Voltammetric Determination of the Total Content of the Most Commonly Occurring Estrogens in Water Media
Abstract
1. Introduction
2. Results and Discussion
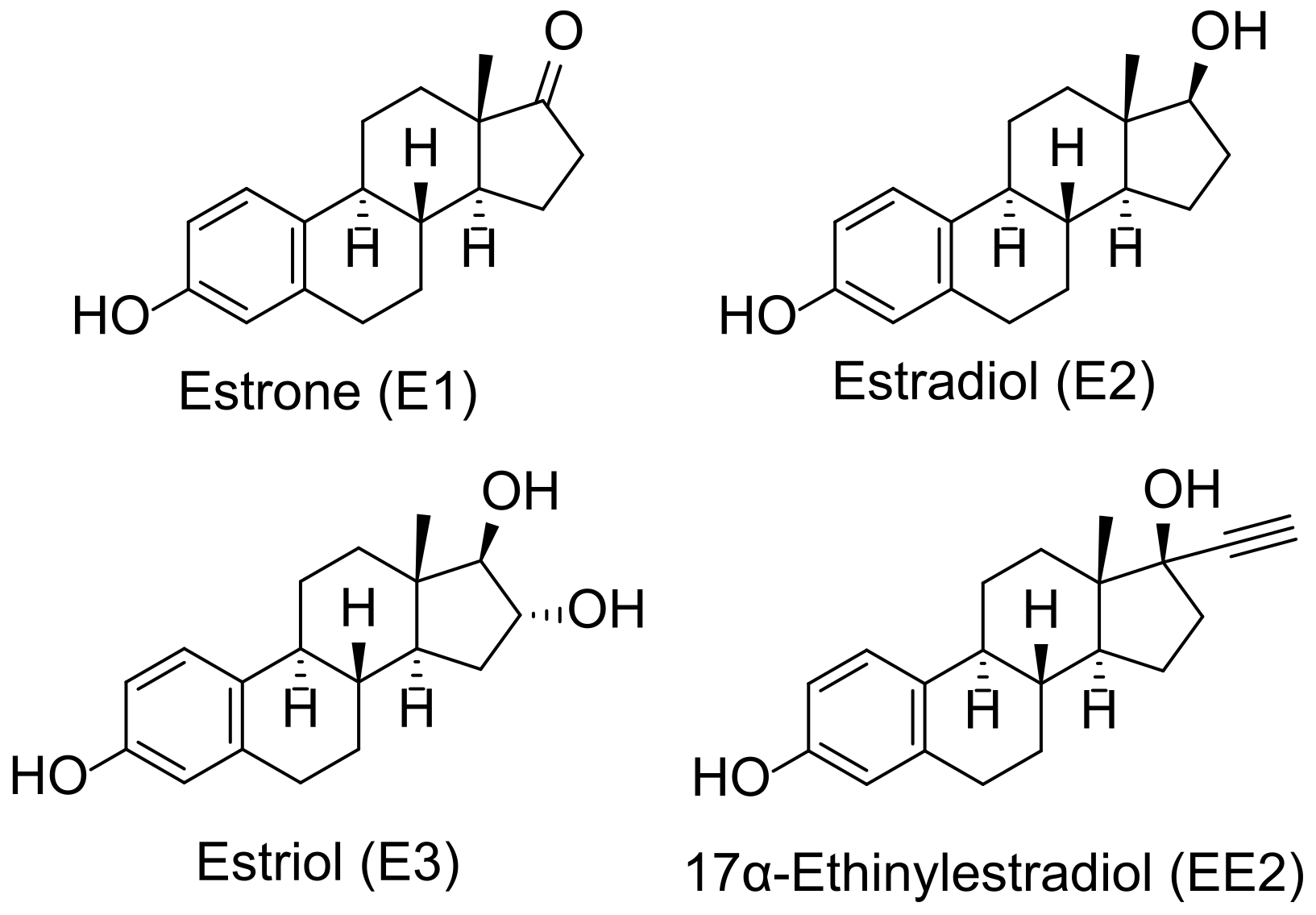
2.1. Electrochemical Behavior of 17α-Ethinylestradiol, Estrone, 17β-Estradiol, and Estriol
2.2. Effect of pH on EE2 Oxidation
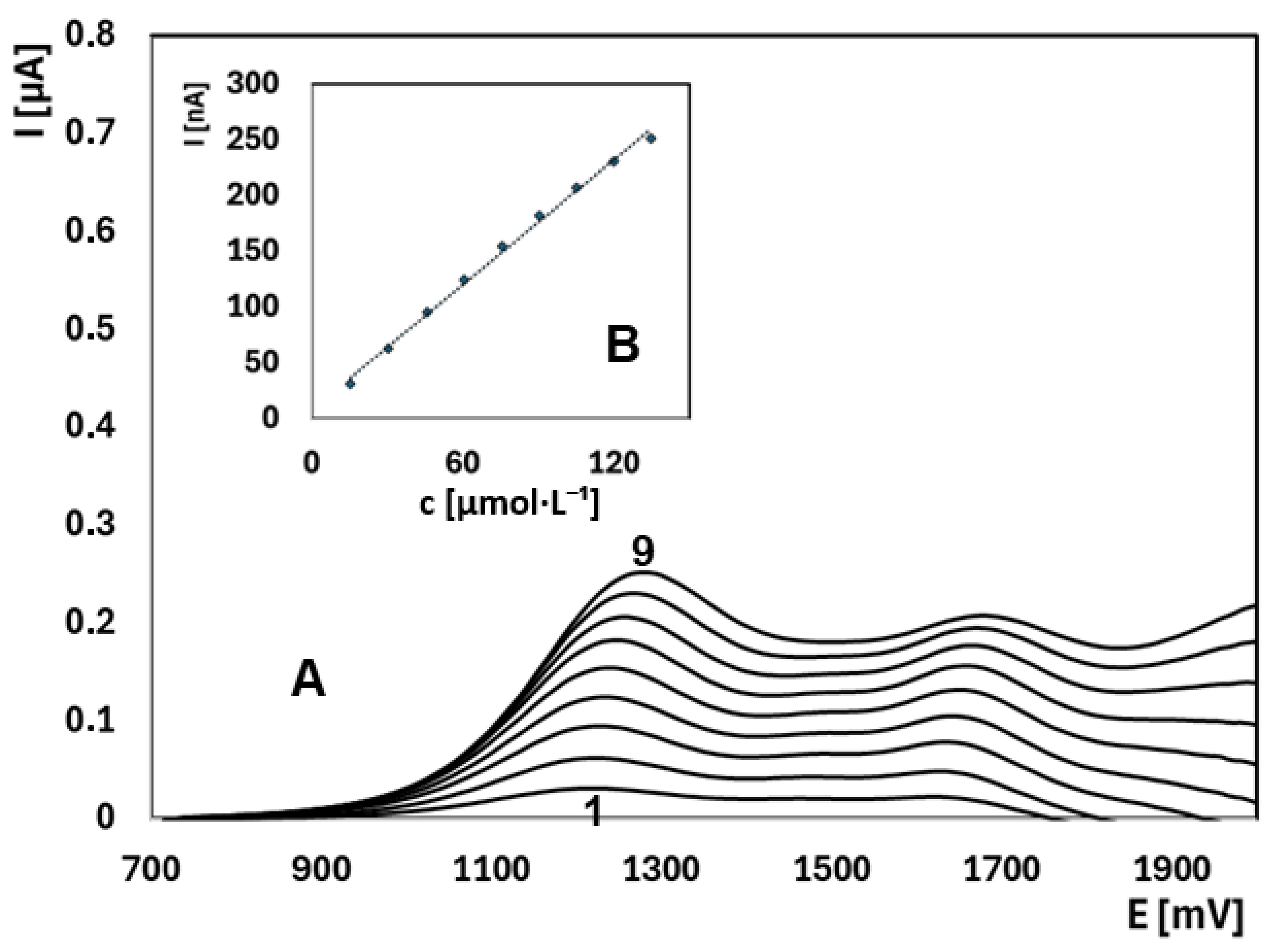
2.3. Quantitative Determination of EE2 Under Optimized Conditions
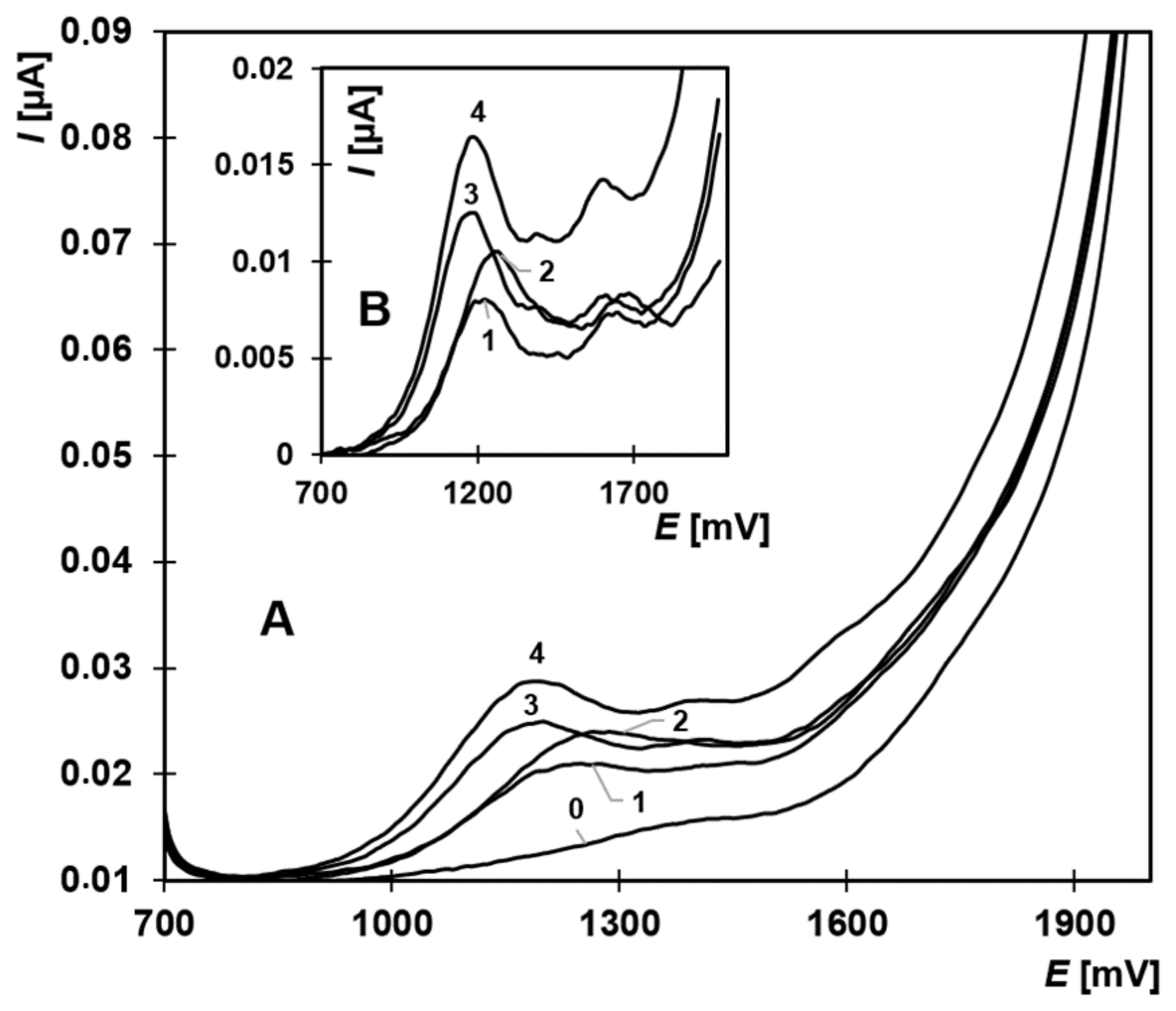
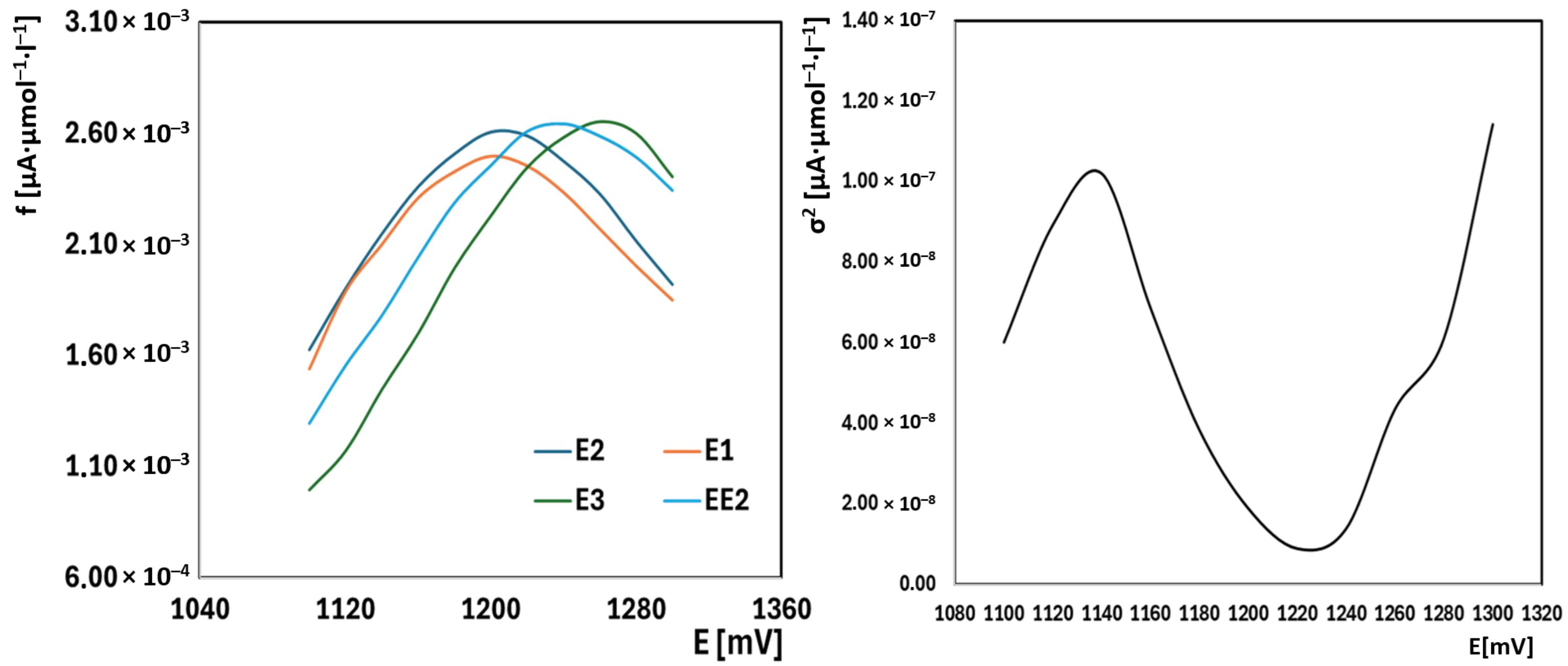
2.4. Simultaneous Analysis of Estrone, 17β-Estradiol, Estriol, and EE2
2.5. Application to Real Water Samples
3. Conclusions
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Instrumentation
4.3. Voltammetric Measurements
4.4. pH Study and Supporting Electrolyte Composition
4.5. Calibration and Quantification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, Personal Care Products and Endocrine Disrupting Agents in the Environment—A Review. CLEAN—Soil Air Water 2009, 37, 277–303. [Google Scholar] [CrossRef]
- D’Ascenzo, G.; Di Corcia, A.; Gentili, A.; Mancini, R.; Mastropasqua, R.; Nazzari, M.; Samperi, R. Fate of Natural Estrogen Conjugates in Municipal Sewage Transport and Treatment Facilities. Sci. Total Environ. 2003, 302, 199–209. [Google Scholar] [CrossRef]
- Hill, R.L.; Janz, D.M. Developmental Estrogenic Exposure in Zebrafish (Danio rerio): I. Effects on Sex Ratio and Breeding Success. Aquat. Toxicol. 2003, 63, 417–429. [Google Scholar] [CrossRef]
