Unlocking the Alkaloid Biological Potential of Chili Pepper (Capsicum spp.), Cacao (Theobroma cacao L.), and Coffee (Coffea spp.) Byproducts: Characterization, Non-Conventional Extraction, Applications, and Future Perspectives
Abstract
1. Introduction
2. Literature Search Strategy and Eligibility Criteria
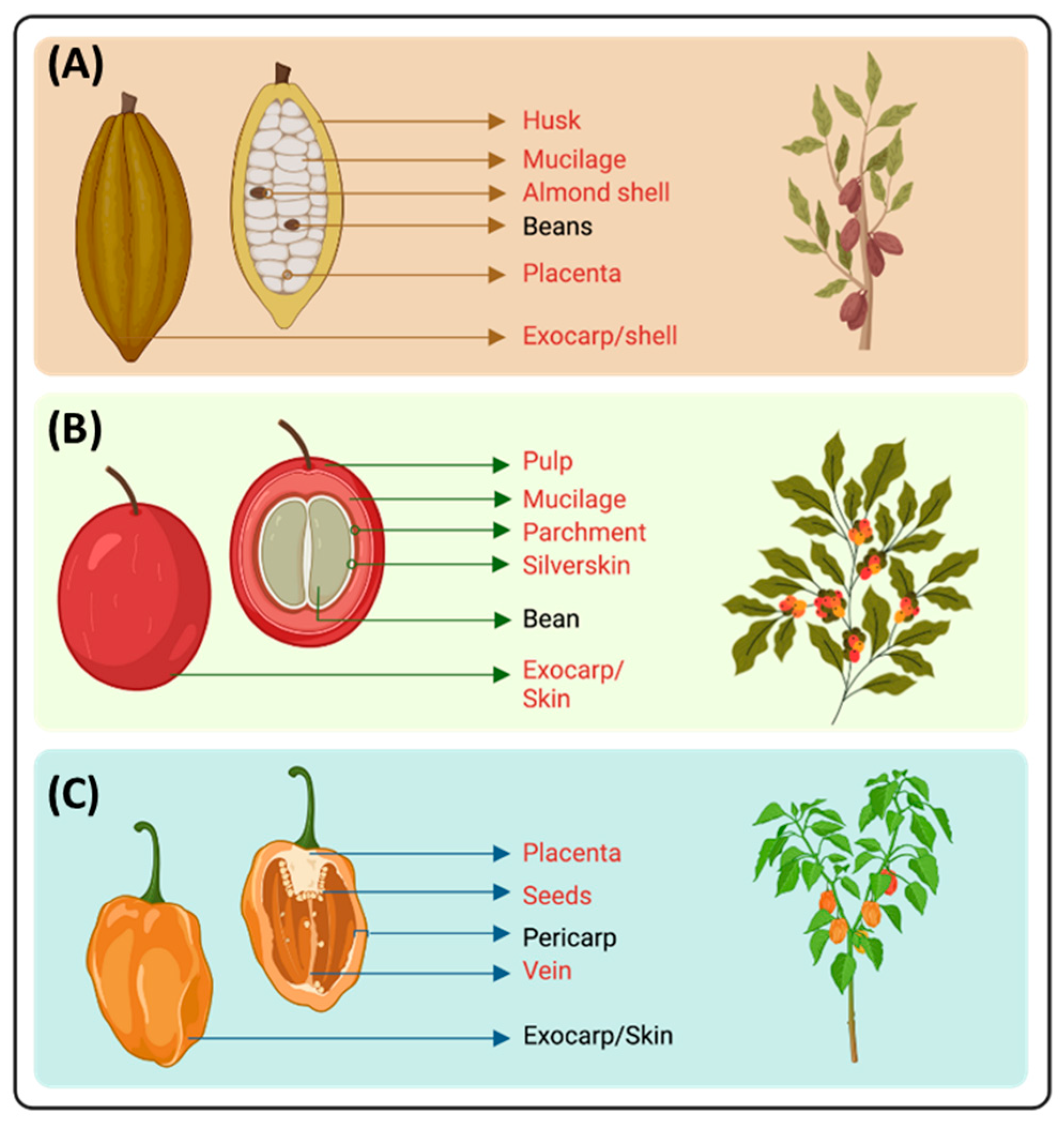
3. Byproducts of Chili Pepper, Cocoa, and Coffee Cultivation
3.1. Chili Pepper
3.2. Cacao
3.3. Coffee
4. Factors Influencing Alkaloid Content
4.1. Chili Pepper Alkaloids
4.2. Cacao Alkaloids
4.3. Coffee Alkaloids
5. Alkaloid Functions and Biological Potential
5.1. Capsaicin
5.2. Theobromine
5.3. Caffeine
| Alkaloid | Function | Model | Assay | Dose | Result | Reference |
|---|---|---|---|---|---|---|
| Capsaicin | Anti-inflammatory | Myoblast cells | LPS-induced inflammation | 50 µM | ↓Calpain-1, ↓TNF-α, and ↓Capase-3 | [48] |
| RAW 264.7 cells | LPS-induced inflammation | 3.05 µM | ↓IL-1β, ↓IL-2, and ↓IL-6 | [49] | ||
| Anti-obesity | C57BL/6 mice | Body weight | HFD + 0.01% | ↑Glucose homeostasis, ↓body weight gain | [46] | |
| Mice | RT-PCR, Western blot, body growth, and fat growth | HFD + 5% capsinoids | ↓Body weight, ↓fat mass, ↑HMG-CoA reductase, ↑CPT-1, ↑FAT/CD36, and ↑GLUT4 | [50] | ||
| Antidiabetic | Type 1 diabetic male Sprague Dawley (SD) rats | Apparent digestibility determination of total sugar and biochemical index determination | 6 mg/kg body weight | ↑Glucose metabolism, ↑insulin level, ↑glycogen content | [51] | |
| Hepatocellular carcinoma HepG2 | Fluorescence indicated by 2-NBDG | 25–200 µM | ↑Glucose consumption, dose-dependent | [67] | ||
| Antiproliferative | Colon adenocarcinoma Caco-2 and esophageal OE19 cells | MTT assay | 81.86 μM | ↑Inhibition of cell proliferation by IC50 | [68] | |
| Hepatocellular carcinoma HepG2 | MTT assay | 172.8 μM | ↑Inhibition of cell proliferation by IC50 | [69] | ||
| Theobromine | Antiproliferative | Colorectal cancer line HT-29 cells | Clonogenic assay | 100 μg extract/mL | Antiproliferative effect | [56] |
| Lung carcinoma epithelial A549 and human colon cancer HTC-116 | MTT assay | 40.20 µM and 34.05 µM, respectively (theobromine derivative T-1-NCA) | Inhibition by IC50 | [58] | ||
| Anti-inflammatory | SW1353 cells induced by IL-1β | RT-PCR, Western blot, ELISA, Luciferase assay, ROS, and intracellular NO measurement | 50 μM | ↓ROS production, ↓COX-2, ↓PGE2, ↓iNOS, ↓NO, ↓TNF-α, ↓MCP-1, ↓NF-κβ, ↓II-collagen degradation | [52] | |
| Human colorectal adenocarcinoma CaCo-2 cells induced by oxysterols | Cell death, immunoblotting, Cytokine by ELISA | 10 μM | ↓ IL-8, ↓MCP-1, ↓tight junction protein level decrease, ↓apoptotic activity | [57] | ||
| Cardiovascular protection | Overweight and obese adults aged 40–55 years old | Anthropometric indices, blood pressure, lipid profile, and glycemic indices | 450 mg/day | ↓Waist circumference, ↓LDL-c/HDL-c, ↓TG/HDL-c | [53] | |
| C57BL/6 mice | Blood sampling, immunoblot analysis, RT-PCR | 200 mg/kg body weight | ↓Body weight gain, ↓PDE4D activity, and ↑energy expenditure | [55] | ||
| Caffeine | Antidepressant | Reserpine-induced rat model of depression | Neurotransmitter analysis | 30 mg/kg | Regulated serotonin, norepinephrine, and dopamine; ↑AchE activity | [60] |
| Antidepressant Anti-inflammatory | Male inpatients age 31–59 years old | Depressive symptoms measurement, Montreal Cognitive Assessment | 60 mg/day | ↑Enhance antidepressant medication, ↑cognitive function, ↓HPA hyperactivation | [61] | |
| Adult Swiss male mice | LPS-induced inflammation on vastus lateralis muscle | 6 mg/kg of body weight | Attenuated LPS-induced catabolic state, ↓NLRP3, ↑adenosinergic receptors Adora1 and Adora 2A | [70] | ||
| Anti-inflammatory Neuroprotective | RAW 264.7 cells | Nitric oxide assay, cytokine assay, nitrite determination, RT-PCR, and Western blot analysis | 200 μM | ↓ NO production, ↓iNOS, ↓COX-2, ↓IL-6, ↓IL-3, ↓IL-12, ↓NF-κB, ↓phospho-p38MAPK i | [62] | |
| Human Neuroblastoma cells treated with HIV-1 Tat1–72 | Aβ level quantification, immunoblotting, and immunoelution | 200 μM | ↓ Aβ levels, decreased vacuolar ATPase, and increased cathepsin D | [71] | ||
