Abstract
Herein, we prepared nanoparticles of chitosan–manganese(II) complexes in different molar ratios (1:2, 1:1, and 2:1) and fully characterized them using dynamic and electrophoretic light scattering, X-ray diffraction, SEM, FTIR, and thermal analysis. Nanoparticles Chitosan + Mn2+ (1:1) have a high catalytic activity in the oxidative coupling of benzylamine, resulting in the imine formation and also in selective aldol reaction. Chitosan + Mn2+ (1:1) catalyze the reactions in the greenest solvents: water and water/ethanol mixture. Moreover, Chitosan + Mn2+ (1:1) is very easy to prepare and convenient to use. The catalyst is separated from the reaction mixture by a simple nanoporous filter or centrifugation and does not lose catalytic activity after at least ten uses. The chitosan–manganese(II) complexes reduce the milk fermentation time, demonstrating the effectiveness in accelerating the fermentation process by Streptococcus thermophilus. They also contribute to increasing the shelf life of fermented milk products by inhibiting the undesirable post-acidification process. We found that the optimal ratio of chitosan and Mn2+ to manifest the apogee of the desired effects (acceleration of milk fermentation and increase in the shelf life of the fermented product) is 1:2.
1. Introduction
Chitosan is one of the most common natural polymers (second only to cellulose). The key advantages of chitosan over other biopolymers include its biocompatibility, biodegradability, non-toxicity, and simplicity of its chemical modification due to the presence of a primary amino group in the macromolecule []. Chitosan can be successfully used in medicine, pharmaceuticals, cosmetics, agriculture, food industry, ecology, and other fields, and these findings have been thoroughly summarized in recent reviews and books [,,,,,]. On the other hand, manganese(II) is a very attractive metal center that is an important microelement for almost all living organisms []. Coordination compounds of manganese(II) are promising for use in medicinal chemistry [], clinical diagnostics [], catalysis (alkylation, oxidation, hydrosilylation, and CO2 reduction) [], and photochemical end electronic devices, including OLEDs [,].
Mixing manganese(II) and chitosan inevitably leads to the formation of coordination compounds since chitosan is a chelating macromolecular ligand that is prone to complexation of d-metal cations []. The vast majority of the literature data on chitosan/manganese(II) complexes describes the sorption of manganese(II) by chitosan or its derivative-based sorbents [,]. Such complexes suffer from insufficient characterization.
Significantly fewer (but very interesting) studies have focused on the targeted synthesis of chitosan/manganese(II) complexes and their attractive applications in various fields. In view of our general interest in the application of metal complexes (i) in catalysis of organic transformations and (ii) in dairy biotechnological processes (stimulation of fermentation and inhibition of post-acidification), we found the following aspects in the literature regarding chitosan/manganese(II) complexes. First, regarding catalysis, chitosan/manganese(II)-based systems are attractive for oxidation of methyl phenyl sulfide [], ethyl benzene oxidation [,], enzymatic catalysis [], and dies oxidation []. However, in all cases, chitosan derivatives or complex Mn(II) compounds on a chitosan matrix are used. This, in turn, makes such studies less attractive in terms of simplicity and elegance of synthesis. Second, regarding dairy biotechnological processes, the literature contains examples of the use of chitosan for stimulating fermentation [] and inhibition of the undesirable process of post-acidification [,]. However, the literature currently lacks an evaluation of the effects of manganese salts and chitosan/manganese(II) complex on both fermentation and post-acidification processes.
To fill these gaps, we set out to prepare and characterize chitosan and manganese(II) complexes in various molar ratios (1:2, 1:1, and 2:1). In initial experiments, we first found that these complexes formed as nanoparticles. In view of our interest in catalytic reactions, we studied the catalytic activity of the complexes in oxidative coupling of benzylamine and in aldol reaction. Based on our experience and curiosity in dairy biotechnological processes, we evaluated the effect of chitosan and manganese(II) complexes on both milk fermentation and post-acidification processes. Thus, this work consists of a predominantly chemical part (synthesis, characterization, and study of the catalytic properties of chitosan/manganese(II) complexes) and a biological part (study of effects of the complexes on milk fermentation and post-acidification). The results of these studies and their detailed discussion are presented in the sections that follow.
2. Results and Discussion
2.1. Chemistry
2.1.1. Synthesis of Complexes
For synthesis of chitosan/manganese complexes, we used high molecular weight chitosan (200 kDa). Since high molecular weight chitosans are insoluble in water, we used 1% acetic acid as a solvent for the synthetic procedures. As a source of the metal center (Mn2+ ions), we chose highly water-soluble manganese(II) chloride tetrahydrate. A polymer/Mn2+ ratio was 1:2, 1:1, or 2:1 mono-mol/mol.
All resultant chitosan-based complexes (named Chitosan + Mn2+ (1:2), Chitosan + Mn2+ (1:1), and Chitosan + Mn2+ (2:1)) provided transparent suspensions (Figure 1A). After freeze-drying, the complexes appeared as fine pale, porous, cotton-like materials (Figure 1B). The synthesized complexes are easily redispersed in water after freeze-drying. The resultant suspensions are stable for a month and then undergo gradual coagulation.

Figure 1.
Suspension (A) and pale, porous, cotton-like freeze-dried Chitosan + Mn2+ (1:1) complex (B).
ICP analysis confirmed quantitative content of manganese in the samples. Thus, the percentage in the samples Chitosan + Mn2+ (1:2), Chitosan + Mn2+ (1:1), and Chitosan + Mn2+ (2:1), respectively, is as follows: 32.18%, 21.36%, and 12.52%.
2.1.2. Dynamic and Electrophoretic Light Scattering Studies
Stability of the suspensions is largely determined by the size of dispersed microparticles and their zeta potential []. Generally, a decrease in hydrodynamic diameter of microparticles results in an increase in stability of their suspensions []. Zeta potential is an extremely important indicator of the stability of microparticles in a liquid medium. A high (ca. 25 mV and more) absolute value of the zeta potential determines increased stability of the colloidal system, i.e., microsuspension [].
In this study, we evaluated the hydrodynamic diameter and zeta potential of the prepared chitosan-based complexes immediately after synthesis, after redispersion, and also during 150 days of suspension in the dark at 5 °C.
Analysis of the starting chitosan solution by dynamic light scattering expectedly showed a rapidly changing pattern of polymodal size distribution with sharply changing intensities and peak positions. This fact is not surprising and is explained by changes in sizes of macromolecular coils as a result of thermal motion due to pronounced segmental mobility, as well as molecular-mass and compositional heterogeneity of chitosan [].
Almost immediately after mixing the chitosan and manganese(II) salt solutions, the situation changed dramatically; we detected the unimodal size distribution with constancy of intensity and peak position. These changes indicate formation of chitosan–metal complexes. Thus, a few minutes after starting mixing chitosan and manganese chloride solutions, the reaction mixtures gave rise to microparticles with a hydrodynamic diameter of ca. 150 nm for Chitosan + Mn2+ 1:2, ca. 274 nm for Chitosan + Mn2+ 1:1, and ca. 174 nm for Chitosan + Mn2+ 2:1 with unimodal size distribution. Scanning electron microscopy (see example in Figure 2) confirms the unimodal distribution and also shows the spherical shape of the resultant microparticles. All formed microparticles have a fairly high positive zeta potential value. (ca. 20 mV for Chitosan + Mn2+ (1:2), ca. 30 mV for Chitosan + Mn2+ (1:1), and ca. 20 mV for Chitosan + Mn2+ (2:1)). After lyophilization, the prepared microparticles completely recover their size, shape, and zeta potential within 30 min, and this indicates their high redispersibility.