- Hanselman, T.A.; Graetz, D.A.; Wilkie, A.C. Manure-Borne Estrogens as Potential Environmental Contaminants: A Review. Environ. Sci. Technol. 2003, 37, 5471–5478. [Google Scholar] [CrossRef]
- Irwin, L.K.; Gray, S.; Oberdörster, E. Vitellogenin Induction in Painted Turtle, Chrysemys picta, as a Biomarker of Exposure to Environmental Levels of Estradiol. Aquat. Toxicol. 2001, 55, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Svenson, A.; Allard, A.-S.; Ek, M. Removal of Estrogenicity in Swedish Municipal Sewage Treatment Plants. Water Res. 2003, 37, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Cargouët, M.; Perdiz, D.; Mouatassim-Souali, A.; Tamisier-Karolak, S.; Levi, Y. Assessment of River Contamination by Estrogenic Compounds in Paris Area (France). Sci. Total Environ. 2004, 324, 55–66. [Google Scholar] [CrossRef]
- Laganà, A.; Bacaloni, A.; De Leva, I.; Faberi, A.; Fago, G.; Marino, A. Analytical Methodologies for Determining the Occurrence of Endocrine Disrupting Chemicals in Sewage Treatment Plants and Natural Waters. Anal. Chim. Acta 2004, 501, 79–88. [Google Scholar] [CrossRef]
- Parkkonen, J.; Larsson, D.G.J.; Adolfsson-Erici, M.; Pettersson, M.; Berg, A.H.; Olsson, P.-E.; Förlin, L. Contraceptive Pill Residues in Sewage Effluent Are Estrogenic to Fish. Mar. Environ. Res. 2000, 50, 198. [Google Scholar] [CrossRef]
- Ying, G.-G.; Williams, B.; Kookana, R. Environmental Fate of Alkylphenols and Alkylphenol Ethoxylates—A Review. Environ. Int. 2002, 28, 215–226. [Google Scholar] [CrossRef]