| Neuroprotective | AlCl3-intoxicated male albino rats | Measurement of lipid peroxidation, reduced glutathione (GHS), nitric oxide, and AchE, Na+/K+-ATPase activity | 20 mg/kg | ↑AchE, ↑Na+/K+-ATPase activity, regulated TNF-α, ↓GHS, | [72] |
6. Alkaloid Extraction Techniques
| Raw Material | Extract | Method | Solvent | Conditions | Yield | Reference |
|---|---|---|---|---|---|---|
| Coffee pulp | Caffeine | UAE | Water | 348.15 K–363.1 K, 396 W, 5.5 min | 206.6 ± 0.1 mg/L | [76,90] |
| Crude guarana and guarana waste | Caffeine | Enzyme-assisted PLE | Water | 323 K, pH 5.0, cellulase | 46.2 ± 0.14 g/100 g extract and 43.53 ± 0.13 g/100 g extract | [83] |
| Spent coffee grounds | Caffeine | HPTE | Ethanol and water 50% v/v | 423.15 K, 700 kPa and 60 min | 31.7 ± 2.9 mg/100 mL extract | [12] |
| Spent coffee grounds | Caffeine and coffee oils | SFE | Carbon dioxide and ethanol | 333 K, 30 MPa, 10% co-solvent | 3.96% molar | [77] |
| Green tea | Caffeine | SFE | Carbon dioxide | 333.15 K, 25 MPa, 3 h | 100% | [81] |
| Green tea | Caffeine | SFE | CO2 + water | 343.15 K, 30 MPa, 4 h | 460.0 mg/g | [80] |
| Habanero (Campsicum chinense var. Mayapan) | Capsaicinoids | SFE | Ethanol 20% w/w | 318.15 K, 10 MPa, 60 min | 37.09 ± 0.84 mg/g extract | [79] |
| Trinidad Scorpion Moruga fruit | Capsaicin | PHWE | Water | 473.15 K, 20 MPa, and 10 + 20 min of static extraction time | 46.45 ± 0.410 mg/g dried sample | [91] |
| Trinidad Scorpion Moruga fruit | Capsaicinoids | Soxhlet | Methanol | 2 h, 0.1 g chili, 150 mL methanol | 42.875 ± 0.403 mg/g dried sample | [92] |
| Habanero chili pepper | Capsaicin and phenolic compounds | EAE | Enzyme solution 1:15 w/v | 318.15 K, 150 min | 310.23 µg/mL | [84] |
| Cacao shell | Theobromine and caffeine | PHWE | Water | Ethanol 15%, 363.15 K, 5 extraction cycles, 6 min static time | 2.06 ± 0.06 and 0.48 ± 0.02 g/100 g | [89] |
| Cacao shell | Caffeine | SFE | CO2 | 41.3 MPa, 313 K, 90 min | 91.80% | [87] |
| Cacao shell | Theobromine and caffeine | PEFAE | Ethanol and water 40% v/v | 1:30 g/mL, 313.15 K, 2 h, 3 kW/cm, and 20 kJ/kg | +25% and +34% | [93] |
| Cocoa shell | Theobromine and caffeine | SFE + PLE | Ethanol | 313.15 K, 20 MPa, 343.15 K, 10 MPa | - | [88] |
7. Alkaloids: Current and Potential Applications in the Industry
8. Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamda, A.S.; Abo, L.D.; Jayakumar, M.; Selvakumar, K.V.; Periyasamy, S.; Emana, A.N. Introduction: Growth of Agricultural Waste, Its Disposal, and Related Environmental Issues. In Agricultural Waste to Value-Added Products: Technical, Economic and Sustainable Aspects; Neelancherry, R., Gao, B., Wisniewski, A., Jr., Eds.; Springer Nature: Singapore, 2023; pp. 1–19. ISBN 978-981-9944-72-9. [Google Scholar]
- Mateos-Aparicio, I.; Matias, A. Food Industry Processing by-Products in Foods. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–281. ISBN 978-0-12-816453-2. [Google Scholar]
- Galanakis, C.M. (Ed.) 12—Food Waste Recovery: Prospects and Opportunities. In Sustainable Food Systems from Agriculture to Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 401–419. ISBN 978-0-12-811935-8. [Google Scholar]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-Industrial by-Products: Valuable Sources of Bioactive Compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Lippi, D. Sin and Pleasure: The History of Chocolate in Medicine. J. Agric. Food Chem. 2015, 63, 9936–9941. [Google Scholar] [CrossRef]
- Patay, É.B.; Bencsik, T.; Papp, N. Phytochemical Overview and Medicinal Importance of Coffea Species from the Past until Now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of Plant Alkaloids on Human Health: A Review of Biological Activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, J.; Chen, J.; Ercisli, S.; Chen, L.; Zhang, S.; Jin, J.; Chen, J.; Ercisli, S.; Chen, L. Purine Alkaloids in Tea Plants: Component, Biosynthetic Mechanism and Genetic Variation. Beverage Plant Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. Biochemistry and Molecular Biology of Capsaicinoid Biosynthesis: Recent Advances and Perspectives. Plant Cell Rep. 2019, 38, 1017–1030. [Google Scholar] [CrossRef]
- Maharjan, A.; Vasamsetti, B.M.K.; Park, J.-H. A Comprehensive Review of Capsaicin: Biosynthesis, Industrial Productions, Processing to Applications, and Clinical Uses. Heliyon 2024, 10, e39721. [Google Scholar] [CrossRef]