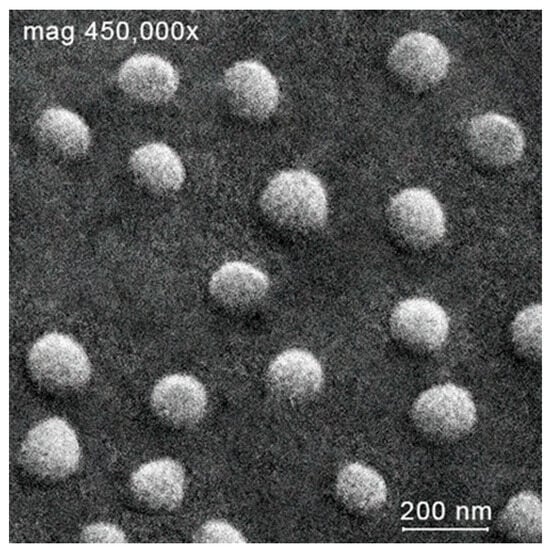
Figure 2.
Scanning electron microscopy of microparticles Chitosan + Mn2+ (1:2).
The hydrodynamic radii are a relatively constant value and fluctuated around 273 ± 64 nm (Chitosan + Mn2+ (2:1)), 233 ± 41 nm (Chitosan + Mn2+ (1:1)) and 261 ± 63 nm (Chitosan + Mn2+ (1:2)) during the experiment (150 days, Figure 3A).

Figure 3.
Hydrodynamic radii (A) and ζ-potentials (B) of the chitosan–Mn2+ systems (the relative standard deviation for all points was below 5%).
Zeta potential of microparticles is more subject to fluctuations than their sizes. The zeta potential of all microparticles of the obtained complexes had a tendency to change over time from 37 mV (2 day) to 5 mV (150 day) for Chitosan + Mn2+ 2:1, from 35 mV (2 day) to 5 mV (150 day) for Chitosan + Mn2+ 1:1, and from 37 mV (2 day) to 5 mV (150 day) for Chitosan + Mn2+ 2:1 (Figure 3B).
A month after the start of the experiment, we observed gradual weak turbidity and coagulation as a result of monotonous destabilization of the complexes due to a decrease in the aggregate stability of the particles. Aggregate stability, in turn, decreases due to a decrease in the absolute value of the ζ-potential (Figure 3B).
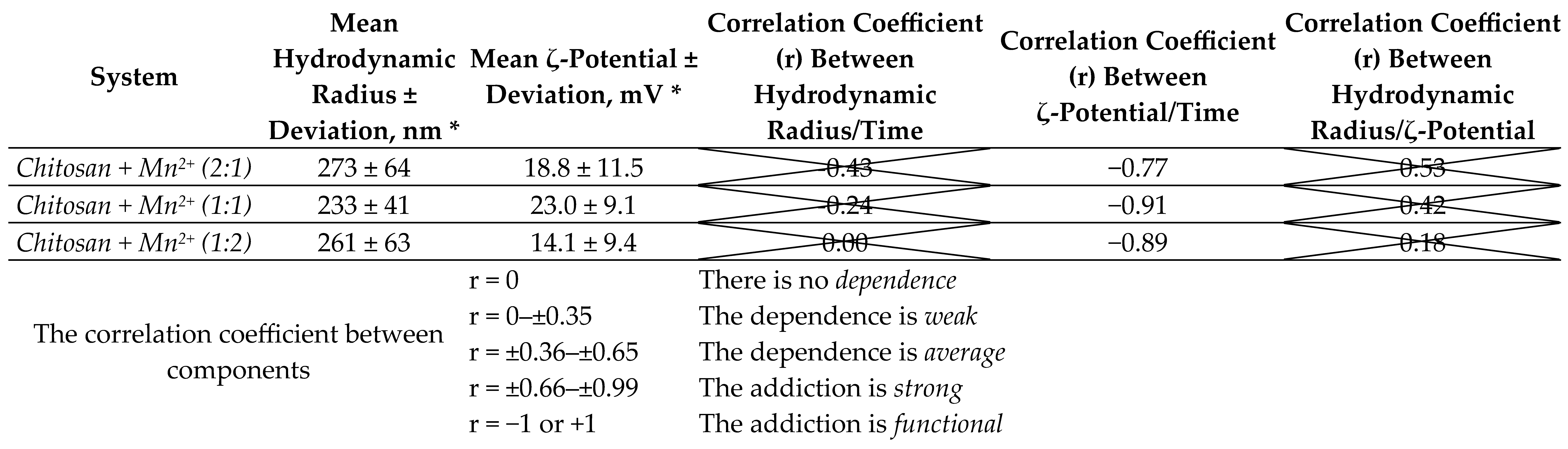
We summarized the statistical and correlation analysis data for the components “hydrodynamic radius–time”, “ζ-potential–time” and “hydrodynamic radius–ζ-potential” in Table 1 over 150 days of experiment.

Table 1.
Statistical analysis of the characteristics of chitosan–metal systems.
Statistical and correlation analysis of experimental data showed that there is no relationship between the parameters “hydrodynamic radius” and “time”, as well as “hydrodynamic radius” and “ζ-potential” for the microparticles of the synthesized complexes since the p-value is unreliable (p < 0.05) and poorly describes the nature of the relationship between these quantities. In addition, the correlation coefficients have a low value (r < 0.65). Statistically unreliable values of the correlation coefficient (crossed out in the table) at p < 0.05 for the obtained systems mean the absence of any statistically significant relationship between the parameters, which in turn means the impossibility of further mathematical processing and functional approximation.
On the contrary, there is a clear statistical dependence between the parameters “ζ-potential” and “time”. All microparticles of the synthesized complexes are described by a medium-strong inverse dependence, which means a statistically reliable decrease in the value of the ζ-potential over time. A strong correlation dependence (r = ±0.66–±0.99) presupposes the possibility of approximating the value of the ζ-potential from time by a mathematical function.
The obtained results indicate that the synthesized systems are not equilibrium. This can be explained by rearrangements in the polymer chain of chitosan, for example, processes of gradual depolymerization of the macromolecule, reactions of rare cross-linking, partial oxidative degradation of chitosan, etc. The manganese(II) center, in turn, can also affect the stability of polymer–metal systems (competitive interaction of solvent molecules with the metal center, and change in the reactivity of the ligand []).
We consider the obtained systems as potential extenders of the shelf life of products based on fermented milk (e.g., yoghurts). The conventional shelf life of such products is 7–10 days. The microparticles of the synthesized complexes are stable for at least a month (see Figure 3); therefore, they are suitable for the chosen purpose. However, since the complexes are nonequilibrium systems, they should be produced as a lyophilizate and redispersed immediately before use.
2.1.3. FTIR Analysis
We also used FTIR spectroscopy to further investigate the interactions between chitosan and the manganese(II) center in the prepared complexes. We used a set of previously published data [,,] to identify the absorption bands in the obtained spectra (Figure 4). The wave numbers of the absorption maxima are presented in Table 2.
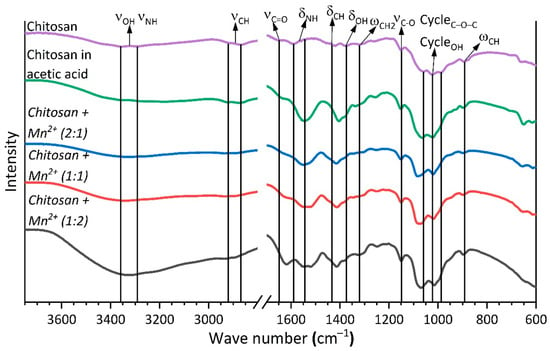
Figure 4.