- Balaguer, P.; François, F.; Comunale, F.; Fenet, H.; Boussioux, A.-M.; Pons, M.; Nicolas, J.-C.; Casellas, C. Reporter Cell Lines to Study the Estrogenic Effects of Xenoestrogens. Sci. Total Environ. 1999, 233, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, Z. Determination of Hormones in Human Urine by Ultra-High-Performance Liquid Chromatography/Triple-Quadrupole Mass Spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8583. [Google Scholar] [CrossRef]
- Martins de Barros, R.; Lissalde, S.; Guibal, R.; Guibaud, G. Development of a Multi-Hormone Analysis Method by LC-MS/MS for Environmental Water Application Using Diffusive Gradient in Thin Films. Talanta 2022, 243, 123390. [Google Scholar] [CrossRef] [PubMed]
- Ferenčík, M.; Schovánková, J.; Stojanová, S. Stanovení a Výskyt Estrogenních Hormonů a Dalších Látek s Estrogenním Účinkem ve Vodách Pomocí LCMS/MS a GC/MS. In Proceedings of the Sborník 8. Konference Hydroanalytika 2019, Hradec Králové, Czech Republic, 17–18 September 2019; pp. 21–26. [Google Scholar]
- Klikarová, J.; Chromá, M.; Sýs, M. Simultaneous Voltammetric Determination of Female Hormones Using Different Carbonaceous Electrodes in a Non-Aqueous Environment. Microchem. J. 2023, 193, 109219. [Google Scholar] [CrossRef]
- Scala-Benuzzi, M.L.; Soler-Illia, G.J.A.A.; Raba, J.; Battaglini, F.; Schneider, R.J.; Pereira, S.V.; Messina, G.A. Immunosensor Based on Porous Gold and Reduced Graphene Platform for the Determination of EE2 by Electrochemical Impedance Spectroscopy. J. Electroanal. Chem. 2021, 897, 115604. [Google Scholar] [CrossRef]
