’s disease: Molecular mechanisms and clinical implications. Neurochem. Int. 2020, 132, 104612. [Google Scholar]
- Gonçalves, V.C.; de la Rosa, T.; de Almeida, A.-C.G.; Scorza, F.A.; Scorza, C.A. Propolis as a Potential Disease-Modifying Strategy in Parkinson’s disease: Cardioprotective and Neuroprotective Effects in the 6-OHDA Rat Model. Nutrients 2020, 12, 1551. [Google Scholar] [CrossRef] [PubMed]
- Goes, A.T.; Jesse, C.R.; Antunes, M.S.; Ladd, F.V.L.; Ladd, A.A.L.; Luchese, C.; Paroul, N.; Boeira, S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Interact. 2018, 279, 111–120. [Google Scholar] [CrossRef]
- Safari, M.; Sameni, H.R.; Badban, L.; Bandegi, A.R.; Vafaei, A.A.; Pour, A.R.; Ghahari, L. Protective Effects of Water Extract of Propolis on Dopaminergic Neurons, Brain Derived Neurotrophic Factor and Stress Oxidative Factors in the Rat Model of Parkinson’s Disease. Int. J. Pharmacol. 2015, 11, 300–308. [Google Scholar]
- Maillet, A.; Krack, P.; Lhommée, E.; Météreau, E.; Klinger, H.; Favre, E.; Le Bars, D.; Schmitt, E.; Bichon, A.; Pelissier, P.; et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 2016, 139, 2486–2502. [Google Scholar] [PubMed]
- Upneja, A.; Paul, B.S.; Jain, D.; Choudhary, R.; Paul, G. Anxiety in Parkinson’s Disease: Correlation with Depression and Quality of Life. J. Neurosci. Rural. Pract. 2021, 12, 323–328. [Google Scholar]
- Carey, G.; Görmezoğlu, M.; de Jong, J.J.; Hofman, P.A.; Backes, W.H.; Dujardin, K.; Leentjens, A.F. Neuroimaging of Anxiety in Parkinson’s Disease: A Systematic Review. Mov. Disord. 2021, 36, 327–339. [Google Scholar]
- Bareeqa, S.B.; Samar, S.S.; Kamal, S.; Masood, Y.; Allahyar; Ahmed, S.I.; Hayat, G. Prodromal Depression and Subsequent Risk of Developing Parkinson’s Disease: A Systematic Review With meta-analysis. Neurodegener. Dis. Manag. 2022, 12, 155–164. [Google Scholar] [CrossRef]
- Cong, S.; Xiang, C.; Zhang, S.; Zhang, T.; Wang, H.; Cong, S. Prevalence and clinical aspects of depression in Parkinson’s disease: A systematic review and meta-analysis of 129 studies. Neurosci. Biobehav. Rev. 2022, 141, 104749. [Google Scholar] [CrossRef]
- Prange, S.; Klinger, H.; Laurencin, C.; Danaila, T.; Thobois, S. Depression in Patients with Parkinson’s Disease: Current Understanding of its Neurobiology and Implications for Treatment. Drugs Aging 2022, 39, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Agarwal, P. Depression and Anxiety in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Broen, M.P.G.; Narayen, N.E.; Kuijf, M.L.; Dissanayaka, N.N.W.; Leentjens, A.F.G. Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2016, 31, 1125–1133. [Google Scholar] [CrossRef]
- Haggard, P. Sense of agency in the human brain. Nat. Rev. Neurosci. 2017, 18, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Murueta-Goyena, A.; Andikoetxea, A.; Gómez-Esteban, J.C.; Gabilondo, I. Contribution of the GABAergic System to Non-Motor Manifestations in Premotor and Early Stages of Parkinson’s Disease. Front. Pharmacol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Pasquini, J.; Brooks, D.J.; Pavese, N. The Cholinergic Brain in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2021, 8, 1012–1026. [Google Scholar] [CrossRef]
- Caggiu, E.; Arru, G.; Hosseini, S.; Niegowska, M.; Sechi, G.; Zarbo, I.R.; Sechi, L.A. Inflammation, Infectious Triggers, and Parkinson’s Disease. Front. Neurol. 2019, 10, 122. [Google Scholar] [CrossRef]
- Lindqvist, D.; Kaufman, E.; Brundin, L.; Hall, S.; Surova, Y.; Hansson, O. Non-Motor Symptoms in Patients with Parkinson’s Disease—Correlations with Inflammatory Cytokines in Serum. PLoS ONE 2012, 7, e47387. [Google Scholar] [CrossRef]
- Chen, H.; O’Reilly, E.J.; Schwarzschild, M.A.; Ascherio, A. Peripheral Inflammatory Biomarkers and Risk of Parkinson’s Disease. Am. J. Epidemiol. 2007, 167, 90–95. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Yao, Z.; Zhang, T.; Li, Z. Dietary inflammatory potential and the incidence of depression and anxiety: A meta-analysis. J. Health Popul. Nutr. 2022, 41, 24. [Google Scholar] [PubMed]
- Varzaghani, V.; Sharifi, M.; Hajiaghaee, R.; Bagheri, S.; Momtaz, S.; Tarassoli, Z.; Razmi, A. Propolis add-on therapy alleviates depressive symptoms; A randomized placebo-controlled clinical trial. Phytother. Res. 2022, 36, 1258–1267. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s Disease: A Focus on the Effects of Propolis and Royal Jelly. Oxidative Med. Cell. Longev. 2020, 2020, 1727142. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.C.; Cuenca-Bermejo, L.; Fernandez-Villalba, E.; Martin-Balbuena, S.; da Silva Fernandes, M.J.; Scorza, C.A.; Herrero, M.-T. Heart Matters: Cardiac Dysfunction and Other Autonomic Changes in Parkinson’s Disease. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2022, 28, 530–542. [Google Scholar]
- Reis, J.S.; Oliveira, G.B.; Monteiro, M.C.; Machado, C.S.; Torres, Y.R.; Prediger, R.D.; Maia, C.S. Antidepressant- and anxiolytic-like activities of an oil extract of propolis in rats. Phytomedicine 2014, 21, 1466–1472. [Google Scholar]
- Yildiz, O.; Karahalil, F.; Can, Z.; Sahin, H.; Kolayli, S. Total monoamine oxidase (MAO) inhibition by chestnut honey, pollen and propolis. J. Enzym. Inhib. Med. Chem. 2014, 29, 690–694. [Google Scholar] [CrossRef]
- Celebi, G.; Gocmez, S.S.; Ozer, C.; Duruksu, G.; Yazır, Y.; Utkan, T. Propolis prevents vascular endothelial dysfunction by attenuating inflammation and oxidative damage in the chronic unpredictable stress model of depression in rats. J. Pharm. Pharmacol. 2023, 75, 1418–1429. [Google Scholar] [PubMed]
- Ali, S.; Corbi, G.; Maes, M.; Scapagnini, G.; Davinelli, S. Exploring the Impact of Flavonoids on Symptoms of Depression: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1644. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaokun, O.O. The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations. Biomedicines 2024, 12, 178. [Google Scholar] [CrossRef]
- Pannu, A.; Sharma, P.C.; Thakur, V.K.; Goyal, R.K. Emerging Role of Flavonoids as the Treatment of Depression. Biomolecules 2021, 11, 1825. [Google Scholar] [CrossRef]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Protective Effects of Quercetin on Anxiety-Like Symptoms and Neuroinflammation Induced by Lipopolysaccharide in Rats. Evid.-Based Complement. Altern. Med. 2020, 2020, 4892415. [Google Scholar]
- Filho, C.; Jesse, C.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; Antunes, M.d.S.; de Gomes, M.; Goes, A.; Boeira, S.; Prigol, M.; et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar]