IR spectrum of chitosan and complexes with Mn2+.

Table 2.
Wave numbers of maxima (cm−1) of absorption bands of chitosan complexes with Mn2+.
The coordination of chitosan to the manganese(II) center does not cause any noticeable changes in the positions of the characteristic bands due to vibrations of the C–O–C bonds of the pyranose ring and the ring-bound O–H groups (∆ 10 cm−1). However, the interaction of chitosan with Mn2+ results in a noticeable shift of the C=O group vibration band (∆ 30 cm−1) and the vibration bands of the N–H and C–H bonds, and also O–H bonds not bound directly to the pyranose ring (∆ 5–15 cm−1 for deformation vibrations, ∆ 50 cm−1 for stretching vibrations of the N–H, O–H bonds, and ∆ 20 cm−1 for stretching vibrations of the C–H bonds). These results indicate the coordination of chitosan to the manganese(II) center through the C=O, N–H, and not bound directly to the pyranose ring O–H functional groups [,,].
2.1.4. X-Ray Diffraction Studies
The obtained complexes were also characterized by X-ray structural analysis (powder diffraction). The diffraction patterns (Figure 5) demonstrate that the synthesized complexes, as well as the starting chitosan, are X-ray amorphous. However, the intensity of the main broadened peak of chitosan is significantly higher than that of chitosan in acetic acid (chitosan acetate). This fact indirectly, at a qualitative level, indicates a significantly higher amorphism of chitosan acetate than that of the starting chitosan. Thus, protonation of chitosan with acetic acid leads to a very noticeable increase in amorphism. The introduction of a Mn(II) center into the protonated chitosan macromolecule also leads to a further slight increase in amorphism, although this effect is significantly less pronounced than the effect of protonation. This is evidenced by a monotonic decrease in the intensity of the peak centered at ca. 20–22° 2θ in the series Chitosan + Mn2+ (2:1) − Chitosan + Mn2+ (1:1) − Chitosan + Mn2+ (2:2) (Figure 5).

Figure 5.
Diffractograms of chitosan and chitosan complexes with Mn2+.
The diffraction patterns also show a change in the peak profile of chitosan in the sample after dissolution in acetic acid compared to the starting polymer. We observed a shift in the main peak of chitosan, and this indicates a rearrangement of the polymer structure because of the formation of chitosan acetate [,]. After the interaction of chitosan with manganese(II) chloride, we did not find any peaks in the diffraction patterns of the obtained complexes corresponding to the original salt.
2.1.5. Differential Thermal and Thermogravimetric Analysis
We used differential thermal and thermogravimetric analysis in N2 atmosphere to study the thermal stability of the obtained sample complexes and to evaluate the effect of introducing Mn2+ ion into the polymer matrix. Thermal curves of the synthesized complexes (Figure 6 and Figure 7) show that the decomposition of their chitosan occurs in two main stages [,,,].

Figure 6.
TGA curves of chitosan and chitosan–Mn2+.
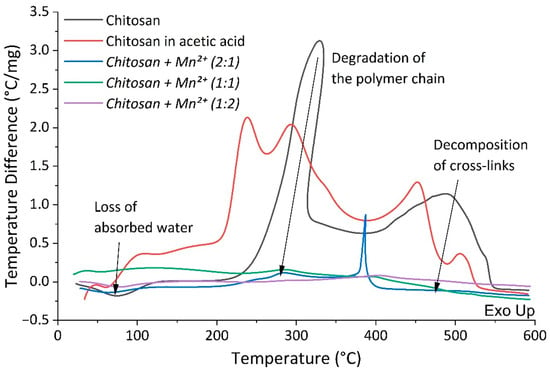
Figure 7.
DTA curves of chitosan and chitosan–Mn2+.
The first stage occurs at a temperature of about 60 °C and is characterized by a mass loss from 7% to 20%. This stage is accompanied by an endothermic effect due to the evaporation of water bound to the polymer matrix and/or coordinated to the manganese(II) center []. Table 3 demonstrates a marked increase in water content in the complexes associated with an increase in manganese content. This is not surprising since the manganese(II) center prefers to coordinate the so-called hard Lewis ligands, primarily H2O.

Table 3.
Thermal parameters of chitosan complexes with Mn2+.
The second stage begins at ca. 225 °C and continues up to 580 °C. This stage results in mass loss of 92% for chitosan, 73.29% for Chitosan + Mn2+ (2:1), 67.95% for Chitosan + Mn2+ (1:1), and 50.81% for Chitosan + Mn2+ (1:2). It is associated with gradual degradation of the polymer chain and the burning of its decay products. The degradation of chitosan is manifested by the cleavage of glycosidic bonds, then the resulting oligomers decompose with the subsequent formation of acetic, butyric acids, as well as lower fatty acids [].
2.1.6. Catalytic Activity of Chitosan–Mn2+ Systems in Oxidative Coupling of Benzylamine
Imines are very attractive for organic chemistry because they are building blocks in the synthesis of many compounds that are important in technology, medicine, agriculture, ecology, and so on [], that is, imines are universal molecules in organic synthesis, especially in the preparation of various heterocyclic systems []. Conventional protocols for the preparation of imines are based on the nucleophilic addition–elimination (AdN-E) reaction of primary amines (acting as the nucleophilic reagent) with aldehydes (the electrophilic substrate). Yet recently, we have witnessed a steady trend toward expanding approaches to imine synthesis (dehydrogenation of secondary amines, coupling of alcohols and amines, and oxidative coupling of amines). Most of these processes require catalysts that are typically used in metal complex catalysis []. The development of these approaches results in new, simple, and preparatively convenient routes to imines and significantly improves the yields of the target products and their isolation procedure. In this study, we used chitosan–Mn2+ systems as catalysts for model reaction of imine synthesis from benzylamine (Scheme 1).
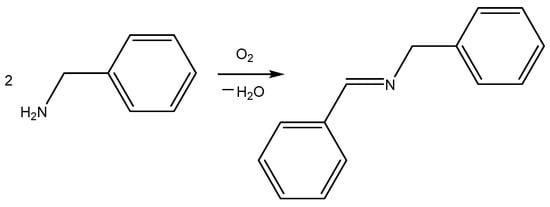
Scheme 1.
The model reaction of imine synthesis from benzylamine.
We evaluated the catalytic properties of the elaborated nanoparticles under the following reaction conditions: heating the reaction mixture for 48 h at 80 °C in the presence of 3 mol% (based on Mn) of the tested catalyst. We first decided to evaluate the catalytic effect of the prepared Chitosan–Mn2+ systems in the greenest solvent, i.e., water (Table 4, Entries 1–3). However, we achieved only low yields (about 20%). We decided to lower the solvent polarity and used methanol (Table 4, Entries 4–6). In this case, the stating materials remained intact, and we observed only traces of the desired product. Thus, the low yields in aqueous media are not due to the low solubility of benzylamine in water, as benzylamine is soluble in methanol, but the yield of the target imine in methanol is lower than in water. Furthermore, we attempted to use 10% trimethylbenzylammonium chloride as a phase-transfer catalyst to improve the yield of the heterogeneous reaction in water. However, this attempt did not result in any noticeable increase in the imine yield. We believe that the low yields in water and, especially, methanol are due to the O-coordination of these solvents to the Mn(II) center, which can lead to deactivation of the so-called catalytically active sites. The catalytic reaction in toluene afforded slightly higher yields (27–34%). Thus, switching to an aromatic solvent increased the conversion of the starting amine to the target imine (Table 4, Entries 7–9). Significant success was achieved when we completely eliminated the solvent (Table 4, Entries 10–12). The solvent-free catalytic reaction resulted in high imine yields (53–80%). Solvent-free transformations, along with reactions in the greenest solvent (water) are of paramount importance in so-called green chemistry [].