- da Silva, M.F.; Pettinato, M.; Casazza, A.A.; Maciel, M.I.S.; Perego, P. Design and Evaluation of Non-Conventional Extraction for Bioactive Compounds Recovery from Spent Coffee (Coffea arabica L.) Grounds. Chem. Eng. Res. Des. 2022, 177, 418–430. [Google Scholar] [CrossRef]
- Sridhar, A.; Vaishampayan, V.; Senthil Kumar, P.; Ponnuchamy, M.; Kapoor, A. Extraction Techniques in Food Industry: Insights into Process Parameters and Their Optimization. Food Chem. Toxicol. 2022, 166, 113207. [Google Scholar] [CrossRef]
- Mendoza-Meneses, C.J.; Feregrino-Pérez, A.A.; Gutiérrez-Antonio, C. Potential Use of Industrial Cocoa Waste in Biofuel Production. J. Chem. 2021, 2021, e3388067. [Google Scholar] [CrossRef]
- Mohammadnezhad, P.; Valdés, A.; Álvarez-Rivera, G. Bioactivity of Food by-Products: An Updated Insight. Curr. Opin. Food Sci. 2023, 52, 101065. [Google Scholar] [CrossRef]
- Yasin, M.; Li, L.; Donovan-Mak, M.; Chen, Z.-H.; Panchal, S.K. Capsicum Waste as a Sustainable Source of Capsaicinoids for Metabolic Diseases. Foods 2023, 12, 907. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) Pod Husk: Renewable Source of Bioactive Compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.H. Proximate Composition, Extraction, and Purification of Theobromine from Cacao Pod Husk (Theobroma cacao L.). Technologies 2017, 5, 14. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized Liquid Extraction of Flavanols and Alkaloids from Cocoa Bean Shell Using Ethanol as Solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Forcina, A.; Petrillo, A.; Travaglioni, M.; di Chiara, S.; De Felice, F. A Comparative Life Cycle Assessment of Different Spent Coffee Ground Reuse Strategies and a Sensitivity Analysis for Verifying the Environmental Convenience Based on the Location of Sites. J. Clean. Prod. 2023, 385, 135727. [Google Scholar] [CrossRef]
- Machado, M.; Espírito Santo, L.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Oliveira, M.B.P.P.; Ferreira, H.; Alves, R.C. Bioactive Potential and Chemical Composition of Coffee By-Products: From Pulp to Silverskin. Foods 2023, 12, 2354. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.B.; Rocha, L.M.; Soares, M.S.; Good God, P.I.V.; da Silva, S.A.; Moreira, D.B.; Silva, G.H. Validation of Methodology for Quantifying Caffeic and Ferulic Acids in Raw and Roasted Coffee Extracts by High-Performance Liquid Chromatography. J. Multidiscip. Sci. J. 2025, 8, 8. [Google Scholar] [CrossRef]
- Suryana, E.A.; Kamsiati, E.; Somantri, A.S. Characteristics of Organoleptic Quality of Several Long-Grain and Bold-Grain Rice Varieties in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1024, 012058. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Elmas, C.; Gezer, C. Capsaicin and Its Effects on Body Weight. J. Am. Nutr. Assoc. 2022, 41, 831–839. [Google Scholar] [CrossRef]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of Different Capsicum Varieties by Evaluation of Their Capsaicinoids Content by High Performance Liquid Chromatography, Determination of Pungency and Effect of High Temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef]
- Rathnayaka, R.M.S.M.B.; Kondo, F.; Prabandaka, S.S.; Nemoto, K.; Matsushima, K. Drought Stress Induced an Increase in the Pungency and Expression of Capsaicinoid Biosynthesis Genes in Chili Pepper (Capsicum annuum L.). Hortic. J. 2021, 90, 410–419. [Google Scholar] [CrossRef]
- Guillen, N.G.; Tito, R.; Mendoza, N.G. Capsaicinoids and Pungency in Capsicum chinense and Capsicum Baccatum Fruits. Pesqui. Agropecu. Trop. 2018, 48, 237–244. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Gaspari, A.; Graziani, G.; Santini, A.; Ritieni, A. Fast Analysis of Polyphenols and Alkaloids in Cocoa-Based Products by Ultra-High Performance Liquid Chromatography and Orbitrap High Resolution Mass Spectrometry (UHPLC-Q-Orbitrap-MS/MS). Food Res. Int. 2018, 111, 229–236. [Google Scholar] [CrossRef]
- Jean-Marie, E.; Bereau, D.; Robinson, J.-C. Benefits of Polyphenols and Methylxanthines from Cocoa Beans on Dietary Metabolic Disorders. Foods 2021, 10, 2049. [Google Scholar] [CrossRef]