- Martínez, N.A.; Schneider, R.J.; Messina, G.A.; Raba, J. Modified Paramagnetic Beads in a Microfluidic System for the Determination of Ethinylestradiol (EE2) in River Water Samples. Biosens. Bioelectron. 2010, 25, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Wong, A.; Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Sotomayor, M.D.P.T.; Moraes, F.C. Voltammetric Determination of Ethinylestradiol Using Screen-Printed Electrode Modified with Functionalized Graphene, Graphene Quantum Dots and Magnetic Nanoparticles Coated with Molecularly Imprinted Polymers. Talanta 2021, 224, 121804. [Google Scholar] [CrossRef] [PubMed]
- Triviño, J.J.; Gómez, M.; Valenzuela, J.; Vera, A.; Arancibia, V. Determination of a Natural (17β-Estradiol) and a Synthetic (17α-Ethinylestradiol) Hormones in Pharmaceutical Formulations and Urine by Adsorptive Stripping Voltammetry. Sens. Actuators B Chem. 2019, 297, 126728. [Google Scholar] [CrossRef]
- Torrinha, Á.; Carneiro, P.; Dias, D.; Delerue-Matos, C.; Morais, S. The Simpler the Better: Highly Sensitive 17α-Ethinylestradiol Sensor Based on an Unmodified Carbon Paper Transducer. Talanta 2022, 245, 123457. [Google Scholar] [CrossRef]
- Martínez, N.A.; Pereira, S.V.; Bertolino, F.A.; Schneider, R.J.; Messina, G.A.; Raba, J. Electrochemical Detection of a Powerful Estrogenic Endocrine Disruptor: Ethinylestradiol in Water Samples through Bioseparation Procedure. Anal. Chim. Acta 2012, 723, 27–32. [Google Scholar] [CrossRef]