Table 4.
Catalytic activity of chitosan–Mn2+ systems in oxidative coupling of benzylamine *.
In all entries, we observed that Chitosan + Mn2+ (1:1) exhibited the greatest catalytic effect. We believe that this may be due to the smaller size of the nanoparticles (see Table 1). Furthermore, the increase in reaction yields demonstrated a dramatic difference in the catalytic effect of the tested nanoparticles (Chitosan + Mn2+ (2:1), Chitosan + Mn2+ (1:1), and Chitosan + Mn2+ (1:2)). Chitosan + Mn2+ (1:1) nanoparticles exhibited the highest catalytic activity.
However, even the solvent-free protocol and the Chitosan + Mn2+ (1:1) catalytic system did not provide a 100% imine yield. This prompted us to increase the catalyst content to 2 mol% (Table 4, Entry 14). As expected, this increase in catalyst concentration to 2 mol% resulted in a higher product yield (93%). Finally, 3 mol% of Chitosan + Mn2+ (1:1) enabled complete conversion of the starting benzylamine to the target imine (Table 4, Entry 14).
The tested catalytic reaction does not proceed under anaerobic conditions. Thus, oxygen is essential, as it obviously functions as an oxidizing agent in the organic transformation.
We also performed a blank experiment, i.e., without any catalyst (Table 4, Entry 13), and detected a very low (10%) yield of imine. To evaluate the necessity of combining Mn2+ and chitosan for the functioning of the integrated catalytic ensemble, we used 1 mol% of manganese(II) acetate in its tetrahydrate form (Table 4, Entry 16) or chitosan (Table 4, Entry 17). Using manganese(II) acetate, we achieved a yield of 15%, and this is only slightly higher than under catalyst-free conditions. The use of chitosan, on the contrary, reduces the imine yield even compared to the complete absence of catalyst (6%). Thus, pure chitosan without any Mn(II) center inhibits the oxidative coupling, and only the complex of chitosan and Mn2+ (1:1) provides the highest yields.
It is also important that the catalyst can be separated from the reaction mixture by simple filtration or centrifugation. After centrifugation, Chitosan + Mn2+ (1:1) can be freeze-dried and reused for catalysis at least 10 times without loss or reduction in catalytic effect.
2.1.7. Catalytic Activity of Chitosan–Mn2+ Systems in Aldol Reaction
Aldol reaction is one of the simplest and the oldest tools for carbon(sp3)–carbon(sp3) bond formation. The reaction includes the coupling of two carbonyl compounds. One of them contains an active methylene component (α-CH acid reaction center) and acts as a nucleophile, attacking the electrophilic carbon atom of the carbonyl group of the second carbonyl compound. Thus, the reaction affords β-hydroxycarbonyl compound []. Classic aldol reaction protocols require the use of strong bases (usually sodium and potassium alkoxides), non-aqueous toxic solvents (toluene and xylene), and prolonged exposure to high temperatures. This, in turn, results in dramatic drawbacks (environmental hazards, and side reactions, such as the elimination of water to form α,β-unsaturated compounds, followed by oxidation and polymerization) []. These complications have led to new protocols that utilize improved catalytic systems and mild conditions []. Most of these metal-based catalysts, although offering high selectivity and mild conditions, are sophisticated and their synthesis requires laborious methods. Here, we propose a new, efficient aldol reaction catalyst, which is prepared by simply mixing chitosan and a manganese(II) salt.
To evaluate the catalytic effect of the synthesized Chitosan–Mn2+ systems, we turned to the classical model aldol reaction of coupling 4-nitrobenzaldehyde with acetone (Scheme 2).
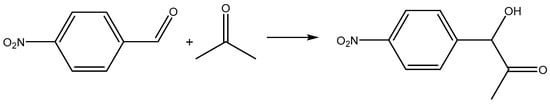
Scheme 2.
The model aldol reaction.
To study the catalytic activity of the tested catalysts for coupling 4-nitrobenzaldehyde aldehyde with acetone, we attempted to use water, the greenest solvent. Furthermore, we intended to avoid heating the reaction mixture and conducted experiments at room temperature (ca. 20 °C). We used 2 mol% of the catalysts (based on Mn), and the reaction was monitored by thick-layer chromatography (TLC) for 1 h (60 min). We considered the reaction complete when the TLC spot of the starting aldehyde disappeared, leaving only the spot of the aldol coupling product, i.e., the corresponding β-hydroxycarbonyl compound. We also monitored the reaction with 1H NMR spectroscopy to quantify the conversion of the starting 4-nitrobenzaldehyde to the desired product. The results are presented in Table 5.

Table 5.
Catalytic activity of Chitosan–Mn2+ systems in aldol reaction *.
The model reaction at room temperature in water proceeded unsatisfactorily. A total 60 min after the start of the experiment, we detected only small amounts of the aldol (Table 5, Entries 1–3). This is apparently due to the low solubility of the starting 4-nitrobenzaldehyde in water. To increase the solubility of the aromatic aldehyde, we added a small amount of ethanol to the reaction mixture (we used a solvent system H2O/EtOH 5/1 (volume/volume)). It should be noted that ethanol is an environmentally friendly organic solvent []. The starting aldehyde is soluble in the solvent system, which significantly improves the yields of the target aldol. The use of Chitosan + Mn2+ (1:1) nanoparticles as a catalyst allows for complete conversion of the starting aldehyde to the desired product (Table 5, Entries 4–6). Moreover, Chitosan + Mn2+ (1:1) nanoparticles are also easily separated from the reaction mixture and do not lose catalytic activity, as in the case of the aldol reaction (see Section 2.1.7). Other nanoparticles proved less active. However, among Chitosan + Mn2+ (2:1) and Chitosan + Mn2+ (1:2), the nanoparticles with a higher manganese(II) content exhibit slightly greater catalytic activity. The reaction proceeds selectively and under mild conditions. In the absence of a catalyst, the studied reaction is impossible, and we did not detect even a trace of the product (Table 5, Entry 7). We also demonstrated that combining both chitosan and Mn2+ is essential for the catalytic effect, since neither chitosan nor Mn2+ alone can catalyze the model aldol reaction (Table 5, Entries 8 and 9).
It is logical to assume that the low yields in an aqueous medium are due to the poor solubility of the starting aldehyde in water. Indeed, the use of 10% phase-transfer catalyst (trimethylbenzylammonium chloride, Table 5, Entry 10) led to a nearly fourfold increase in the aldol yield (up to 45%). However, we believe that the use of an H2O/EtOH solvent system is more appropriate because (i) H2O/EtOH is significantly more environmentally friendly than trimethylbenzylammonium chloride, (ii) all other things being equal, H2O/EtOH provides higher yields of the product, and (iii) H2O/EtOH is cheaper and more readily available.
2.2. Biology
Intensification of enzymatic processes, enhancing the growth and activity of desirable producing microorganisms, is a task of paramount importance in dairy biotechnology []. On the other hand, post-acidification is an undesirable phenomenon in fermented milk products, characterized by acidification outside the optimal pH range due to the activity of microflora []. This process triggers an increase in hydrophobic and electrostatic interactions between protein molecules, leading to (i) the enlargement and aggregation of casein particles and (ii) partial restructuring of the protein network within the product []. Consequently, post-acidification significantly reduces the shelf life of fermented dairy products. Thus, the development of systems capable of intensifying fermentation and inhibiting post-acid is of great importance for dairy biotechnology.