- Cruz, J.F.M.; Leite, P.B.; Soares, S.E.; Bispo, E.D.S. Bioactive Compounds in Different Cocoa (Theobroma cacao, L) Cultivars During Fermentation. Food Sci. Technol. 2015, 35, 279–284. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [PubMed]
- Utrilla-Vázquez, M.; Rodríguez-Campos, J.; Avendaño-Arazate, C.H.; Gschaedler, A.; Lugo-Cervantes, E. Analysis of Volatile Compounds of Five Varieties of Maya Cocoa During Fermentation and Drying Processes by Venn diagram and PCA. Food Res. Int. 2020, 129, 108834. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, J.G.B.; Tellez, H.B.H.; Atuesta, G.C.P.; Aldana, A.P.S.; Arteaga, J.J.M. Antioxidant Activity, Total Polyphenol Content and Methylxantine Ratio in Four Materials of Theobroma cacao L. from Tolima, Colombia. Heliyon 2022, 8, e09402. [Google Scholar] [CrossRef]
- Velásquez-Reyes, D.; Rodríguez-Campos, J.; Avendaño-Arrazate, C.; Gschaedler, A.; Alcázar-Valle, M.; Lugo-Cervantes, E. Forastero and Criollo Cocoa Beans, Differences on the Profile of Volatile and Non-Volatile Compounds in the Process from Fermentation to Liquor. Heliyon 2023, 9, e15129. [Google Scholar] [CrossRef]
- Peláez, P.; Bardón, I.; Camasca, P. Methylxanthine and Catechin Content of Fresh and Fermented Cocoa Beans, Dried Cocoa Beans, and Cocoa Liquor. Sci. Agropecu. 2016, 7, 355–365. [Google Scholar] [CrossRef]
- Dang, Y.K.T.; Nguyen, H.V.H. Effects of Maturity at Harvest and Fermentation Conditions on Bioactive Compounds of Cocoa Beans. Plant Foods Hum. Nutr. 2019, 74, 54–60. [Google Scholar] [CrossRef]
- Mengistu, M.W.; Workie, M.A.; Mohammed, A.S. Biochemical Compounds of Arabica Coffee (Coffea arabica L.) Varieties Grown in Northwestern Highlands of Ethiopia. Cogent Food Agric. 2020, 6, 1741319. [Google Scholar] [CrossRef]
- Gebeyehu, B.T. Determination of Caffeine Content and Antioxidant Activity of Coffee. Am. J. Appl. Chem. 2015, 3, 69. [Google Scholar] [CrossRef]
- Gamonal, L.E.; Vallejos-Torres, G.; López, L.A. Sensory Analysis of Four Cultivars of Coffee (Coffea arabica L.), Grown at Different Altitudes in the San Martin Region—Peru. Cienc. Rural. 2017, 47, e20160882. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Q.; Chen, C.; Yang, X.; Xie, R.; Xu, J. The Role of Transient Receptor Potential Vanilloid 1 in Common Diseases of the Digestive Tract and the Cardiovascular and Respiratory System. Front. Physiol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Meza, R.C.; Ancatén-González, C.; Chiu, C.Q.; Chávez, A.E. Transient Receptor Potential Vanilloid 1 Function at Central Synapses in Health and Disease. Front. Cell. Neurosci. 2022, 16, 864828. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Shen, M.; Zhao, X.; Zhu, H.; Yang, Y.; Lu, S.; Tan, Y.; Li, G.; Li, M.; Wang, J.; et al. Anti-Obesity Effect of Capsaicin in Mice Fed with High-Fat Diet Is Associated with an Increase in Population of the Gut Bacterium Akkermansia muciniphila. Front. Microbiol. 2017, 8, 272. [Google Scholar] [CrossRef]
- Silva, J.L.; Santos, E.A.; Alvarez-Leite, J.I. Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain? Nutrients 2023, 15, 4469. [Google Scholar] [CrossRef]
- Shang, K.; Amna, T.; Amina, M.; Al-Musayeib, N.; Al-Deyab, S.; Hwang, I. Influence of Capsaicin on Inflammatory Cytokines Induced by Lipopolysaccharide in Myoblast Cells Under in Vitro Environment. Pharmacogn. Mag. 2017, 13, s26–s32. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ferré, H.E.; Martínez-Avila, M.; Antunes-Ricardo, M.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Gutiérrez-Uribe, J.A. In Vitro Evaluation of Anti-Inflammatory Activity of “Habanero” Chili Pepper (Capsicum chinense) Seeds Extracts Pretreated with Cellulase. Plant Foods Hum. Nutr. 2023, 78, 109–116. [Google Scholar] [CrossRef]
- Hong, Q.; Xia, C.; Hu, X.; Quan, Y. Capsinoids Suppress Fat Accumulation via Lipid Metabolism. Mol. Med. Rep. 2015, 11, 1669–1674. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Zhang, L.; Sun, H.; Liu, X. Capsaicin Reduces Blood Glucose by Increasing Insulin Levels and Glycogen Content Better than Capsiate in Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2017, 65, 2323–2330. [Google Scholar] [CrossRef]