- Yang, C.; Sang, Q.; Zhang, S.; Huang, W. Voltammetric Determination of Estrone Based on the Enhancement Effect of Surfactant and a MWNT Film Electrode. Mater. Sci. Eng. C 2009, 29, 1741–1745. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Li, L.; Swain, G.M. Comparative Electrochemical Response of Estrone at Glassy-Carbon, Nitrogen-Containing Tetrahedral Amorphous Carbon and Boron-Doped Diamond Thin-Film Electrodes. J. Electroanal. Chem. 2014, 712, 207–214. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G. Poly (L-Proline) Modified Carbon Paste Electrode as the Voltammetric Sensor for the Detection of Estriol and Its Simultaneous Determination with Folic and Ascorbic Acid. Mater. Sci. Energy Technol. 2019, 2, 365–371. [Google Scholar] [CrossRef]
- Özcan, A.; Topçuoğulları, D. Voltammetric Determination of 17-β-Estradiol by Cysteamine Self-Assembled Gold Nanoparticle Modified Fumed Silica Decorated Graphene Nanoribbon Nanocomposite. Sens. Actuators B Chem. 2017, 250, 85–90. [Google Scholar] [CrossRef]
- da Silveira, J.P.; Piovesan, J.V.; Spinelli, A. Carbon Paste Electrode Modified with Ferrimagnetic Nanoparticles for Voltammetric Detection of the Hormone Estriol. Microchem. J. 2017, 133, 22–30. [Google Scholar] [CrossRef]
- Jin, G.-P.; Lin, X.-Q. Voltammetric Behavior and Determination of Estrogens at Carbamylcholine Modified Paraffin-Impregnated Graphite Electrode. Electrochim. Acta 2005, 50, 3556–3562. [Google Scholar] [CrossRef]