2.2.1. Effect of Chitosan–Mn2+ Systems on Fermentation: Kinetics, pH Changes, and Dynamics of CFU Number Changes
At the first stage, we investigated the effect of the obtained Chitosan–Mn2+ systems (Chitosan + Mn2+ (2:1), Chitosan + Mn2+ (1:1), and Chitosan + Mn2+ (1:2)) on fermented milk pH changes and fermentation kinetics. We compared the effects of the synthesized systems with manganese chloride. We used different masses of the tested samples which contained the same amount (1.3 mg per 1000 mL of milk) of Mn2+ cations. We also carried out a blank experiment which excludes the use of any additives. The rate and depth of pH change dramatically depends on the type of additive used.
The results of the experiment are shown in Figure 8. The highest rate of pH decrease was observed in the case of the control experiment, i.e., without any additives. Eight hours after the start of the fermentation experiment, the pH value reached 4.30. Manganese(II) chloride causes the least acidification of milk in the same period of time (pH = 5.21). According to their ability to reduce the pH of fermented milk, the Chitosan–Mn2+ systems are arranged in the following order: Chitosan + Mn2+ (1:2) (pH after 8 h 4.74) < Chitosan + Mn2+ (1:1) (pH after 8 h 4.45) < Chitosan + Mn2+ (2:1) (pH after 8 h 4.37). Since the amount of manganese(II) is the same in all tested systems, the observed effect is related to the amount of chitosan: the greatest acidification of fermented milk is caused by Chitosan + Mn2+ (2:1) system containing the largest amount of chitosan (Figure 8A). We found a similar pattern for the milk fermentation time. The fermentation time is considered to be when the pH reaches 4.60 [,]. The fermentation time was inversely proportional to the fermentation rate. The shortest fermentation time (see Figure 8B) was observed in the control experiment and in the case of using the Chitosan + Mn2+ (2:1) and Chitosan + Mn2+ (1:1) systems (4 h and 5 h, respectively). Figure 8C displays that the fermentation rate decreases with a decrease in the chitosan content in the system. For the Chitosan + Mn2+ (1:2) system and manganese chloride, the fermentation time is more than 8 h.
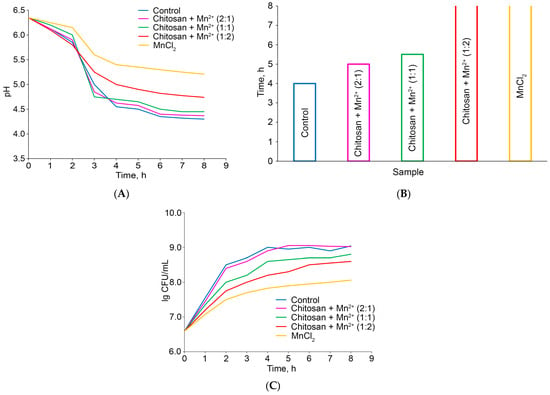
Figure 8.
pH changes of the milk under fermentation (A); fermentation time (B); changes in the number of CFU during fermentation (C).
At the next stage, we assessed the ability of the tested additives to influence the number of colony-forming units (CFU) producing bacteria St. thermophilus in fermented milk in comparison with the control experiment (without any additives). The initial number of CFU in milk was 3.93 × 106 CFU/mL. Over 8 h of the experiment, the maximum increase in the number of CFU was achieved in the case of the control experiment: the number of CFU/mL increased to 1.12 × 109. The use of Chitosan + Mn2+ 2:1 for fermentation leads to a similar result (1.04 × 106 CFU/mL). At the same time, the graphical dependence of lg CFU on time under the influence of the Chitosan + Mn2+ (2:1) system is close in nature to that for the control experiment; even the analysis of variance did not reveal any significant differences between the trends of these two curves (Figure 8C). Manganese(II) chloride has a pronounced inhibitory effect on the growth of producing bacteria. Under the action of MnCl2, we were able to achieve an increase in the CFU/mL value only up to 1.17 × 108, which is an order of magnitude less than in the case of the control experiment or Chitosan + Mn2+ (2:1) system. The other tested Chitosan–Mn2+ systems demonstrate an intermediate position. Their tendency to inhibit the growth of St. thermophilus in fermented milk increases with decreasing chitosan content. Thus, the obtained results are consistent with those for changing the pH and fermentation time: the tested additives with the highest chitosan content not only cause the greatest souring of milk with the highest fermentation rate, but also determine the highest growth rate of St. thermophilus. This result is consistent with the views that the fermentation kinetics are associated with the growth of St. thermophilus [,]. A separate, interesting direction arising from this work is to elucidate the mechanism by which chitosan–Mn2+ complexes influence bacterial growth. This effect may be either nutritional (Mn2+ as a micronutrient) or physicochemical. Our future work will be focused on exploring this issue.
2.2.2. Effect of Chitosan–Mn2+ Systems on Post-Acidification: Dynamics of Changes in the Number of CFU and pH of the Fermented Product During Storage
In the final stages of the experiment, we monitored the change in pH and the number of CFU during the storage of fermented milk for 7 days, conducting the experiment under thermostatting conditions at 25 °C. The number of CFU/mL tends to decrease during the experiment, but this trend is different for the tested samples depending on the additive (Figure 9A). In the control experiment, the CFU/mL value decreases from about 109 to about 108.4, demonstrating a pronounced almost linear downward trend. Under the influence of the Chitosan + Mn2+ (2:1) system, a less pronounced tendency to decrease in the number of producing bacteria is observed and the CFU value decreases only from about 109 to 108.7, with the main decrease observed in the first 3 days of the experiment, and subsequently the level of St. thermophilus remains approximately constant with a tendency only to an insignificant monotonic decrease. In the case of other additives, the level of bacteria decreases significantly, and this effect is most pronounced for the so-called “pure” manganese chloride.

Figure 9.
Monitoring St. thermophilus (A) and pH (B) levels during storage.
The patterns of pH changes in fermented milk during storage are similar (Figure 9B). In all cases, we observed a decrease in pH despite a decrease in the number of bacteria. This effect can apparently be explained by the growth of other microorganisms that cause the so-called post-acidification of fermented milk, as well as a cascade of side biochemical reactions []. The complete and detailed mechanism of the undesirable process of post-acidification still remains unclear and debatable. Some researchers believe that the post-acidification in fermented milks is generally related to the lowering of pH and to the formation of bitter peptides, excluding the growth of unfavorable bacteria. Other publications indicate that undesirable continued growth of microorganisms may be the cause of post-acidification [].
The greatest post-acidification was observed in the control experiment. This results in the product becoming unsuitable on the 4th day of storage at 25 °C, since the pH of the product reaches an undesirable value, below 4.4 [,,]. The best result was achieved using the Chitosan + Mn2+ (2:1) system as an additive: the pH level was almost the same with a slight insignificant tendency to decrease.
Thus, the fermented milk retains its shelf life almost two times longer compared to the control when using the Chitosan + Mn2+ (2:1) system. It is worth noting that yoghurts are usually stored at lower temperatures. However, if we managed to achieve an extension of the shelf life even at 25 °C, then at lower temperatures, obviously, this effect will be undoubtedly more pronounced.
3. Materials and Methods
3.1. Chemicals and Solvents
Chitosan of viscosity-average molecular weight 200 kDa and degree of acetylation 8% was purchased from Bioprogress (Moscow, Russia). By the term “chitosan”, we mean the powder of the commercial chitosan used. By the term “chitosan in acetic acid”, we mean the dry lyophilizate of chitosan obtained by dissolving commercial chitosan in acetic acid, followed by purification by dialysis against distilled water from excess acid and subsequent lyophilic drying. Manganese(II) chloride tetrahydrate were from Reachim (Moscow, Russia). Other chemicals and solvents were also from commercial sources and used as received without any further purification.