- Shi, J.-X.; Cheng, C.; Ruan, H.-N.; Li, J.; Liu, C.-M. Isochlorogenic acid B alleviates lead-induced anxiety, depression and neuroinflammation in mice by the BDNF pathway. NeuroToxicology 2023, 98, 1–8. [Google Scholar] [CrossRef]
- Ni, J.; Wu, Z.; Meng, J.; Zhu, A.; Zhong, X.; Wu, S.; Nakanishi, H. The Neuroprotective Effects of Brazilian Green Propolis on Neurodegenerative Damage in Human Neuronal SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2017, 2017, 7984327. [Google Scholar]
- Guo, J.T.; Li, H.Y.; Cheng, C.; Shi, J.X.; Ruan, H.N.; Li, J.; Liu, C.M. Isochlorogenic acid A ameliorated lead-induced anxiety-like behaviors in mice by inhibiting ferroptosis-mediated neuroinflammation via the BDNF/Nrf2/GPX4 pathways. Food Chem. Toxicol. 2024, 190, 114814. [Google Scholar] [PubMed]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. Cognition Deficits in Parkinson’s Disease: Mechanisms and Treatment. Park. Dis. 2020, 2020, 1–11. [Google Scholar]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurology. 2017, 13, 217–231. [Google Scholar]
- Bohnen, N.I.; Yarnall, A.J.; Weil, R.S.; Moro, E.; Moehle, M.S.; Borghammer, P.; Bedard, M.-A.; Albin, R.L. Cholinergic system changes in Parkinson’s disease: Emerging therapeutic approaches. Lancet Neurol. 2022, 21, 381–392. [Google Scholar]
- Orlando, I.F.; Shine, J.M.; Robbins, T.W.; Rowe, J.B.; O’callaghan, C. Noradrenergic and cholinergic systems take centre stage in neuropsychiatric diseases of ageing. Neurosci. Biobehav. Rev. 2023, 149, 105167. [Google Scholar]
- Rizzi, G.; Tan, K.R. Dopamine and Acetylcholine, a Circuit Point of View in Parkinson’s Disease. Front. Neural Circuits 2017, 11, 110. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Biundo, R.; Dobkin, R.; Goldman, J.; Lewis, S. Management of psychiatric and cognitive complications in Parkinson’s disease. BMJ 2022, 379, e068718. [Google Scholar] [CrossRef]
- Asama, T.; Hiraoka, T.; Ohkuma, A.; Okumura, N.; Yamaki, A.; Urakami, K. Cognitive Improvement and Safety Assessment of a Dietary Supplement Containing Propolis Extract in Elderly Japanese: A Placebo-Controlled, Randomized, Parallel-Group, Double-Blind Human Clinical Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6664217. [Google Scholar] [CrossRef]
- Zhu, A.; Wu, Z.; Zhong, X.; Ni, J.; Li, Y.; Meng, J.; Du, C.; Zhao, X.; Nakanishi, H.; Wu, S. Brazilian Green Propolis Prevents Cognitive Decline into Mild Cognitive Impairment in Elderly People Living at High Altitude. J. Alzheimer’s Dis. 2018, 63, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Nanaware, S.; Shelar, M.; Sinnathambi, A.; Mahadik, K.; Lohidasan, S. Neuroprotective effect of Indian propolis in β-amyloid induced memory deficit: Impact on behavioral and biochemical parameters in rats. Biomed. Pharmacother. 2017, 93, 543–553. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Alrashidi, H.; Eaton, S.; Heales, S. Biochemical characterization of proliferative and differentiated SH-SY5Y cell line as a model for Parkinson’s disease. Neurochem. Int. 2021, 145, 105009. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Sugimoto, Y.; Fujita, A.; Kanouchi, H. Ethanol extract of Brazilian propolis ameliorates cognitive dysfunction and suppressed protein aggregations caused by hyperhomocysteinemia. Biosci. Biotechnol. Biochem. 2015, 79, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Perera, C.O.; Tandean, S. Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines 2021, 9, 1227. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Cheng, Z.; Xi, K.; Huang, X.; Kuang, F.; Wang, W.; Liu, T.; Guo, B.; Wu, S. Quercetin ameliorates memory impairment by inhibiting abnormal microglial activation in a mouse model of paradoxical sleep deprivation. Biochem. Biophys. Res. Commun. 2022, 632, 10–16. [Google Scholar] [CrossRef]
- Videnovic, A.; Golombek, D. Circadian and sleep disorders in Parkinson’s disease. Exp. Neurol. 2013, 243, 45–56. [Google Scholar]
- Parkinson’s Foundation. Sleep Disorders. Available online: https://www.parkinson.org/understanding-parkinsons/non-movement-symptoms/sleep-disorders (accessed on 6 June 2024).
- Schütz, L.; Sixel-Döring, F.; Hermann, W. Management of Sleep Disturbances in Parkinson’s Disease. J. Park. Dis. 2022, 12, 2029–2058. [Google Scholar]
- Zhang, X.; Molsberry, S.A.; Pavlova, M.; Schwarzschild, M.A.; Ascherio, A.; Gao, X. Association of Sleepwalking and REM Sleep Behavior Disorder With Parkinson Disease in Men. JAMA Netw. Open 2021, 4, e215713. [Google Scholar]
- Dodet, P.; Houot, M.; Leu-Semenescu, S.; Corvol, J.C.; Lehéricy, S.; Mangone, G.; Vidailhet, M.; Roze, E. Sleep disorders in Parkinson’s disease, an early and multiple problem. npj Park. Dis. 2024, 10, 46. [Google Scholar]
- Schrempf, W.; Brandt, M.D.; Storch, A.; Reichmann, H. Sleep Disorders in Parkinson’s Disease. J. Park. Dis. 2014, 4, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Hu, X.; Zheng, T.; Liu, L.; Kuang, G.; Liu, H.; Wang, X.; Li, J.; Huang, J.; Wang, T.; et al. Obstructive sleep apnea in Parkinson’s disease: A prevalent, clinically relevant and treatable feature. Park. Relat. Disord. 2023, 115, 105790. [Google Scholar]
- Kaminska, M.; Lafontaine, A.-L.; Kimoff, R.J. The Interaction between Obstructive Sleep Apnea and Parkinson’s Disease: Possible Mechanisms and Implications for Cognitive Function. Park. Dis. 2015, 2015, 849472. [Google Scholar]
- Gong, L.-J.; Wang, X.-Y.; Gu, W.-Y.; Wu, X. Pinocembrin ameliorates intermittent hypoxia-induced neuroinflammation through BNIP3-dependent mitophagy in a murine model of sleep apnea. J. Neuroinflamm. 2020, 17, 337. [Google Scholar] [CrossRef]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar]
- Flores-Ponce, X.; Velasco, I. Dopaminergic neuron metabolism: Relevance for understanding Parkinson’s disease. Metabolomics 2024, 20, 116. [Google Scholar]
- Mitroshina, E.V.; Vedunova, M.V. The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 4581. [Google Scholar] [CrossRef] [PubMed]
- Zuzuárregui, J.R.P.; During, E.H. Sleep Issues in Parkinson’s Disease and Their Management. Neurotherapeutics 2020, 17, 1480–1494. [Google Scholar]
- Wang, X.-L.; Li, L. Circadian Clock Regulates Inflammation and the Development of Neurodegeneration. Front. Cell. Infect. Microbiol. 2021, 11, 696554. [Google Scholar]
- Brüning, F.; Noya, S.B.; Bange, T.; Koutsouli, S.; Rudolph, J.D.; Tyagarajan, S.K.; Cox, J.; Mann, M.; Brown, S.A.; Robles, M.S. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 2019, 366, eaav3617. [Google Scholar]