- Hu, S.; Wu, K.; Yi, H.; Cui, D. Voltammetric Behavior and Determination of Estrogens at Nafion-Modified Glassy Carbon Electrode in the Presence of Cetyltrimethylammonium Bromide. Anal. Chim. Acta 2002, 464, 209–216. [Google Scholar] [CrossRef]
- Xu, J.; Granger, M.C.; Chen, Q.; Strojek, J.W.; Lister, T.E.; Swain, G.M. Peer Reviewed: Boron-Doped Diamond Thin-Film Electrodes. Anal. Chem. 1997, 69, 591A–597A. [Google Scholar] [CrossRef]
- Kraft, A. Doped Diamond: A Compact Review on a New, Versatile Electrode Material. Int. J. Electrochem. Sci. 2007, 2, 355–385. [Google Scholar] [CrossRef]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.H.; Santos, G.d.O.S.; Romanholo Ferreira, L.F.; Américo-Pinheiro, J.H.P.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Environmental Aspects of Hormones Estriol, 17β-Estradiol and 17α-Ethinylestradiol: Electrochemical Processes as next-Generation Technologies for Their Removal in Water Matrices. Chemosphere 2021, 267, 128888. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, T.; Du, L.; Zhang, Z.; Nian, Q.; Wang, M. Fast and Simple Determination of Estrogens in Milk Powders by Magnetic Solid-Phase Extraction Using Carbon Nitride Composites Prior to HPLC. Anal. Bioanal. Chem. 2021, 413, 215–223. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Zhen, H.; Chen, X.; Sheng, M.; Li, K.; Xue, W.; Zhao, H.; Meng, S.; Cao, G. Determination of Estrogens and Estrogen Mimics by Solid-Phase Extraction with Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B 2021, 1168, 122559. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Jia, J.; Tong, K.; Ouyang, J.; Jiang, W.; Zhu, X. Determination of Synthetic Estrogens in Milk by a Novel Hyper-Crosslinked Polymer SPME Coupled with HPLC-MS. Microchem. J. 2022, 181, 107700. [Google Scholar] [CrossRef]
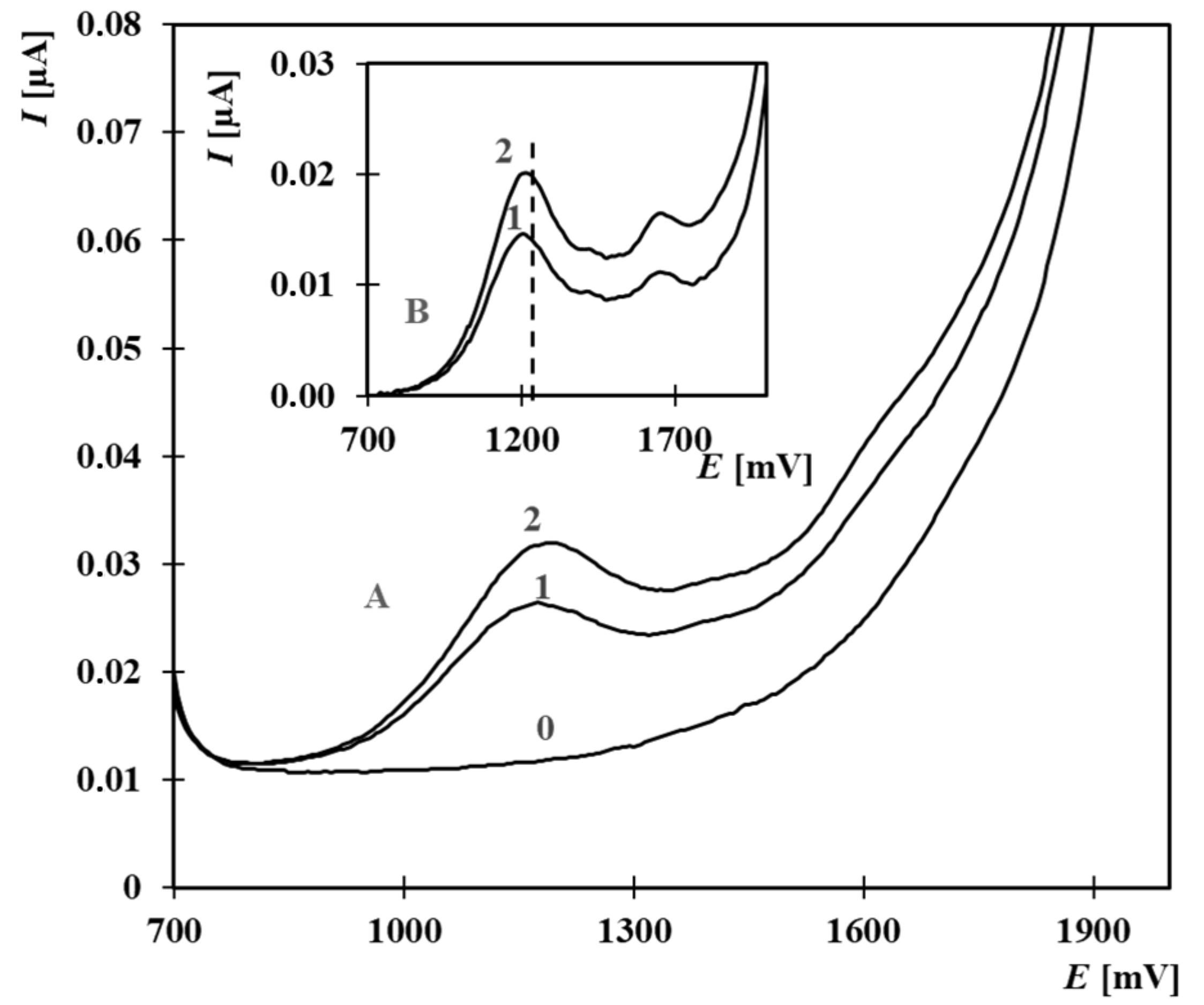
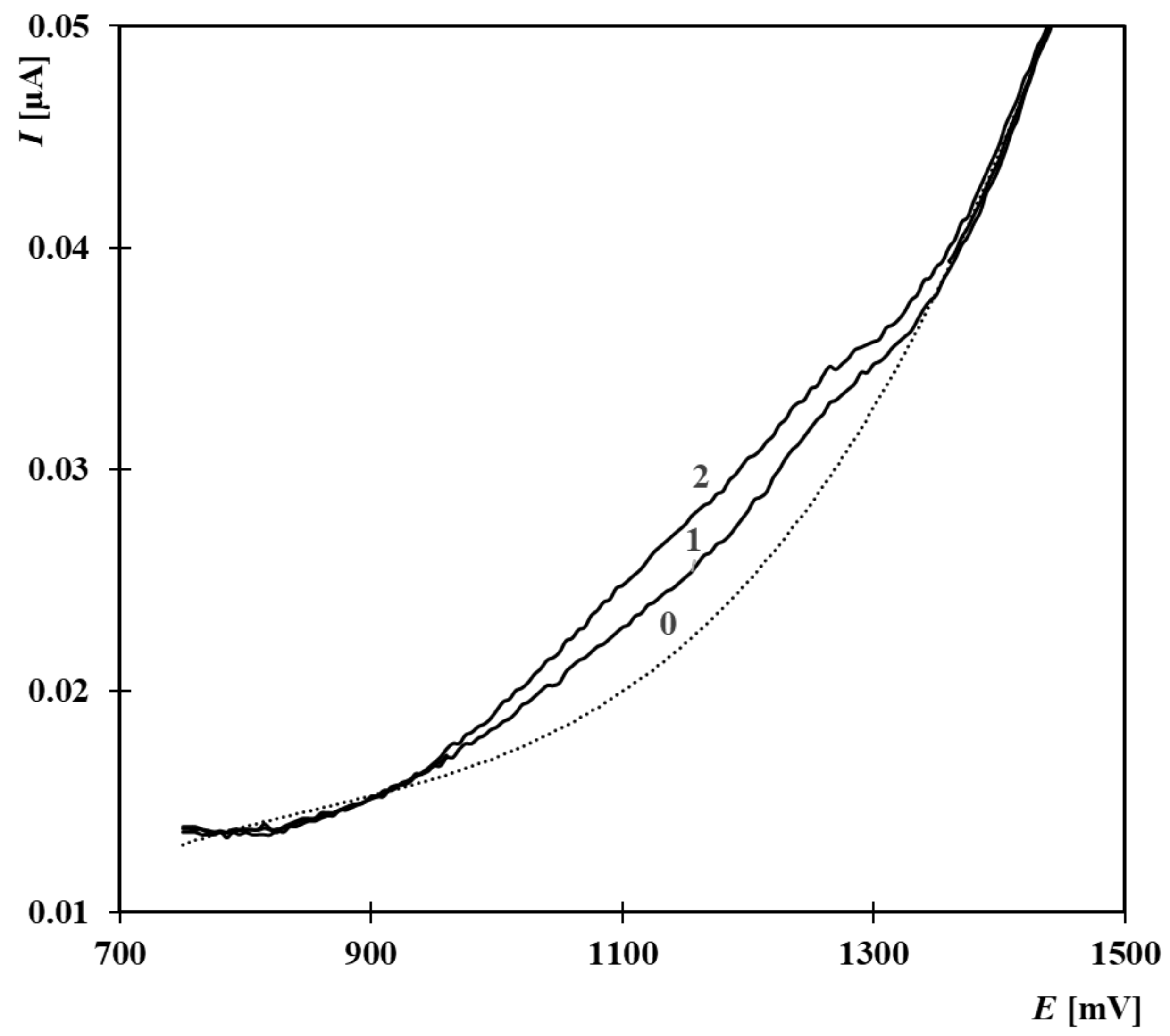
| Composition of the Supporting Electrolyte | Concentration Range [µmol·L−1] | Calibration Equation | R2 |
|---|---|---|---|
| 15 mL BR | 15.35–105.33 | I = 1.88c − 6.76 | 0.998 |
| 10 mL BR + 5 mL ACN | 15.35–134.55 | I = 1.90c + 6.38 | 0.999 |
| 5 mL BR + 10 mL ACN | 15.35–134.55 | I = 1.75c + 11.11 | 0.998 |
| pH | Calibration Equation | R2 |
|---|---|---|
| 2.04 | I = −0.003c2 + 1.07c − 1.73 | 0.9995 |
| 4.03 | I = −0.0024c2 + 3.77c + 2.47 | 0.9980 |
| 6.06 | I = −0.008c2 + 2.77c − 10.50 | 0.9911 |
| 9.04 | I = 1.86c + 8.39 | 0.9984 |
| 10.00 | I = 1.78c + 8.87 | 0.9956 |
| Analyzed [µmol·L−1] | Found [µmol·L−1] | Δ [%] | ||||
|---|---|---|---|---|---|---|
| E1 | E2 | E3 | EE2 | Σ Estrogens | Σ Estrogens | |
| 1.34 | 1.92 | 0.53 | 1.66 | 5.45 | 5.95 | +9.17 |
| 1.34 | 3.84 | 0.53 | 3.33 | 9.03 | 8.47 | −6.28 |
| 1.34 | 1.92 | 0.53 | 3.33 | 7.06 | 7.12 | +0.85 |
| 1.87 | 2.69 | 0.74 | 3.33 | 8.63 | 8.73 | +1.37 |
| 2.68 | 3.84 | 1.06 | 3.33 | 10.90 | 11.14 | +2.11 |
| No. | Found Σ Estrogens [µmol·L−1] | Δ [µmol·L−1] | Δ [%] |