3.2. Instrumentation
The apparent hydrodynamic radii and ζ-potential of nanoparticles in water were estimated at room temperature (about 25 °C) using a Photocor Compact-Z instrument (Moscow, Russia) at λ = 638 nm, θ = 90° for radii (10 scans, each one for 10 s), and θ = 20° for ζ-potential (three scans, each one for 60 s).
pH was measured potentiometrically using a PHS-3C device (Shanghai Precision Scientific Instrument Co., Ltd., Shanghai, China).
Inductively coupled plasma-atomic emission spectroscopy measurements (ICP) were performed at Leeman ICP-AES Prodigy XP spectrometer (Hudson, NH, USA).
IR spectroscopy was recorded on a Shimadzu IRSpirit (Nakagyo-ku, Japan) at 4000 to 400 cm−1.
X-ray diffraction analysis was carried out on a Dron-7 X-ray diffractometer (Guangzhou Betop Scientific Technology Co., Ltd., Guangzhou, China), using a 2θ angle interval from 5° to 50° with scanning step ∆2θ = 0.02° and exposure of 3 s per point. Cu Kα radiation (Ni filter) was used, which was subsequently decomposed into Kα1 and Kα2 components during the processing of the spectra.
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were performed on the SDT Q600 (TA Instruments, New Castle, DE, USA) using a heating rate of 10 °C/min in the temperature range from 30 °C to 600 °C in N2 atmosphere.
The 1H NMR spectra were recorded on a Bruker Avance II spectrometer (Billerica, MA, USA) at an operating frequency of 400 MHz.
Thin-layer chromatography (TLC) was performed on Merck 60 F254SiO2 plates (Rahway, NJ, USA) with 1:1 (v:v) acetone–chloroform as the eluent.
3.3. Preparation of Chitosan/Manganese(II) Complexes
Chitosan (0.025 g) was dissolved in 25 mL of 1% CH3COOH and stirred for 30 min with a mechanical stirrer. MnCl2 × 4H2O (0.1979 g) was dissolved in 5 mL of distilled water and stirred for 30 min to produce 0.2M solution. The manganese(II) salt solution was poured as a thin stream to the vigorously stirred solution of chitosan to produce 1:2, 1:1, or 2:1 mono-mol/mol polymer/Mn2+ ratio solutions, and the resulting mixtures was intensely stirred for 5 min (mono-mol is a structural unit in the polymer chain of chitosan–glucosamine or acetylglucosamine unit). The resultant suspensions were frozen and freeze-dried.
3.4. Catalytic Synthesis of Imine by Oxidative Coupling of Benzylamine
The reaction flask was charged with benzylamine (0.050 mol), solvent (30 mL, see Table 4: H2O, MeOH, or MePh), internal standard hexamethyl disiloxane (0.010 mol), the tested catalyst (see Table 4: 1, 2, or 3 mol% based on Mn; the content of Mn in the chitosan/manganese(II) complexes tested as catalysts is described in Section 2.1). The reaction mixture was stirred during 48 h under air at 80 °C. The solvent was evaporated under vacuum. The aliquot 10.0 mg was dissolved in CDCl3 and analyzed by 1H NMR spectroscopy. The conversion of benzylamine into the corresponding imine was calculated as 100 × I(H from CH=N of the imine), while I(H of hexamethyl disiloxane) = 18.
3.5. Catalytic Aldol Reaction
The reaction vial was charged by 4-nitrobenzaldehyde (0.010 mol), acetone (0.030 mol), catalyst (see Table 5; 3 mol% based on Mn; the content of Mn in the chitosan/manganese(II) complexes tested as catalysts is described in Section 2.1) and solvent (30 mL, H2O, or H2O/EtOH 5/1, v/v). The reaction mixture was stirred at room temperature for 5, 15, 30, or 60 min. The catalyst was immediately removed by filtration. The solvent was evaporated under vacuum and the solid residue was quantitatively analyzed by 1H NMR spectroscopy in DMSO-d6 solution. The conversion of 4-nitrobenzaldehyde into the aldol was calculated as 100/(I(H1) + 1), while I(H2) = 1 (where I(H1) is the integral intensity of the aldehyde group proton of p-nitrobenzaldehyde, and I(H2) is integral intensity of α-CH proton the aldol).
3.6. Fermentation Study
Milk purchased from Neva-Milk Company (Saint Petersburg, Russia) was used for experiments. A total of 1000 mL of the milk was poured into a flat-bottomed flask, and 4.64 mg MnCl2 × 4H2O or 12.30 mg Chitosan + Mn2+ (1:2) or 8.12 mg Chitosan + Mn2+ (1:1) or 6.13 mg Chitosan + Mn2+ (2:1) was added to the milk and stirred over 20 min under room temperature. Thus, we added different amounts of the tested samples which contain the same mass of Mn2+, i.e., 1.30 mg. The flasks were charged by Streptococcus thermophilus (St. thermophilus, 20 g per 1000 mL of the milk) and treated for 8 h at 42 °C for fermentation. For pH monitoring, 50 mL of milk was taken at 2, 3, 4, 5, 6, 7, and 8 h of the fermentation experiment. When the pH value decreased to 4.60, fermentation was considered complete. The remaining fermented milk was immediately cooled to stop the fermentation process and was used in further experiments.
3.7. Post-Acidification Study
For post-acidification assessment, the cooled fermented milk was kept in plastic sterile plastic yogurt containers (1 portion was 85 mL) at 25 °C. pH and the number of colony-forming units (CFU) of St. thermophilus monitoring was conducted every 24 h for one week. For determination of CFU, 25 mL of the fermented milk was poured into 200 mL of 0.15% peptone water. Tenfold dilutions were used for inoculation on a nutrient medium (tryptone, sucrose, yeast extract, and potassium hydrophosphate, pH = 6.8). Colonies were counted after incubation of the inoculations at 37 °C for 24 h.
3.8. Statistics
The statistical significance of differences between the samples was determined by a one-way analysis of variance (ANOVA) using JMP 5.0.1 software (SAS Campus Drive, Cary, NC, USA). Mean values, where appropriate, were compared by applying the Student’s t-test at a significance level p < 0.05.
4. Conclusions
The results of this work can be considered from the following few perspectives.
(1) Development and Characterization of Chitosan/Mn(II) Nanoparticles:
- We successfully synthesized and fully characterized nanoparticles based on chitosan and manganese(II) ions in various molar ratios: Chitosan + Mn2+ (1:2), Chitosan + Mn2+ (1:1), and Chitosan + Mn2+ (2:1);
- These nanoparticles exhibited microscale sizes and a progressively decreasing zeta potential in aqueous medium, indicating sedimentation instability;
- ICP analysis verified the quantitative manganese content, while IR spectroscopy demonstrated the coordination of manganese(II) to the chitosan matrix.
(2) Catalytic Activity of Chitosan/Mn(II) Nanoparticles:
- Nanoparticles Chitosan + Mn2+ (1:1) high catalytic activity in oxidative coupling of benzylamine resulting the imine formation. The reaction proceeds selectively under green solvent-free conditions and affords quantitative yields of the product;
- Moreover, Chitosan + Mn2+ (1:1) nanoparticles are efficient catalysts for selective aldol reaction at room temperature in the greenest solvent system H2O/EtOH 5/1 (volume/volume);
- Catalyst Chitosan + Mn2+ (1:1) is very easy to prepare and convenient to use. The catalyst is separated from the reaction mixture by nanoporous filter or centrifugation and does not lose catalytic activity after at least ten uses.