- Drăgoi, C.M.; Nicolae, A.C.; Ungurianu, A.; Margină, D.M.; Grădinaru, D.; Dumitrescu, I.B. Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis—Molecular Mechanisms in Human Health. Cells 2024, 13, 138. [Google Scholar] [CrossRef]
- Xu, H.; Huang, L.; Zhao, J.; Chen, S.; Liu, J.; Li, G. The circadian clock and inflammation: A new insight. Clin. Chim. Acta 2021, 512, 12–17. [Google Scholar] [PubMed]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar]
- Camberos-Barraza, J.; Camacho-Zamora, A.; Bátiz-Beltrán, J.C.; Osuna-Ramos, J.F.; Rábago-Monzón, Á.R.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; Guadrón-Llanos, A.M.; Picos-Cárdenas, V.J.; Calderón-Zamora, L.; et al. Sleep, Glial Function, and the Endocannabinoid System: Implications for Neuroinflammation and Sleep Disorders. Int. J. Mol. Sci. 2024, 25, 3160. [Google Scholar] [CrossRef]
- Jackson, F.R. Glial cell modulation of circadian rhythms. Glia 2011, 59, 1341–1350. [Google Scholar] [CrossRef]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian Rhythm Connections to Oxidative Stress: Implications for Human Health. Antioxid. Redox Signal 2013, 19, 192–208. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Jyoti, S. Alteration in biochemical parameters in the brain of transgenic Drosophila melanogaster model of Parkinson’s disease exposed to apigenin. Integr. Med. Res. 2017, 6, 245–253. [Google Scholar] [PubMed]
- Chow, N.K.; Fretz, M.; Hamburger, M.; Butterweck, V. Telemetry as a Tool to Measure Sedative Effects of a Valerian Root Extract and Its Single Constituents in Mice. Planta Med. 2011, 77, 795–803. [Google Scholar]
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 2000, 71, S117–S123. [Google Scholar]
- Kramer, D.J.; Johnson, A.A. Apigenin: A natural molecule at the intersection of sleep and aging. Front. Nutr. 2024, 11, 1359176. [Google Scholar]
- Kim, J.-W.; Kim, C.-S.; Hu, Z.; Han, J.-Y.; Kim, S.K.; Yoo, S.-K.; Yeo, Y.M.; Chong, M.S.; Lee, K.; Hong, J.T.; et al. Enhancement of pentobarbital-induced sleep by apigenin through chloride ion channel activation. Arch. Pharmacol. Res. 2012, 35, 367–373. [Google Scholar]
- Kambe, D.; Kotani, M.; Yoshimoto, M.; Kaku, S.; Chaki, S.; Honda, K. Effects of quercetin on the sleep–wake cycle in rats: Involvement of gamma-aminobutyric acid receptor type A in regulation of rapid eye movement sleep. Brain Res. 2010, 1330, 83–88. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yu, L.-H.; Zhang, J.; Xie, D.-J.; Zhang, X.-X.; Yu, J.-M. Network pharmacology-based and molecular docking-based analysis of Suanzaoren decoction for the treatment of Parkinson’s disease with sleep disorder. BioMed Res. Int. 2021, 2021, 1752570. [Google Scholar]
- Godos, J.; Ferri, R.; Castellano, S.; Angelino, D.; Mena, P.; Del Rio, D.; Caraci, F.; Galvano, F.; Grosso, G. Specific Dietary (Poly)phenols Are Associated with Sleep Quality in a Cohort of Italian Adults. Nutrients 2020, 12, 1226. [Google Scholar] [CrossRef]
- Conti, B.J.; Búfalo, M.C.; Golim, M.D.A.; Bankova, V.; Sforcin, J.M. Cinnamic Acid Is Partially Involved in Propolis Immunomodulatory Action on Human Monocytes. Evid.-Based Complement. Altern. Med. 2013, 2013, 109864. [Google Scholar]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- Oishi, K.; Yamamoto, S.; Oike, H.; Ohkura, N.; Taniguchi, M. Cinnamic acid shortens the period of the circadian clock in mice. Biochem. Biophys. Rep. 2017, 9, 232–237. [Google Scholar] [CrossRef]
- Hey, G.; Nair, N.; Klann, E.; Gurrala, A.; Safarpour, D.; Mai, V.; Ramirez-Zamora, A.; Vedam-Mai, V. Therapies for Parkinson’s disease and the gut microbiome: Evidence for bidirectional connection. Front. Aging Neurosci. 2023, 15, 1151850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, B.; Guo, J. Parkinson’s disease and gut microbiota: From clinical to mechanistic and therapeutic studies. Transl. Neurodegener. 2023, 12, 59. [Google Scholar] [CrossRef]
- Schaeffer, E.; Kluge, A.; Böttner, M.; Zunke, F.; Cossais, F.; Berg, D.; Arnold, P. Alpha Synuclein Connects the Gut-Brain Axis in Parkinson’s Disease Patients—A View on Clinical Aspects, Cellular Pathology and Analytical Methodology. Front. Cell Dev. Biol. 2020, 8, 573696. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural. Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study. Brain 2020, 143, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? npj Park. Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Warnecke, T.; Schäfer, K.H.; Claus, I.; del Tredici, K.; Jost, W.H. Gastrointestinal involvement in Parkinson’s disease: Pathophysiology, diagnosis, and management. npj Park. Dis. 2022, 8, 31. [Google Scholar] [CrossRef]
- Kakino, M.; Izuta, H.; Tsuruma, K.; Araki, Y.; Shimazawa, M.; Ichihara, K.; Hara, H. Laxative effects and mechanism of action of Brazilian green propolis. BMC Complement. Altern. Med. 2012, 12, 192. [Google Scholar] [CrossRef]
- Konings, B.; Villatoro, L.; Eynde, J.V.D.; Barahona, G.; Burns, R.; McKnight, M.; Hui, K.; Yenokyan, G.; Tack, J.; Pasricha, P.J. Gastrointestinal syndromes preceding a diagnosis of Parkinson’s disease: Testing Braak’s hypothesis using a nationwide database for comparison with Alzheimer’s disease and cerebrovascular diseases. Gut 2023, 72, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sjölander, A.; Pedersen, N.L.; Ludvigsson, J.F.; Chen, H.; Fang, F.; Wirdefeldt, K. Irritable bowel syndrome and Parkinson’s disease risk: Register-based studies. npj Park. Dis. 2021, 7, 5. [Google Scholar]
- Zhang, X.; Svn, Z.; Liv, M.; Yang, Y.; Zeng, R.; Huang, Q.; Sun, Q. Association Between Irritable Bowel Syndrome and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 720958. [Google Scholar] [CrossRef] [PubMed]
- Miryan, M.; Soleimani, D.; Alavinejad, P.; Abbaspour, M.; Ostadrahimi, A. Effects of propolis supplementation on irritable bowel syndrome with constipation (IBS-C) and mixed (IBS-M) stool pattern: A randomized, double-blind clinical trial. Food Sci. Nutr. 2022, 10, 1899–1907. [Google Scholar] [PubMed]
- Soleimani, D.; Miryan, M.; Tutunchi, H.; Navashenaq, J.G.; Sadeghi, E.; Ghayour-Mobarhan, M.; Ferns, G.A.; Ostadrahimi, A. A systematic review of preclinical studies on the efficacy of propolis for the treatment of inflammatory bowel disease. Phytother. Res. 2021, 35, 701–710. [Google Scholar]