|---|---|---|---|
| 1 | 11.24 | +0.33 | +3.02 |
| 2 | 11.07 | +0.16 | +1.47 |
| 3 | 10.98 | +0.07 | +0.64 |
| 4 | 10.95 | +0.04 | +0.37 |
| 5 | 10.93 | +0.02 | +0.18 |
| Mean: 11.03 µmol·L−1 SD: 0.60 µmol·L−1 CI: 11.03 ± 0.74 µmol·L−1 | |||
| No. | Sample | Analyzed [mol·L−1] | Found [mol·L−1] | Δ [mol·L−1] | Δ [%] |
|---|---|---|---|---|---|
| 1 | Model | 9.99 × 10–8 | 9.99 × 10–8 | 0 | 0 |
| 2 | Model | 2.47 × 10–8 | 2.54 × 10–8 | +0.07 | +2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chýlková, J.; Bartáček, J.; Měchová, N.; Sedlák, M.; Váňa, J. Voltammetric Determination of the Total Content of the Most Commonly Occurring Estrogens in Water Media. Molecules 2025, 30, 751. https://doi.org/10.3390/molecules30030751
Chýlková J, Bartáček J, Měchová N, Sedlák M, Váňa J. Voltammetric Determination of the Total Content of the Most Commonly Occurring Estrogens in Water Media. Molecules. 2025; 30(3):751. https://doi.org/10.3390/molecules30030751
Chicago/Turabian StyleChýlková, Jaromíra, Jan Bartáček, Natálie Měchová, Miloš Sedlák, and Jiří Váňa. 2025. "Voltammetric Determination of the Total Content of the Most Commonly Occurring Estrogens in Water Media" Molecules 30, no. 3: 751. https://doi.org/10.3390/molecules30030751
APA StyleChýlková, J., Bartáček, J., Měchová, N., Sedlák, M., & Váňa, J. (2025). Voltammetric Determination of the Total Content of the Most Commonly Occurring Estrogens in Water Media. Molecules, 30(3), 751. https://doi.org/10.3390/molecules30030751