(3) Stimulation of Milk Fermentation and Shelf-Life Assessment of Fermented Milk Products:
- Chitosan + Mn2+ (2:1) complex significantly reduced the milk fermentation time, demonstrating its efficiency in promoting faster fermentation;
- Over a 7-day storage period, the Chitosan + Mn2+ (2:1) system exhibited the least pronounced decline in colony-forming units (CFUs) of Streptococcus thermophilus;
- The pH of the fermented milk products treated with this system remained nearly constant, with only a slight decrease, indicating enhanced stability;
- Using the Chitosan + Mn2+ (2:1) system nearly doubled the shelf life of fermented milk products compared to the control.
Finally, the synthesized nanoparticles are promising candidates for further chemical and biological studies. In addition, the elaborated nanoparticles are of interest for reinforcement chitosan films with their subsequent treatment with cold plasma to prepare new film materials with attractive physicochemical and biological properties. Both these projects are underway in our group.
Author Contributions
Conceptualization, R.A.G., A.A.N., L.V.N. and A.S.K.; methodology, R.A.G., A.A.N., D.I.S., R.H.N. and A.A.K.; software, T.V.S. and I.S.G.; validation, R.H.N. and W.L.; formal analysis, T.V.S. and I.S.G.; investigation, A.R.E. and A.G.T.; resources, A.A.K., V.V.R. and A.G.T.; data curation, A.R.E. and D.I.S.; writing—original draft preparation, R.A.G., A.A.N., D.I.S., A.R.E. and L.V.N.; writing—review and editing, R.H.N., A.A.K., V.V.R., T.V.S., I.S.G., W.L., A.G.T. and A.S.K.; visualization, L.V.N.; supervision, I.S.G., A.G.T. and A.A.K.; project administration, W.L. and A.S.K.; funding acquisition, A.R.E., W.L. and A.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out with the support of the Ministry of Science and Higher Education of the Russian Federation (State Assignment No. FSSF-2026-0043) within the framework of the federal project “Development of technologies for controlled thermonuclear fusion and innovative plasma technologies”. This publication also has been supported by the RUDN University Scientific Projects Grant System, project № 202785-2-000. This work was financially supported by the National Natural Science Foundation of China (32271422, W2431042, 32311530766, 32411540226), Shanghai Frontiers Science Center of Advanced Textiles, Donghua University (24S10102, 23S10115), and the Fundamental Research Funds for the Science and Technology Commission of Shanghai Municipality (No. 20DZ2254900). The work was also carried out with the financial support of the state research assignment Polymer and composite materials for advanced technologies, Yudin V.E., 2024–2026, No. 1023031700040-5-1.4.4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chicea, D.; Nicolae-Maranciuc, A. A Review of Chitosan-Based Materials for Biomedical, Food, and Water Treatment Applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef]
- Wiącek, A.E. (Ed.) Chitosan, Chitosan Derivatives and Their Applications; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2024. [Google Scholar]
- Kumari, S.; Kishor, R. Chapter 1—Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Mabrouk, M.; Hammad, S.F.; Mansour, F.R.; Abdella, A.A. A Critical Review of Analytical Applications of Chitosan as a Sustainable Chemical with Functions Galore. Crit. Rev. Anal. Chem. 2024, 54, 840–856. [Google Scholar] [CrossRef]
- Reshad, R.; Jishan, T.; Chowdhury, N. Chitosan and its Broad Applications: A Brief Review. J. Clin. Trials Exp. Investig. 2021, 12, em00779. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Ainyanbhor, I.E.; Edo, G.I.; Akpoghelie, P.O.; Owheruo, J.O.; Sumer Gaaz, T.S.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Essaghah, A.E.A.; et al. A review on manganese and its effect on health and distribution in selected African countries. J. Trace Elem. Med. Biol. 2025, 91, 127707. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Iqbal, M.A. Coordination Complexes of Manganese and Their Biomedical Applications. ChemistrySelect 2017, 2, 1586–1604. [Google Scholar] [CrossRef]
- Daksh, S.; Kaul, A.; Deep, S.; Datta, A. Current advancement in the development of manganese complexes as magnetic resonance imaging probes. J. Inorg. Biochem. 2022, 237, 112018. [Google Scholar] [CrossRef] [PubMed]
- Vanden Broeck, S.M.P.; Cazin, C.S.J. Manganese-N-heterocyclic carbene (NHC) complexes—An overview. Polyhedron 2021, 205, 115204. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Kronenberger, S.; Naumann, R.; Förster, C.; East, N.R.; Klett, J.; Heinze, K. A manganese(I) complex with a 190 ns metal-to-ligand charge transfer lifetime. Nat. Commun. 2025, 16, 7850. [Google Scholar] [CrossRef]
- Kozieł, S.; Wojtala, D.; Szmitka, M.; Sawka, J.; Komarnicka, U.K. Can Mn coordination compounds be good candidates for medical applications? Front. Chem. Biol. 2024, 3, 1337372. [Google Scholar] [CrossRef]
- Kluczka, J. Removal of boron and manganese ions from wet-flue gas desulfurizationwastewater by hybrid chitosan-zirconium sorbent. Polymers 2020, 12, 635. [Google Scholar] [CrossRef]
- Febriana, E.; Pitriani, P.; Handayani, M.; Irawan, J.; Prasetyo, A.B.; Sulistiyono, E.; Firdiyono, F. Adsorption of Metal Ion Manganese (Mn) in Sodium Silicate Solution using Chitosan. J. Phys. Conf. Ser. 2021, 1912, 012037. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, C.; Shen, P.; Liu, Z.; Hu, J.; Zhang, Y. Facile synthesis of recyclable Mn-crosslinked chitosan schiff base composites as heterogeneous catalysts for selective oxidation of methyl phenyl sulfide with H2O2. Int. J. Biol. Macromol. 2025, 311, 144119. [Google Scholar] [CrossRef]
- Mo, L.Q.; Huang, X.F.; Wang, G.C.; Huang, G.; Liu, P. Full use of factors promoting catalytic performance of chitosan supported manganese porphyrin. Sci. Rep. 2020, 10, 14132. [Google Scholar] [CrossRef]
- Huang, G.; Yan, C.; Cai, J.L.; Mo, L.Q.; Zhao, S.K.; Guo, Y.A.; Wei, S.J.; Shen, Y.L. Practicably efficient ethylbenzene oxidation catalyzed by manganese tetrakis(4-sulfonatophenyl)porphyrin grafted to powdered chitosan. J. Porph. Phthalocyanines 2018, 22, 481–490. [Google Scholar] [CrossRef]
- IşIk, C.; Teke, M. Polycaprolactone/chitosan/manganese (II) oxide nanofiber: Improving pH stability of arginase enzyme. Polym. Bull. 2025, 82, 7827–7850. [Google Scholar] [CrossRef]
- Mohammad Hosseini, N.; Sheshmani, S.; Shahvelayati, A.S. Manganese ferrite-graphite oxide-chitosan nanocomposite for efficient dye removal from aqueous and textile wastewater under UV and sunlight irradiation. Sci. Rep. 2025, 15, 866. [Google Scholar] [CrossRef] [PubMed]