- Yue, B.; Ren, J.; Yu, Z.; Luo, X.; Ren, Y.; Zhang, J.; Mani, S.; Wang, Z.; Dou, W. Pinocembrin alleviates ulcerative colitis in mice via regulating gut microbiota, suppressing TLR4/MD2/NF-κB pathway and promoting intestinal barrier. Biosci. Rep. 2020, 40, BSR20200986. [Google Scholar] [CrossRef]
- Salim, S.; Ahmad, F.; Banu, A.; Mohammad, F. Gut microbiome and Parkinson’s disease: Perspective on pathogenesis and treatment. J. Adv. Res. 2023, 50, 83–105. [Google Scholar]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut bacterial profiles in Parkinson’s disease: A systematic review. CNS Neurosci. Ther. 2023, 29, 140–157. [Google Scholar]
- Shannon, K.M. Gut-Derived Sterile Inflammation and Parkinson’s Disease. Front. Neurol. 2022, 13, 831090. [Google Scholar]
- Yoo, J.; Groer, M.; Dutra, S.; Sarkar, A.; McSkimming, D. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Xue, M.; Liu, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393. [Google Scholar]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar]
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomed. Pharmacother. 2022, 148, 112759. [Google Scholar]
- Zheng, Y.; Wu, Y.; Tao, L.; Chen, X.; Jones, T.J.; Wang, K.; Hu, F. Chinese Propolis Prevents Obesity and Metabolism Syndromes Induced by a High Fat Diet and Accompanied by an Altered Gut Microbiota Structure in Mice. Nutrients 2020, 12, 959. [Google Scholar] [CrossRef]
- Aabed, K.; Bhat, R.S.; Al-Dbass, A.; Moubayed, N.; Algahtani, N.; Merghani, N.M.; Alanazi, A.; Zayed, N. Bee pollen and propolis improve neuroinflammation and dysbiosis induced by propionic acid, a short chain fatty acid in a rodent model of autism. Lipids Health Dis. 2019, 18, 200. [Google Scholar] [PubMed]
- Guan, R.; Ma, N.; Liu, G.; Wu, Q.; Su, S.; Wang, J.; Geng, Y. Ethanol extract of propolis regulates type 2 diabetes in mice via metabolism and gut microbiota. J. Ethnopharmacol. 2023, 310, 116385. [Google Scholar]
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; et al. Brazilian green propolis improves gut microbiota dysbiosis and protects against sarcopenic obesity. J. Cachex-Sarcopenia Muscle 2022, 13, 3028–3047. [Google Scholar]
- Palacios, N.; Wilkinson, J.; Bjornevik, K.; Schwarzschild, M.A.; McIver, L.; Ascherio, A.; Huttenhower, C. Metagenomics of the Gut Microbiome in Parkinson’s Disease: Prodromal Changes. Ann. Neurol. 2023, 94, 486–501. [Google Scholar]
- Ning, J.; Huang, S.-Y.; Chen, S.-D.; Zhang, Y.-R.; Huang, Y.-Y.; Yu, J.-T. Investigating Casual Associations Among Gut Microbiota, Metabolites, and Neurodegenerative Diseases: A Mendelian Randomization Study. J. Alzheimer’s Dis. 2022, 87, 211–222. [Google Scholar]
- Feng, J.; Li, Z.; Ma, H.; Yue, Y.; Hao, K.; Li, J.; Xiang, Y.; Min, Y. Quercetin alleviates intestinal inflammation and improves intestinal functions via modulating gut microbiota composition in LPS-challenged laying hens. Poult. Sci. 2023, 102, 102433. [Google Scholar]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.; Conlon, M.A.; Topping, D.L. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin Enhances Intestinal Barrier Function through the Assembly of Zonnula Occludens-2, Occludin, and Claudin-1 and the Expression of Claudin-4 in Caco-2 Cells. J. Nutr. 2009, 139, 965–974. [Google Scholar]
- Park, J.; Kim, T.J.; Song, J.H.; Jang, H.; Kim, J.S.; Kang, S.H.; Kim, H.-R.; Hwangbo, S.; Shin, H.Y.; Na, D.L.; et al. Helicobacter pylori Infection Is Associated with Neurodegeneration in Cognitively Normal Men. J. Alzheimer’s Dis. 2021, 82, 1591–1599. [Google Scholar]
- Noori, M.; Mahboobi, R.; Nabavi-Rad, A.; Jamshidizadeh, S.; Fakharian, F.; Yadegar, A.; Zali, M.R. Helicobacter pylori infection contributes to the expression of Alzheimer’s disease-associated risk factors and neuroinflammation. Heliyon 2023, 9, e19607. [Google Scholar]
- Wei, B.R.; Zhao, Y.J.; Cheng, Y.F.; Huang, C.; Zhang, F. Helicobacter pylori infection and Parkinson’s Disease: Etiology, pathogenesis and levodopa bioavailability. Immun. Ageing 2024, 21, 1. [Google Scholar]
- Baltas, N.; Karaoglu, S.A.; Tarakci, C.; Kolayli, S. Effect of propolis in gastric disorders: Inhibition studies on the growth of Helicobacter pylori and production of its urease. J. Enzym. Inhib. Med. Chem. 2016, 31, 46–50. [Google Scholar]
- Song, M.-Y.; Lee, D.-Y.; Han, Y.-M.; Kim, E.-H. Anti-Inflammatory Effect of Korean Propolis on Helicobacter pylori-Infected Gastric Mucosal Injury Mice Model. Nutrients 2022, 14, 4644. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Derejian, S.; Koumanova, R.; Katsarov, N.; Gergova, G.; Mitov, I.; Nikolov, R.; Krastev, Z. Inhibition of Helicobacter pylori growth in vitro by Bulgarian propolis: Preliminary report. J. Med. Microbiol. 2003, 52, 417–419. [Google Scholar]
- Cui, K.; Lu, W.; Zhu, L.; Shen, X.; Huang, J. Caffeic acid phenethyl ester (CAPE), an active component of propolis, inhibits Helicobacter pylori peptide deformylase activity. Biochem. Biophys. Res. Commun. 2013, 435, 289–294. [Google Scholar] [PubMed]
- Governa, P.; Romagnoli, G.; Albanese, P.; Rossi, F.; Manetti, F.; Biagi, M. Effect of in vitro simulated digestion on the anti- Helicobacter pylori activity of different Propolis extracts. J. Enzym. Inhib. Med. Chem. 2023, 38, 2183810. [Google Scholar]
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Bozhadze, A.; Jokhadze, M.; Korona-Głowniak, I. Correlation between Chemical Profile of Georgian Propolis Extracts and Their Activity against Helicobacter pylori. Molecules 2023, 28, 1374. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, X.; Jiang, Z.; Jiang, Z. Association of small intestinal bacterial overgrowth with Parkinson’s disease: A systematic review and meta-analysis. Gut Pathog. 2021, 13, 25. [Google Scholar]
- Sabuncuoglu, M.Z. Propolis reduces bacterial translocation and intestinal villus atrophy in experimental obstructive jaundice. World J. Gastroenterol. 2007, 13, 5226–5231. [Google Scholar]
- Scorza, F.A.; Fiorini, A.C.; Scorza, C.A.; Finsterer, J. Cardiac abnormalities in Parkinson’s disease and Parkinsonism. J. Clin. Neurosci. 2018, 53, 1–5. [Google Scholar]
- Potashkin, J.; Huang, X.; Becker, C.; Chen, H.; Foltynie, T.; Marras, C. Understanding the Links Between Cardiovascular Disease and Parkinson’s Disease. Mov. Disord. 2020, 35, 55–74. [Google Scholar]
- Cuenca-Bermejo, L.; Almela, P.; Navarro-Zaragoza, J.; Villalba, E.F.; González-Cuello, A.-M.; Laorden, M.-L.; Herrero, M.-T. Cardiac Changes in Parkinson’s Disease: Lessons from Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 13488. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef] [PubMed]