- Gu, R.; Shi, Y.; Huang, W.; Lao, C.; Zou, Z.; Pan, S.; Huang, Z. Theobromine Mitigates IL-1β-Induced Oxidative Stress, Inflammatory Response, and Degradation of Type II Collagen in Human Chondrocytes. Int. Immunopharmacol. 2020, 82, 106226. [Google Scholar] [CrossRef]
- Sharifi-Zahabi, E.; Rezvani, N.; Hajizadeh-Sharafabad, F.; Hosseini-Baharanchi, F.S.; Shidfar, F.; Rahimi, M. Theobromine Supplementation in Combination with a Low-Calorie Diet Improves Cardiovascular Risk Factors in Overweight and Obese Subjects with Metabolic Syndrome: A Randomized Controlled Trial. Food Funct. 2023, 14, 8431–8441. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Zonfrillo, M.; Villivà, C.; Bonmassar, E.; Ravagnan, G. Inflammatory Microenvironment and Adipogenic Differentiation in Obesity: The Inhibitory Effect of Theobromine in a Model of Human Obesity In Vitro. Mediat. Inflamm. 2019, 2019, 1515621. [Google Scholar] [CrossRef]
- Jang, M.H.; Mukherjee, S.; Choi, M.J.; Kang, N.H.; Pham, H.G.; Yun, J.W. Theobromine Alleviates Diet-Induced Obesity in Mice via Phosphodiesterase-4 Inhibition. Eur. J. Nutr. 2020, 59, 3503–3516. [Google Scholar] [CrossRef]
- Cadoná, F.C.; Machado, A.K.; Azzolin, V.F.; Barbisan, F.; Dornelles, E.B.; Glanzner, W.; Gonçalves, P.B.D.; Assmann, C.E.; Ribeiro, E.E.; da Cruz, I.B.M. Guaraná a Caffeine-Rich Food Increases Oxaliplatin Sensitivity of Colorectal HT-29 Cells by Apoptosis Pathway Modulation. Anti-Cancer Agents Med. Chem. 2016, 16, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Iaia, N.; Rossin, D.; Sottero, B.; Venezia, I.; Poli, G.; Biasi, F. Efficacy of Theobromine in Preventing Intestinal CaCo-2 Cell Damage Induced by Oxysterols. Arch. Biochem. Biophys. 2020, 694, 108591. [Google Scholar] [CrossRef] [PubMed]
- Eissa, I.H.; Yousef, R.G.; Elkaeed, E.B.; Alsfouk, A.A.; Husein, D.Z.; Ibrahim, I.M.; Alesawy, M.S.; Elkady, H.; Metwaly, A.M. Anticancer Derivative of the Natural Alkaloid, Theobromine, Inhibiting EGFR Protein: Computer-Aided Drug Discovery Approach. PLoS ONE 2023, 18, e0282586. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The Relevance of Theobromine for the Beneficial Effects of Cocoa Consumption. Front. Pharmacol. 2015, 6, 30. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Sawie, H.G.; Hosny, E.N.; Mourad, H.H. Assessment of the Antidepressant Effect of Caffeine Using Rat Model of Depression Induced by Reserpine. Bull. Natl. Res. Cent. 2018, 42, 36. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Deng, R.; Fan, Y.; Li, K.; Meng, F.; Li, X.; Liu, R. Low Dose of Caffeine Enhances the Efficacy of Antidepressants in Major Depressive Disorder and the Underlying Neural Substrates. Mol. Nutr. Food Res. 2017, 61, 1600910. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Kim, K.-J.; Ryu, S.-J.; Lee, B.-Y. Caffeine Prevents LPS-Induced Inflammatory Responses in RAW264.7 Cells and Zebrafish. Chem. Biol. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef]
- Pinho, L.S.; da Silva, M.P.; Thomazini, M.; Cooperstone, J.L.; Campanella, O.H.; da Costa Rodrigues, C.E.; Favaro-Trindade, C.S. Guaraná (Paullinia cupana) by-Product as a Source of Bioactive Compounds and as a Natural Antioxidant for Food Applications. J. Food Process. Preserv. 2021, 45, e15854. [Google Scholar] [CrossRef]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H.; et al. Methylxanthines Enhance the Effects of Cocoa Flavanols on Cardiovascular Function: Randomized, Double-masked Controlled Studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef]
- Aksoy, C.S.; Avci, F.G.; Ugurel, O.M.; Atas, B.; Sayar, N.A.; Akbulut, B.S. Potentiating the Activity of Berberine for Staphylococcus aureus in a Combinatorial Treatment with Thymol. Microb. Pathog. 2020, 149, 104542. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Zeng, H.; Shi, N.; Peng, W.; Yang, Q.; Ren, J.; Yang, H.; Chen, L.; Chen, Y.; Guo, J. Effects of Capsaicin on Glucose Uptake and Consumption in Hepatocytes. Molecules 2023, 28, 5258. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, M.; Orlo, E.; Nugnes, R.; Piscitelli, C.; Russo, C.; Isidori, M. Capsaicin in Hot Chili Peppers: In Vitro Evaluation of Its Antiradical, Antiproliferative and Apoptotic Activities. Plant Foods Hum. Nutr. 2019, 74, 164–170. [Google Scholar] [CrossRef]