- Dysin, A.P.; Egorov, A.R.; Godzishevskaya, A.A.; Kirichuk, A.A.; Tskhovrebov, A.G.; Kritchenkov, A.S. Biologically Active Supplements Affecting Producer Microorganisms in Food Biotechnology: A Review. Molecules 2023, 28, 1413. [Google Scholar] [CrossRef] [PubMed]
- Badsha, I.; Namasivayam, S.K.R.; Jayaprakash, C.; Nachiyar, C.V.; Bharani, R.S.A. Polylysine. In Handbook of Nutraceuticals and Natural Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 257–272. [Google Scholar]
- Yousefi, M.; Khanniri, E.; Khorshidian, N.; Sohrabvandi, S.; Mortazavian, A.M. Development of Probiotic Apple Juice using Encapsulated Probiotics in Xanthan-Chitosan Based Hydrogels. Appl. Food Biotechnol. 2023, 10, 205–213. [Google Scholar]
- Tamura, Y.; Hayashi, H.; Nishimura, Y.; Ikeda, M. Reactions of 1-alkylbenzimidazolium 3-imines with acetylenic-compounds and benzaldehyde. J. Heterocycl. Chem. 1975, 12, 225–230. [Google Scholar] [CrossRef]
- Hill, A.J.; Walton, A.; Mazzeo, F.A. Suspension Stability; Why Particle Size, Zeta Potential and Rheology are Important. Ann. Trans. Nord. Rheol. Soc. 2011, 20, 209–214. [Google Scholar]
- Belenkii, D.; Balakhanov, D.; Lesnikov, E. Measurement of the zeta potential. Brief review of the main methods. Analytics 2017, 34, 82–89. [Google Scholar] [CrossRef]
- Sustmann, R. A simple model for substituent effects in cycloaddition reactions. I. 1,3-dipolar cycloadditions. Tetrahedron Lett. 1971, 12, 2717–2720. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Liu, H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohydr. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: Hoboken, NJ, USA, 1986; p. 536. [Google Scholar]
- Sustmann, R. A simple model for substituent effects in cycloaddition reactions. II. The diels-alder reaction. Tetrahedron Lett. 1971, 12, 2721–2724. [Google Scholar] [CrossRef]
- Moyano, A.; Pericas, M.A.; Valenti, E. A theoretical-study on the mechanism of the thermal and the acid-catalyzed decarboxylation of 2-oxetanones (beta-lactones). J. Org. Chem. 1989, 54, 573–582. [Google Scholar] [CrossRef]
- Lecea, B.; Arrieta, A.; Roa, G.; Ugalde, J.M.; Cossio, F.P. Catalytic and solvent effects on the cycloaddition reaction between ketenes and carbonyl-compounds to form 2-oxetanones. J. Am. Chem. Soc. 1994, 116, 9613–9619. [Google Scholar] [CrossRef]
- Ritthidej, G.C.; Phaechamud, T.; Koizumi, T. Moist heat treatment on physicochemical change of chitosan salt films. Int. J. Pharm. 2002, 232, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.Z.A.; Harun, M.K.; Ali, A.M.M.; Mohammat, M.F.; Hanafiah, M.A.K.M.; Ibrahim, S.C.; Mustaffa, M.; Darus, Z.M.; Latif, F. XRD and Surface Morphology Studies on Chitosan-Based Film Electrolytes. J. Appl. Sci. 2006, 6, 3150–3154. [Google Scholar] [CrossRef]
- Nieto, J.M.; Peniche-Covas, C.; Padro’n, G. Characterization of chitosan by pyrolysis-mass spectrometry, thermal analysis and differential scanning calorimetry. Thermochim. Acta 1991, 176, 63–68. [Google Scholar] [CrossRef]
- López, F.A.; Mercê, A.L.R.; Alguacil, F.J.; López-Delgado, A. A kinetic study on the thermal behaviour of chitosan. J. Therm. Anal. Calorim. 2007, 91, 633–639. [Google Scholar] [CrossRef]
- de Britto, D.; Campana-Filho, S.P. Kinetics of the thermal degradation of chitosan. Thermochim. Acta 2007, 465, 73–82. [Google Scholar] [CrossRef]
- Wanjun, T.; Cunxin, W.; Donghua, C. Kinetic studies on the pyrolysis of chitin and chitosan. Polym. Degrad. Stabil. 2005, 87, 389–394. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Park, L.H.; Leitao, E.M.; Weber, C.C. Green imine synthesis from amines using transition metal and micellar catalysis. Org. Biomol. Chem. 2023, 22, 202–227. [Google Scholar] [CrossRef]
- Singh, G.S. Chapter 15—Green chemistry of evergreen imines in the synthesis of nitrogen-containing heterocycles. In Green Synthetic Approaches for Biologically Relevant Heterocycles, 2nd ed.; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 655–687. [Google Scholar]
- Chetty, L.C.; Kruger, H.G.; Arvidsson, P.I.; Naicker, T.; Govender, T. Investigating the efficacy of green solvents and solvent-free conditions in hydrogen-bonding mediated organocatalyzed model reactions. RSC Adv. 2024, 14, 7992–7998. [Google Scholar] [CrossRef]
- Cho, J.; Weck, M.; Hwang, S.; Jang, S.S. Multiscale Modeling Approach for the Aldol Addition Reaction in Multicompartment Micelle-Based Nanoreactor. J. Phys. Chem. B 2023, 127, 10067–10076. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, S.; Ghosh, S.K.; Ghosh, A.; Saha, R.; Banerjee, S.; Saha, B. Review of the aldol reaction. Synth. Commun. 2016, 46, 1327–1342. [Google Scholar] [CrossRef]
- Cordes, M.; Kalesse, M. Very recent advances in vinylogous mukaiyama aldol reactions and their applications to synthesis. Molecules 2019, 24, 3040. [Google Scholar] [CrossRef]
- Tekin, K.; Hao, N.; Karagoz, S.; Ragauskas, A.J. Ethanol: A Promising Green Solvent for the Deconstruction of Lignocellulose. ChemSusChem 2018, 11, 3559–3575. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Tiwari, S.; Kumar, A.; Raman, R.K.; Kadyan, S. Review on factors affecting and control of post-acidification in yoghurt and related products. Trends Food Sci. Technol. 2021, 109, 499–512. [Google Scholar] [CrossRef]
- Guenard-Lampron, V.; St-Gelais, D.; Villeneuve, S.; Turgeon, S.L. Short communication: Effect of stirring operations on changes in physical and rheological properties of nonfat yogurts during storage. J. Dairy Sci. 2020, 103, 210–214. [Google Scholar] [CrossRef]
- Ibarra, A.; Acha, R.; Calleja, M.T.; Chiralt-Boix, A.; Wittig, E. Optimization and shelf life of a low-lactose yogurt with Lactobacillus rhamnosus HN001. J. Dairy Sci. 2012, 95, 3536–3548. [Google Scholar] [CrossRef]
- Julijana, T.; Nikola, G.; Borche, M. Examination of pH, Titratable Acidity and Antioxidant Activity in Fermented Milk. J. Mater. Sci. Eng. A 2016, 6, 326–333. [Google Scholar] [CrossRef]
- Shene, C.; Canquil, N.; Bravo, S.; Rubilar, M. Production of the exopolysaccharides by Streptococcus thermophilus: Effect of growth conditions on fermentation kinetics and intrinsic viscosity. Int. J. Food Microbiol. 2008, 124, 279–284. [Google Scholar] [CrossRef]
- Han, M.; Wu, Y.; Guo, X.; Jiang, L.; Wang, X.; Gai, Z. Milk fermentation by monocultures or co-cultures of Streptococcus thermophilus strains. Front. Bioeng. Biotechnol. 2022, 10, 1097013. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, T.; O’Mahony, J.A.; McCarthy, N.A. pH, the Fundamentals for Milk and Dairy Processing: A Review. Dairy 2023, 4, 395–409. [Google Scholar] [CrossRef]
- Popescu, L.; Bulgaru, V.; Siminiuc, R. Effect of Temperature, pH and Amount of Enzyme Used in the Lactose Hydrolysis of Milk. Food Nutr. Sci. 2021, 12, 1243–1254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).