- Tulbă, D.; Cozma, L.; Bălănescu, P.; Buzea, A.; Băicuș, C.; Popescu, B.O. Blood Pressure Patterns in Patients with Parkinson’s Disease: A Systematic Review. J. Pers. Med. 2021, 11, 129. [Google Scholar] [CrossRef]
- Grosu, L.; Grosu, A.I.; Crisan, D.; Zlibut, A.; Perju-Dumbrava, L. Parkinson’s disease and cardiovascular involvement: Edifying insights (Review). Biomed. Rep. 2023, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Duz, O.A.; Yilmaz, N.H. Nocturnal blood pressure changes in Parkinson’s disease: Correlation with autonomic dysfunction and vitamin D levels. Acta Neurol. Belg. 2020, 120, 915–920. [Google Scholar]
- Posada, I.J.; Benito-León, J.; Louis, E.D.; Trincado, R.; Villarejo, A.; Medrano, M.J.; Bermejo-Pareja, F. Mortality from Parkinson’s disease: A population-based prospective study (NEDICES). Mov. Disord. 2011, 26, 2522–2529. [Google Scholar] [CrossRef]
- Kumar, A.; Avishay, D.M.; Jones, C.R.; Shaikh, J.D.; Kaur, R.; Aljadah, M.; Kichloo, A.; Shiwalkar, N.; Keshavamurthy, S. Sudden cardiac death: Epidemiology, pathogenesis and management. Rev. Cardiovasc. Med. 2021, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.A.; Guimarães-Marques, M.; Nejm, M.; de Almeida, A.C.G.; Scorza, C.A.; Fiorini, A.C.; Finsterer, J. Sudden unexpected death in Parkinson’s disease: Insights from clinical practice. Clinics 2022, 77, 100001. [Google Scholar] [CrossRef]
- Ryu, D.-W.; Han, K.; Cho, A.-H. Mortality and causes of death in patients with Parkinson’s disease: A nationwide population-based cohort study. Front. Neurol. 2023, 14, 1236296. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Lin, L.; Jia, X.; Hu, C.; Qi, H.; Lin, H.; Zheng, R.; Li, M.; Xu, Y.; et al. Association between triglyceride glucose index and breast cancer in 142,184 Chinese adults: Findings from the REACTION study. Front. Endocrinol. 2024, 15, 1321622. [Google Scholar] [CrossRef]
- Hu, G.; Jousilahti, P.; Bidel, S.; Antikainen, R.; Tuomilehto, J. Type 2 Diabetes and the Risk of Parkinson’s Disease. Diabetes Care 2007, 30, 842–847. [Google Scholar] [CrossRef]
- Pagano, G.; Polychronis, S.; Wilson, H.; Giordano, B.; Ferrara, N.; Niccolini, F.; Politis, M. Diabetes mellitus and Parkinson disease. Neurology 2018, 90, e1654–e1662. [Google Scholar] [CrossRef] [PubMed]
- Maluf, F.C.; Feder, D.; Carvalho, A.A.d.S. Analysis of the Relationship between Type II Diabetes Mellitus and Parkinson’s Disease: A Systematic Review. Park. Dis. 2019, 2019, 1–14. [Google Scholar]
- Wang, S.-Y.; Wu, S.-L.; Chen, T.-C.; Chuang, C.-S. Antidiabetic Agents for Treatment of Parkinson’s Disease: A Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 4805. [Google Scholar] [CrossRef]
- Meissner, W.G.; Remy, P.; Giordana, C.; Maltête, D.; Derkinderen, P.; Houéto, J.-L.; Anheim, M.; Benatru, I.; Boraud, T.; Brefel-Courbon, C.; et al. Trial of Lixisenatide in Early Parkinson’s Disease. N. Engl. J. Med. 2024, 390, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In Vitro, and Clinical Studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef]
- Silva, H.; Francisco, R.; Saraiva, A.; Francisco, S.; Carrascosa, C.; Raposo, A. The Cardiovascular Therapeutic Potential of Propolis—A Comprehensive Review. Biology 2021, 10, 27. [Google Scholar] [CrossRef]
- Taylor, E.; Kim, Y.; Zhang, K.; Chau, L.; Nguyen, B.C.; Rayalam, S.; Wang, X. Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress. Molecules 2022, 27, 4396. [Google Scholar] [CrossRef]
- Borriello, M.; Iannuzzi, C.; Sirangelo, I. Pinocembrin Protects from AGE-Induced Cytotoxicity and Inhibits Non-Enzymatic Glycation in Human Insulin. Cells 2019, 8, 385. [Google Scholar] [CrossRef]
- Xu, W.; Lu, H.; Yuan, Y.; Deng, Z.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods 2022, 11, 2439. [Google Scholar] [CrossRef]
- Zulhendri, F.; Perera, C.O.; Tandean, S.; Abdulah, R.; Herman, H.; Christoper, A.; Chandrasekaran, K.; Putra, A.; Lesmana, R. The Potential Use of Propolis as a Primary or an Adjunctive Therapy in Respiratory Tract-Related Diseases and Disorders: A Systematic Scoping Review. Biomed. Pharmacother. 2022, 146, 112595. [Google Scholar]
- Oršolić, N.; Jurčević, I.L.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Junaković, E.P.; Terzić, S.; Jutrić, D. Effect of Propolis on Diet-Induced Hyperlipidemia and Atherogenic Indices in Mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Zakerkish, M.; Jenabi, M.; Zaeemzadeh, N.; Hemmati, A.A.; Neisi, N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 7289. [Google Scholar] [CrossRef]
- Nikbaf-Shandiz, M.; Tutunchi, H.; Khoshbaten, M.; Bonab, H.N.; Ebrahimi-Mameghani, M. Propolis supplementation in obese patients with non-alcoholic fatty liver disease: Effects on glucose homeostasis, lipid profile, liver function, anthropometric indices and meta-inflammation. Food Funct. 2022, 13, 11568–11578. [Google Scholar] [PubMed]
- Chen, Y.; Wang, J.; Wang, Y.; Wang, P.; Zhou, Z.; Wu, R.; Xu, Q.; You, H.; Liu, Y.; Wang, L.; et al. A propolis-derived small molecule ameliorates metabolic syndrome in obese mice by targeting the CREB/CRTC2 transcriptional complex. Nat. Commun. 2022, 13, 246. [Google Scholar]
- Ueda, M.; Hayashibara, K.; Ashida, H. Propolis extract promotes translocation of glucose transporter 4 and glucose uptake through both PI3K- and AMPK-dependent pathways in skeletal muscle. BioFactors 2013, 39, 457–466. [Google Scholar]
- Chen, L.-H.; Chien, Y.-W.; Chang, M.-L.; Hou, C.-C.; Chan, C.-H.; Tang, H.-W.; Huang, H.-Y. Taiwanese Green Propolis Ethanol Extract Delays the Progression of Type 2 Diabetes Mellitus in Rats Treated with Streptozotocin/High-Fat Diet. Nutrients 2018, 10, 503. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.-S.; Juvekar, A.R. Chrysin ameliorates nonalcoholic fatty liver disease in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1617–1628. [Google Scholar]
- Wang, Q.; Chen, R.; Tang, Q.; Chen, J.-Y.; Wang, Y.; Sui, D.-J.; Liu, A.-D. Flavonoid Extract from Propolis Provides Cardioprotection following Myocardial Infarction by Activating PPAR-γ. Evid. -Based Complement. Altern. Med. 2022, 2022, 1–13. [Google Scholar]
- Zhou, Y.; Tao, H.; Xu, N.; Zhou, S.; Peng, Y.; Zhu, J.; Liu, S.; Chang, Y. Chrysin improves diabetic nephropathy by regulating the AMPK -mediated lipid metabolism in HFD/STZ -induced DN mice. J. Food Biochem. 2022, 46, e14379. [Google Scholar] [CrossRef]