- Hacioglu, C. Capsaicin Inhibits Cell Proliferation by Enhancing Oxidative Stress and Apoptosis Through SIRT1/NOX4 Signaling Pathways in HepG2 and HL-7702 Cells. J. Biochem. Mol. Toxicol. 2022, 36, e22974. [Google Scholar] [CrossRef] [PubMed]
- Eichwald, T.; Solano, A.F.; Souza, J.; de Miranda, T.B.; Carvalho, L.B.; dos Santos Sanna, P.L.; da Silva, R.A.F.; Latini, A. Anti-Inflammatory Effect of Caffeine on Muscle under Lipopolysaccharide-Induced Inflammation. Antioxidants 2023, 12, 554. [Google Scholar] [CrossRef]
- Soliman, M.L.; Geiger, J.D.; Chen, X. Caffeine Blocks HIV-1 Tat-Induced Amyloid Beta Production and Tau Phosphorylation. J. Neuroimmune Pharmacol. 2017, 12, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hosny, E.N.; Sawie, H.G.; Elhadidy, M.E.; Khadrawy, Y.A. Evaluation of Antioxidant and Anti-Inflammatory Efficacy of Caffeine in Rat Model of Neurotoxicity. Nutr. Neurosci. 2019, 22, 789–796. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Santos, T.R.J.; de Santana, L.C.L.A. Conventional and Emerging Techniques for Extraction of Bioactive Compounds from Fruit Waste. Braz. J. Food Technol. 2022, 25, e2021130. [Google Scholar] [CrossRef]
- Mussatto, S.I. Chapter 11—Generating Biomedical Polyphenolic Compounds from Spent Coffee or Silverskin. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 93–106. ISBN 978-0-12-409517-5. [Google Scholar]
- Serna-Jiménez, J.A.; Torres-Valenzuela, L.S.; Sanín Villarreal, A.; Roldan, C.; Martín, M.A.; Siles, J.A.; Chica, A.F. Advanced Extraction of Caffeine and Polyphenols from Coffee Pulp: Comparison of Conventional and Ultrasound-Assisted Methods. LWT 2023, 177, 114571. [Google Scholar] [CrossRef]
- Coelho, J.; Filipe, R.; Robalo, M.; Boyadzhieva, S.; Cholakov, G.; STATEVA, R. Supercritical CO2 Extraction of Spent Coffee Grounds. INFLUENCE of Co-Solvents and Characterization of the Extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- Thai, L.Q.; Niwat, C.; Qin, S.; Konsue, N. Supercritical Carbon Dioxide and Ethanol-Assisted Extraction of Bioactive Compounds from Bourbon, Catimor, and Caturra coffee pulp for maximized antioxidant and therapeutic properties. Future Foods 2024, 9, 100381. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Scampicchio, M.; Ferrentino, G.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Evaluation of the Capsaicinoid Extraction Conditions from Mexican Capsicum chinense Var. Mayapan with Supercritical Fluid Extraction (SFE). Processes 2023, 11, 2272. [Google Scholar] [CrossRef]
- Milovanovic, S.; Lukic, I.; Krgovic, N.; Tadic, V.; Radovanovic, Z.; Tyśkiewicz, K.; Konkol, M. Selection of Processing Parameters for the Integrated Supercritical CO2 Extraction from Green Tea Leaves and Extract Impregnation onto Starch-Chitosan Based Films. J. Supercrit. Fluids 2024, 206, 106163. [Google Scholar] [CrossRef]
- Sökmen, M.; Demir, E.; Alomar, S.Y. Optimization of Sequential Supercritical Fluid Extraction (SFE) of Caffeine and Catechins from Green Tea. J. Supercrit. Fluids 2018, 133, 171–176. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Santana, Á.L.; Queirós, L.D.; Martínez, J.; Macedo, G.A. Pressurized Liquid- and Supercritical Fluid Extraction of Crude and Waste Seeds of Guarana (Paullinia cupana): Obtaining of Bioactive Compounds and Mathematical Modeling. Food Bioprod. Process. 2019, 117, 194–202. [Google Scholar] [CrossRef]
- Cortes-Ferre, H.E.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Enzyme-Assisted Extraction of Anti-Inflammatory Compounds from Habanero Chili Pepper (Capsicum chinense) Seeds. Front. Nutr. 2022, 9, 942805. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Mamat, H.; Jusoh, W.M.S.W.; Idham, Z.; Yunus, M.A.C.; Irianto, I. Influence of Particle Size in Supercritical Carbon Dioxide Extraction of Roselle (Hibiscus sabdariffa) on Bioactive Compound Recovery, Extraction Rate, Diffusivity, and Solubility. Sci. Rep. 2023, 13, 10871. [Google Scholar] [CrossRef]
- Yian, L.N.; Putra, N.R.; Idham, Z.; Mohd Idrus, N.F.; Abdul Aziz, A.H.; Mohd Setapar, S.H.; Che Yunus, M.A. Supercritical Carbon Dioxide Extraction of Hevea Brasiliensis Seeds: Influence of Particle Size on to Oil Seed Recovery and its Kinetic. Malays. J. Fundam. Appl. Sci. 2021, 17, 253–261. [Google Scholar] [CrossRef]