- El-Emam, M.M.A.; Behairy, A.; Mostafa, M.; Khamis, T.; Osman, N.M.S.; Alsemeh, A.E.; Mansour, M.F. Chrysin-loaded PEGylated liposomes protect against alloxan-induced diabetic neuropathy in rats: The interplay between endoplasmic reticulum stress and autophagy. Biol. Res. 2024, 57, 45. [Google Scholar] [CrossRef]
- Okutan, H.; Ozcelik, N.; Yilmaz, H.R.; Uz, E. Effects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heart. Clin. Biochem. 2005, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, M.S.; El-Agamy, D.S.; Suddek, G.M.; Nader, M.A. Propolis protects against high glucose-induced vascular endothelial dysfunction in isolated rat aorta. J. Physiol. Biochem. 2014, 70, 247–254. [Google Scholar] [CrossRef]
- Saavedra, N.; Cuevas, A.; Cavalcante, M.F.; Dörr, F.A.; Saavedra, K.; Zambrano, T.; Abdalla, D.S.P.; Salazar, L.A. Polyphenols from Chilean Propolis and Pinocembrin Reduce MMP-9 Gene Expression and Activity in Activated Macrophages. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Ahmed, R.; Tanvir, E.M.; Hossen, S.; Afroz, R.; Ahmmed, I.; Rumpa, N.-E.; Paul, S.; Gan, S.H.; Sulaiman, S.A.; Khalil, I. Antioxidant Properties and Cardioprotective Mechanism of Malaysian Propolis in Rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 5370545. [Google Scholar] [CrossRef]
- Chang, H.; Yuan, W.; Wu, H.; Yin, X.; Xuan, H. Bioactive components and mechanisms of Chinese poplar propolis alleviates oxidized low-density lipoprotein-induced endothelial cells injury. BMC Complement. Altern. Med. 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Braik, A.; Lahouel, M.; Merabet, R.; Djebar, M.R.; Morin, D. Myocardial protection by propolis during prolonged hypothermic preservation. Cryobiology 2019, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef]
- Yang, T.; Liu, H.; Yang, C.; Mo, H.; Wang, X.; Song, X.; Jiang, L.; Deng, P.; Chen, R.; Wu, P.; et al. Galangin Attenuates Myocardial Ischemic Reperfusion-Induced Ferroptosis by Targeting Nrf2/Gpx4 Signaling Pathway. Drug Des. Dev. Ther. 2023, 17, 2495–2511. [Google Scholar] [CrossRef]
- Onbas, R.; Kazan, A.; Nalbantsoy, A.; Yesil-Celiktas, O. Cytotoxic and Nitric Oxide Inhibition Activities of Propolis Extract along with Microencapsulation by Complex Coacervation. Plant Foods Hum. Nutr. 2016, 71, 286–293. [Google Scholar] [CrossRef]
- Alanazi, S.; Alenzi, N.; Fearnley, J.; Harnett, W.; Watson, D.G. Temperate Propolis Has Anti-Inflammatory Effects and Is a Potent Inhibitor of Nitric Oxide Formation in Macrophages. Metabolites 2020, 10, 413. [Google Scholar] [CrossRef]
- Pakdeechote, P.; Poasakate, A.; Prasatthong, P.; Potue, P.; Khamseekaew, J.; Maneesai, P. Mitigation effect of galangin against aortic dysfunction and hypertrophy in rats with metabolic syndrome. Heliyon 2023, 9, e16500. [Google Scholar]
- Inui, S.; Hosoya, T.; Yoshizumi, K.; Sato, H.; Kumazawa, S. Phytochemical and anti-inflammatory properties of Senegalese propolis and isolated compounds. Fitoterapia 2021, 151, 104861. [Google Scholar]
- Ji, C.; Pan, Y.; Xu, S.; Yu, C.; Ji, J.; Chen, M.; Hu, F. Propolis ameliorates restenosis in hypercholesterolemia rabbits with carotid balloon injury by inhibiting lipid accumulation, oxidative stress, and TLR4/NF-κB pathway. J. Food Biochem. 2021, 45, e13577. [Google Scholar]
- El-Hakam, F.E.-Z.A.; Laban, G.A.; El-Din, S.B.; El-Hamid, H.A.; Farouk, M.H. Apitherapy combination improvement of blood pressure, cardiovascular protection, and antioxidant and anti-inflammatory responses in dexamethasone model hypertensive rats. Sci. Rep. 2022, 12, 20765. [Google Scholar]
- Eşrefoğlu, M.; Gül, M.; Ateş, B.; Erdoğan, A. The effects of caffeic acid phenethyl ester and melatonin on age-related vascular remodeling and cardiac damage. Fundam. Clin. Pharmacol. 2011, 25, 580–590. [Google Scholar]
- Ozer, M.; Parlakpinar, H.; Acet, A. Reduction of ischemia–reperfusion induced myocardial infarct size in rats by caffeic acid phenethyl ester (CAPE). Clin. Biochem. 2004, 37, 702–705. [Google Scholar] [PubMed]
- Bruehl, S.; Milne, G.; Schildcrout, J.; Shi, Y.; Anderson, S.; Shinar, A.; Polkowski, G.; Mishra, P.; Billings, F.T.I. Oxidative stress is associated with characteristic features of the dysfunctional chronic pain phenotype. Pain 2022, 163, 786–794. [Google Scholar]
- de Andrade, D.C.; Mylius, V.; Perez-Lloret, S.; Cury, R.G.; Bannister, K.; Moisset, X.; Taricani Kubota, G.; Finnerup, N.B.; Bouhassira, D.; Chaudhuri, K.R.; et al. Pain in Parkinson disease: Mechanistic substrates, main classification systems, and how to make sense out of them. Pain 2023, 164, 2425–2434. [Google Scholar]
- Mylius, V.; Möller, J.C.; Bohlhalter, S.; de Andrade, D.C.; Lloret, S.P. Diagnosis and Management of Pain in Parkinson’s Disease: A New Approach. Drugs Aging 2021, 38, 559–577. [Google Scholar]
- Mylius, V.; Lloret, S.P.; Cury, R.G.; Teixeira, M.J.; Barbosa, V.R.; Barbosa, E.R.; Moreira, L.I.; Listik, C.; Fernandes, A.M.M.; Veiga, D.d.L.; et al. The Parkinson disease pain classification system: Results from an international mechanism-based classification approach. Pain 2021, 162, 1201–1210. [Google Scholar] [PubMed]
- Nardelli, D.; Gambioli, F.; De Bartolo, M.I.; Mancinelli, R.; Biagioni, F.; Carotti, S.; Falato, E.; Leodori, G.; Puglisi-Allegra, S.; Vivacqua, G.; et al. Pain in Parkinson’s disease: A neuroanatomy-based approach. Brain Commun. 2024, 6, fcae210. [Google Scholar]
- Silverdale, M.A.; Kobylecki, C.; Kass-Iliyya, L.; Martinez-Martin, P.; Lawton, M.; Cotterill, S.; Chaudhuri, K.R.; Morris, H.; Baig, F.; Williams, N.; et al. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Park. Relat. Disord 2018, 56, 27–32. [Google Scholar]
- Blood, M.R.Y.; Ferro, M.M.; Munhoz, R.P.; Teive, H.A.G.; Camargo, C.H.F. Classification and Characteristics of Pain Associated with Parkinson’s Disease. Park. Dis. 2016, 2016, 6067132. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar]
- Sokeng, S.D.; Talla, E.; Sakava, P.; Tagne, M.A.F.; Henoumont, C.; Sophie, L.; Mbafor, J.T.; Fohouo, F.-N.T. Anti-Inflammatory and Analgesic Effect of Arachic Acid Ethyl Ester Isolated from Propolis. BioMed Res. Int. 2020, 2020, 8797284. [Google Scholar]
- Sun, L.; Liao, L.; Wang, B. Potential Antinociceptive Effects of Chinese Propolis and Identification on Its Active Compounds. J. Immunol. Res. 2018, 2018, 5429543. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Pahlavani, N.; Ranjbar, G.; Navashenaq, J.G.; Salehi-Sahlabadi, A.; Mahmudiono, T.; Shalaby, M.N.; Jokar, M.; Nematy, M.; Barghchi, H.; et al. Does propolis have any effect on rheumatoid arthritis? A review study. Food Sci. Nutr. 2022, 10, 1003–1020. [Google Scholar]
- Nazari-Bonab, H.; Jamilian, P.; Radkhah, N.; Zarezadeh, M.; Ebrahimi-Mameghani, M. The effect of propolis supplementation in improving antioxidant status: A systematic review and meta-analysis of controlled clinical trials. Phytother. Res. 2023, 37, 3712–3723. [Google Scholar]
- Paulino, N.; Teixeira, C.; Martins, R.; Scremin, A.; Dirsch, V.M.; Vollmar, A.M.; Abreu, S.R.L.; de Castro, S.L.; Marcucci, M.C. Evaluation of the Analgesic and Anti-Inflammatory Effects of a Brazilian Green Propolis. Planta Medica 2006, 72, 899–906. [Google Scholar] [PubMed]
- de Freitas, K.S.; da Silva, L.H.D.; Squarisi, I.S.; Oliveira, L.T.d.S.; Ribeiro, A.B.; Alves, B.S.; Esperandim, T.R.; de Melo, M.R.S.; Ozelin, S.D.; Lemes, D.C.; et al. Red propolis exhibits chemopreventive effect associated with antiproliferative and anti-inflammatory activities. Toxicol. Res. 2022, 11, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Paulino, M.; Alvareda, E.; Iribarne, F.; Miranda, P.; Espinosa, V.; Aguilera, S.; Pardo, H. Toward the understanding of the molecular basis for the inhibition of COX-1 and COX-2 by phenolic compounds present in Uruguayan propolis and grape pomace. J. Biomol. Struct. Dyn. 2016, 34, 2643–2657. [Google Scholar] [PubMed]
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-administration of 5FU and propolis on AOM/DSS induced colorectal cancer in BALB-c mice. Life Sci. 2021, 276, 119390. [Google Scholar]
- Wang, C.-C.; Wang, Y.-X.; Yu, N.-Q.; Hu, D.; Wang, X.-Y.; Chen, X.-G.; Liao, Y.-W.; Yao, J.; Wang, H.; He, L.; et al. Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2. Molecules 2017, 22, 732. [Google Scholar] [CrossRef]
- Zeitoun, R.; Najjar, F.; Wehbi, B.; Khalil, A.; Fayyad-Kazan, M.; Dagher-Hamalian, C.; Faour, W.H.; El-Makhour, Y. Chemical Composition, Antioxidant and Anti-inflammatory Activity Evaluation of the Lebanese Propolis Extract. Curr. Pharm. Biotechnol. 2019, 20, 84–96. [Google Scholar]
- Campos, R.O.P.D.; Paulino, N.; Da Silva, C.H.M.; Scremin, A.; Calixto, J.B. Anti-hyperalgesic Effect of an Ethanolic Extract of Propolis in Mice and Rats. J. Pharm. Pharmacol. 1998, 50, 1187–1193. [Google Scholar]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.; Wong, D.V.T.; Lima-Júnior, R.C.; Ribeiro, R.d.A.; Vale, M.L. The Antioxidant Effects of the Flavonoids Rutin and Quercetin Inhibit Oxaliplatin-Induced Chronic Painful Peripheral Neuropathy. Mol. Pain. 2013, 9, 53. [Google Scholar]
- Cheng, H.; Zhang, Y.; Lu, W.; Gao, X.; Xu, C.; Bao, H. Caffeic acid phenethyl ester attenuates neuropathic pain by suppressing the p38/NF-κB signal pathway in microglia. J. Pain Res. 2018, 11, 2709–2719. [Google Scholar]
- Sharma, S.S.; Kulkarni, N.P.; Vaidya, B.; Narula, A.S. Caffeic Acid Phenethyl Ester (CAPE) Attenuates Paclitaxel-induced Peripheral Neuropathy: A Mechanistic Study. Curr. Neurovasc. Res. 2022, 19, 293–302. [Google Scholar]
- Sun, W.; Xie, W.; Huang, D.; Cui, Y.; Yue, J.; He, Q.; Jiang, L.; Xiong, J.; Sun, W.; Yi, Q. Caffeic acid phenethyl ester attenuates osteoarthritis progression by activating NRF2/HO 1 and inhibiting the NF κB signaling pathway. Int. J. Mol. Med. 2022, 50, 134. [Google Scholar] [CrossRef] [PubMed]
- Pol, O. The role of carbon monoxide, heme oxygenase 1, and the Nrf2 transcription factor in the modulation of chronic pain and their interactions with opioids and cannabinoids. Med. Res. Rev. 2021, 41, 136–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Carcolé, M.; Castany, S.; Leánez, S.; Pol, O. Treatment with a Heme Oxygenase 1 Inducer Enhances the Antinociceptive Effects of µ -Opioid, δ -Opioid, and Cannabinoid 2 Receptors during Inflammatory Pain. J. Pharmacol. Exp. Ther. 2014, 351, 224–232. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Wang, B. Carbon monoxide signaling and soluble guanylyl cyclase: Facts, myths, and intriguing possibilities. Biochem. Pharmacol. 2022, 200, 115041. [Google Scholar] [CrossRef]
- Sachs, D.; Cunha, F.Q.; Ferreira, S.H. Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K + channel pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 3680–3685. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-S.; Feng, J.-H.; Park, J.-S.; Lee, H.-J.; Lee, J.-Y.; Lim, S.-S.; Suh, H.-W. Antinociceptive effect of chrysin in diabetic neuropathy and formalin-induced pain models. Anim. Cells Syst. 2020, 24, 143–150. [Google Scholar] [CrossRef]
- Xu, B.; Xu, Y.; Kong, J.; Liu, Y.; Zhang, L.; Shen, F.; Wang, J.; Shen, X.; Chen, H. Chrysin mitigated neuropathic pain and peripheral sensitization in knee osteoarthritis rats by repressing the RAGE/PI3K/AKT pathway regulated by HMGB1. Cytokine 2024, 180, 156635. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Cui, W.; Zhou, W.; Zhao, X. 5-HT1Areceptor-mediated attenuation of heat hyperalgesia and mechanical allodynia by chrysin in mice with experimental mononeuropathy. Reg. Anesth. Pain. Med. 2020, 45, 610–619. [Google Scholar] [CrossRef]
- Zhai, K.; Hu, L.; Chen, J.; Fu, C.Y.; Chen, Q. Chrysin Induces Hyperalgesia via the GABA A Receptor in Mice. Planta Med. 2008, 74, 1229–1234. [Google Scholar] [CrossRef]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and Derivatives: Useful Tools in Inflammation and Pain Management. Futur. Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Xu, A.-J.; Zhou, Y.-Q.; Liu, C.; Liu, D.-Q.; Tian, Y.-K.; Mei, W.; Tian, X.-B. The Emerging Role of Quercetin in the Treatment of Chronic Pain. Curr. Neuropharmacol. 2022, 20, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Kumar, B.; Sagi, S.S. Hypoxia-mediated alterations in pulmonary surfactant protein expressions: Beneficial effects of quercetin prophylaxis. Respir. Physiol. Neurobiol. 2021, 291, 103695. [Google Scholar] [CrossRef]
- Huang, S.-S.; Liu, S.-M.; Lin, S.-M.; Liao, P.-H.; Lin, R.-H.; Chen, Y.-C.; Chih, C.-L.; Tsai, S.-K. Antiarrhythmic effect of caffeic acid phenethyl ester (CAPE) on myocardial ischemia/reperfusion injury in rats. Clin. Biochem. 2005, 38, 943–947. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Sahna, E.; Acet, A.; Mizrak, B.; Polat, A. Protective effect of caffeic acid phenethyl ester (CAPE) on myocardial ischemia–reperfusion-induced apoptotic cell death. Toxicology 2005, 209, 1–14. [Google Scholar] [CrossRef]
- Chang, G.-J.; Chang, C.-J.; Chen, W.-J.; Yeh, Y.-H.; Lee, H.-Y. Electrophysiological and mechanical effects of caffeic acid phenethyl ester, a novel cardioprotective agent with antiarrhythmic activity, in guinea-pig heart. Eur. J. Pharmacol. 2013, 702, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef]