- González-Alejo, F.A.; Barajas-Fernández, J.; Olán-Acosta, M.D.L.Á.; Lagunes-Gálvez, L.M.; García-Alamilla, P. Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell. Processes 2019, 7, 385. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated Green-Based Processes Using Supercritical CO2 and Pressurized Ethanol Applied to Recover Antioxidant Compouds from Cocoa (Theobroma cacao) Bean Hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Pagliari, S.; Celano, R.; Rastrelli, L.; Sacco, E.; Arlati, F.; Labra, M.; Campone, L. Extraction of Methylxanthines by Pressurized Hot Water Extraction from Cocoa Shell by-Product as Natural Source of Functional Ingredient. LWT 2022, 170, 114115. [Google Scholar] [CrossRef]
- Myo, H.; Nuntawat, K. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Coffee Pulp Using Propylene Glycol as a Solvent and Their Antioxidant Activities. Ultrason. Sonochem. 2022, 89, 106127. [Google Scholar] [CrossRef] [PubMed]
- Bajer, T.; Bajerová, P.; Kremr, D.; Eisner, A.; Ventura, K. Central Composite Design of Pressurised Hot Water Extraction Process for Extracting Capsaicinoids from Chili Peppers. J. Food Compos. Anal. 2015, 40, 32–38. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds from Aromatic Plants and Food by-Products. Front. Nutr. 2022, 8, 792203. [Google Scholar] [CrossRef]
- Tshering, G.; Posadzki, P.; Kongkaew, C. Efficacy and Safety of Topical Capsaicin in the Treatment of Osteoarthritis Pain: A Systematic Review and Meta-Analysis. Phytother. Res. 2024, 38, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Okoro, N.K.; Oseni, B.A.; Okubanjo, O.O.; Adegun, A.A.; Ilomuanya, M.O. Development of Diclofenac and Capsaicin Emulgel for the Management of Inflammation in Rheumatoid Arthritis. Trop. J. Nat. Prod. Res. 2023, 7, 3246–3252. [Google Scholar]
- Diaz Jalaff, L.; Ortega Cancino, E.; Altavilla, M.; Vargas Hurtado, K.; Nolan Mella, N.; Faccini, M. Eco-Friendly Sol–Gel Coatings as Microfouling Barrier for Marine Applications. Coatings 2023, 13, 1755. [Google Scholar] [CrossRef]
- Rakesh, V.; Patgiri, P.; Borah, A.; Nandhini, D.; Gogoi, I. Comparative Study on the Repellency and Chemical Profiles of Different Chilli Peppers Formulations Against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) in Stored Wheat. J. Stored Prod. Res. 2024, 106, 102312. [Google Scholar] [CrossRef]
- Noor Hasyierah, M.S.; Norhidayah, A.; Rahayu, M.A.; Adilah, A.; Nur Humaira, I. Botanical Insecticide of Chili and Ginger Extract on Nilaparvata lugens, Brown Planthopper. IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012001. [Google Scholar] [CrossRef]
- Global Market Insights Inc. Capsaicin Market Size & Share, Global Report 2023–2032. Available online: https://www.gminsights.com/industry-analysis/capsaicin-market (accessed on 2 September 2025).
- Hussain, N.; bt Md Dali, A.Z.; Munawar, N. Effects of Fat Contents and Particle Size of Cocoa Nibs on Alkaloid Composition, Antioxidant Activities, and Volatile Compound of Concentrated Cocoa Drink. J. Food Process. Preserv. 2021, 45, e15748. [Google Scholar] [CrossRef]
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Babić, J.; Pajin, B.; Miličević, B.; Guberac, S.; Vrdoljak, A.; Šubarić, D. Cocoa Husk Application in the Enrichment of Extruded Snack Products. J. Food Process. Preserv. 2019, 43, e13866. [Google Scholar] [CrossRef]
- Agudelo, C.; Bravo, K.; Ramírez-Atehortúa, A.; Torres, D.; Osorio, E.; Carrillo-Hormaza, L. Chemical and Skincare Property Characterization of the Main Cocoa Byproducts: Extraction Optimization by Rsm Approach for Development of Sustainable ingredients. Molecules 2021, 26, 7429. [Google Scholar] [CrossRef]
- Danaswari, M.S.; Yogiartono, R.M.; Rachmadi, P. Rubbing Cacao Bean Extract in Improving Microhardness Enamel on Dental Bleaching. Int. J. Pharm. Res. 2020, 12, 1579–1584. [Google Scholar] [CrossRef]
- Taneja, V.; Nekkanti, S.; Gupta, K.; Hassija, J. Remineralization Potential of Theobromine on Artificial Carious Lesions. J. Int. Soc. Prev. Community Dent. 2019, 9, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Stats Market Research. Global Theobromine Market Research Report 2024 (Status and Outlook). Available online: https://www.statsmarketresearch.com/global-theobromine-2024-297-8012027 (accessed on 2 September 2025).
- Sharma, R. Theobromine Market Report | Global Forecast From 2025 To 2033. Available online: https://dataintelo.com/report/theobromine-market (accessed on 2 September 2025).
- Data Horizzon Research Capsaicin Market Size, Growth Analysis & Forecast Report—2033. Available online: https://datahorizzonresearch.com/capsaicin-market-58589 (accessed on 2 September 2025).
- Dauber, C.; Romero, M.; Chaparro, C.; Ureta, C.; Ferrari, C.; Lans, R.; Frugoni, L.; Echeverry, M.V.; Calvo, B.S.; Trostchansky, A.; et al. Cookies Enriched with Coffee Silverskin Powder and Coffee Silverskin Ultrasound Extract to Enhance Fiber Content and Antioxidant Properties. Appl. Food Res. 2024, 4, 100373. [Google Scholar] [CrossRef]
- Benincá, D.B.; do Carmo, L.B.; Grancieri, M.; Aguiar, L.L.; Lima Filho, T.; Costa, A.G.V.; Oliveira, D.D.S.; Saraiva, S.H.; Silva, P.I. Incorporation of Spent Coffee Grounds in Muffins: A Promising Industrial Application. Food Chem. Adv. 2023, 3, 100329. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Y.; Sun, Y.; Lu, T.; Cao, Q.; Chen, X. Characterization of Kombucha Prepared from Black Tea and Coffee Leaves: A Comparative Analysis of Physiochemical Properties, Bioactive Components, and Bioactivities. J. Food Sci. 2024, 89, 3430–3444. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Rajabi-Moghaddam, S. Encapsulation of Caffeine in Chitosan-Coated Nanoliposomes and Its Application in Drink Formulation. Food Hydrocoll. 2024, 149, 109598. [Google Scholar] [CrossRef]
- Kassem, A.A.; Asfour, M.H.; Abd El-Alim, S.H.; Khattab, M.A.; Salama, A. Topical Caffeine-Loaded Nanostructured Lipid Carriers for Enhanced Treatment of Cellulite: A 32 Full Factorial Design Optimization and in Vivo Evaluation in rats. Int. J. Pharm. 2023, 643, 123271. [Google Scholar] [CrossRef]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of Bioactive Compounds: Challenges and Perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Singh, A.K.; Pal, P.; Pandey, B.; Goksen, G.; Sahoo, U.K.; Lorenzo, J.M.; Sarangi, P.K. Development of “Smart Foods” for Health by Nanoencapsulation: Novel Technologies and Challenges. Food Chem. X 2023, 20, 100910. [Google Scholar] [CrossRef] [PubMed]
- Neha, G.; Rakesh, N.; Siddharth, D. Caffeine Market Report and Forecast 2025–2034. Available online: https://www.expertmarketresearch.com/reports/caffeine-market (accessed on 2 September 2025).
- Mark Wide Research. Caffeine Market Analysis-Industry Size, Share, Research Report, Insights, Covid-19 Impact, Statistics, Trends, Growth and Forecast 2025–2034 | Size, Share, Growth. Available online: https://markwideresearch.com/coffee-market. (accessed on 1 September 2025).
- Research and Markets 2025 Caffeine Market Report—Industry Size, Competition, Trends and Growth Opportunities by Region—Forecast by Types and Applications (2024–2032). Available online: https://www.researchandmarkets.com/reports/5608033/2025-caffeine-market-report-industry-size (accessed on 2 September 2025).
- QY Research. Global Caffeine Industry Research Report, Growth Trends and Competitive Analysis 2024–2030. Available online: https://www.qyresearch.com/reports/2699919/caffeine (accessed on 2 September 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, A.; Mojica, L.; Coronado-Cáceres, L.; Castillo-Herrera, G.A. Unlocking the Alkaloid Biological Potential of Chili Pepper (Capsicum spp.), Cacao (Theobroma cacao L.), and Coffee (Coffea spp.) Byproducts: Characterization, Non-Conventional Extraction, Applications, and Future Perspectives. Molecules 2025, 30, 3795. https://doi.org/10.3390/molecules30183795
Cárdenas A, Mojica L, Coronado-Cáceres L, Castillo-Herrera GA. Unlocking the Alkaloid Biological Potential of Chili Pepper (Capsicum spp.), Cacao (Theobroma cacao L.), and Coffee (Coffea spp.) Byproducts: Characterization, Non-Conventional Extraction, Applications, and Future Perspectives. Molecules. 2025; 30(18):3795. https://doi.org/10.3390/molecules30183795
Chicago/Turabian StyleCárdenas, Anahí, Luis Mojica, Luis Coronado-Cáceres, and Gustavo A. Castillo-Herrera. 2025. "Unlocking the Alkaloid Biological Potential of Chili Pepper (Capsicum spp.), Cacao (Theobroma cacao L.), and Coffee (Coffea spp.) Byproducts: Characterization, Non-Conventional Extraction, Applications, and Future Perspectives" Molecules 30, no. 18: 3795. https://doi.org/10.3390/molecules30183795
APA StyleCárdenas, A., Mojica, L., Coronado-Cáceres, L., & Castillo-Herrera, G. A. (2025). Unlocking the Alkaloid Biological Potential of Chili Pepper (Capsicum spp.), Cacao (Theobroma cacao L.), and Coffee (Coffea spp.) Byproducts: Characterization, Non-Conventional Extraction, Applications, and Future Perspectives. Molecules, 30(18), 3795. https://doi.org/10.3390/molecules30183795