- Goncalves, V.C.; da Fonsêca, V.S.; Faria, D.d.P.; Izidoro, M.A.; Berretta, A.A.; de Almeida, A.-C.G.; Fonseca, F.L.A.; Scorza, F.A.; Scorza, C.A. Propolis induces cardiac metabolism changes in 6-hydroxydopamine animal model: A dietary intervention as a potential cardioprotective approach in Parkinson’s disease. Front. Pharmacol. 2022, 13, 1013703. [Google Scholar] [CrossRef]
- Heimrich, K.G.; Lehmann, T.; Schlattmann, P.; Prell, T. Heart Rate Variability Analyses in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 959. [Google Scholar] [CrossRef]
- Iniguez, M.; Jimenez-Marin, A.; Erramuzpe, A.; Acera, M.; Tijero, B.; Murueta-Goyena, A.; Del Pino, R.; Fernandez, T.; Carmona-Abellan, M.; Cabrera-Zubizarreta, A.; et al. Heart-brain synchronization breakdown in Parkinson’s disease. npj Park. Dis. 2022, 8, 64. [Google Scholar]
- Alonso, A.; Huang, X.; Mosley, T.H.; Heiss, G.; Chen, H. Heart rate variability and the risk of Parkinson disease: The Atherosclerosis Risk in Communities study. Ann. Neurol. 2015, 77, 877–883. [Google Scholar]
- Alizadeh, P.; Terroba-Chambi, C.; Achen, B.; Bruno, V. Pain in monogenic Parkinson’s disease: A comprehensive review. Front. Neurol. 2023, 14, 1248828. [Google Scholar]
- Antonini, A.; Tinazzi, M. Targeting pain in Parkinson’s disease. Lancet Neurol. 2015, 14, 1144–1145. [Google Scholar] [CrossRef]
- Naisby, J.; Lawson, R.A.; Galna, B.; Alcock, L.; Burn, D.J.; Rochester, L.; Yarnall, A.J. Trajectories of pain over 6 years in early Parkinson’s disease: ICICLE-PD. J. Neurol. 2021, 268, 4759–4767. [Google Scholar]
- Tai, Y.-C.; Lin, C.-H. An overview of pain in Parkinson’s disease. Clin. Park. Relat. Disord. 2020, 2, 1–8. [Google Scholar] [PubMed]
- McMahon, L.; Blake, C.; Lennon, O. A systematic review and meta-analysis of respiratory dysfunction in Parkinson’s disease. Eur. J. Neurol. 2023, 30, 1481–1504. [Google Scholar]
- Docu Axelerad, A.; Stroe, A.Z.; Arghir, O.C.; Docu Axelerad, D.; Gogu, A.E. Respiratory dysfunctions in Parkinson’s disease patients. Brain Sci. 2021, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Kaczyńska, K.; Orłowska, M.E.; Andrzejewski, K. Respiratory Abnormalities in Parkinson’s Disease: What Do We Know from Studies in Humans and Animal Models? Int. J. Mol. Sci. 2022, 23, 3499. [Google Scholar] [CrossRef]
- Won, J.H.; Byun, S.J.; Oh, B.-M.; Park, S.J.; Gil Seo, H. Risk and mortality of aspiration pneumonia in Parkinson’s disease: A nationwide database study. Sci. Rep. 2021, 11, 6597. [Google Scholar]
- Aquino, Y.C.; Cabral, L.M.; Miranda, N.C.; Naccarato, M.C.; Falquetto, B.; Moreira, T.S.; Takakura, A.C. Respiratory disorders of Parkinson’s disease. J. Neurophysiol. 2022, 127, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Falquetto, B.; Thieme, K.; Malta, M.B.; e Rocha, K.C.; Tuppy, M.; Potje, S.R.; Antoniali, C.; Rodrigues, A.C.; Munhoz, C.D.; Moreira, T.S.; et al. Oxidative stress in the medullary respiratory neurons contributes to respiratory dysfunction in the 6-OHDA model of Parkinson’s disease. J. Physiol. 2020, 598, 5271–5293. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Oliveira, M.A.; Moriya, H.T.; Moreira, T.S.; Takakura, A.C. Respiratory disturbances in a mouse model of Parkinson’s disease. Exp. Physiol. 2019, 104, 729–739. [Google Scholar] [CrossRef]
- Ding, W.; Liu, Y. Genistein attenuates genioglossus muscle fatigue under chronic intermittent hypoxia by down-regulation of oxidative stress level and up-regulation of antioxidant enzyme activity through ERK1/2 signaling pathway. Oral Dis. 2011, 17, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.H.; Li, W.; Chen, X.Y.; Shi, J.J. The study of genistein attenuating genioglossus muscle fatigue under chronic intermittent hypoxia. Chin. J. Stomatol. 2016, 51, 46–50. [Google Scholar]
- Ding, W.; Chen, X.; Li, W.; Fu, Z.; Shi, J. Genistein Protects Genioglossus Myoblast Against Hypoxia-induced Injury through PI3K-Akt and ERK MAPK Pathways. Sci. Rep. 2017, 7, 5085. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.; Sepúlveda, P.; Castillo, R.L.; Salazar, L.A. Relationship between Hypoxic and Immune Pathways Activation in the Progression of Neuroinflammation: Role of HIF-1α and Th17 Cells. Int. J. Mol. Sci. 2023, 24, 3073. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, A.; Takayama, F.; Okada, R.; Liu, Y.; Harada, Y.; Wu, S.; Nakanishi, H. Brazilian Green Propolis Suppresses the Hypoxia-Induced Neuroinflammatory Responses by Inhibiting NF-κB Activation in Microglia. Oxidative Med. Cell. Longev. 2013, 2013, 103695. [Google Scholar] [CrossRef]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell’Agli, M. The antiviral and immunomodulatory activities of propolis: An update and future perspectives for respiratory diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M. The Effects of Propolis on Viral Respiratory Diseases. Molecules 2023, 28, 359. [Google Scholar] [CrossRef]
- Yuksel, S.; Akyol, S. The consumption of propolis and royal jelly in preventing upper respiratory tract infections and as dietary supplementation in children. J. Intercult. Ethnopharmacol. 2016, 5, 308–311. [Google Scholar] [PubMed]
- Bachevski, D.; Damevska, K.; Simeonovski, V.; Dimova, M. Back to the basics: Propolis and COVID-19. Dermatol. Ther. 2020, 33, e13780. [Google Scholar]
- Berretta, A.A.; Silveira, M.A.D.; Capcha, J.M.C.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar]
- Dilokthornsakul, W.; Kosiyaporn, R.; Wuttipongwaragon, R.; Dilokthornsakul, P. Potential effects of propolis and honey in COVID-19 prevention and treatment: A systematic review of in silico and clinical studies. J. Integr. Med. 2022, 20, 114–125. [Google Scholar] [PubMed]
- Ripari, N.; Sartori, A.A.; Honorio, M.d.S.; Conte, F.L.; Tasca, K.I.; Santiago, K.B.; Sforcin, J.M. Propolis antiviral and immunomodulatory activity: A review and perspectives for COVID-19 treatment. J. Pharm. Pharmacol. 2021, 73, 281–299. [Google Scholar] [PubMed]
- Yang, J.-Y.; Ma, Y.-X.; Liu, Y.; Peng, X.-J.; Chen, X.-Z. A Comprehensive Review of Natural Flavonoids with Anti-SARS-CoV-2 Activity. Molecules 2023, 28, 2735. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; de Jong, D.; Berretta, A.A.; dos Santos Galvão, E.B.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; da Conceição, L.F.M.R.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).