3. Materials and Methods
Solvents for extractions and chromatography were of technical grade and were distilled prior to use. Extracts were dried over technical grade anhydrous Na
2SO
4. The NMR spectra were obtained on a Bruker Avance DPX 300 at 300 MHz for
1H and 75.5 MHz for
13C nucleus, and on a Bruker UltraShield 500 plus (Bruker, Billerica, MA, USA) at 500 MHz for
1H and 126 MHz for
13C nucleus, using CDCl
3 with TMS as the internal standard, as solvent. Mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA), and IR spectra on a Perkin-Elmer Spectrum BX FTIR spectrophotometer (PerkinElmer, Waltham, MA, USA). Column chromatography (CC) was performed on silica gel (Silica gel 60, particle size: 0.035–0.070 mm (Sigma-Aldrich, St. Louis, MO, USA)). All the commercially available chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3-Carboxypyridinium dichromate (NDC) was freshly prepared and used following the procedure described in the literature [
29,
30].
3.1. Synthesis of (1R,4R)-1-(iodomethyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-one (2) [27]
Compound
2 was prepared according to the procedure described in the literature [
27]. To a solution of (1
S)-(+)-10-camphorsulfonic acid (
1) (1.0 equiv., 172 mmol, 39.95 g) in anhydrous toluene (250 mL) under argon, PPh
3 (4.0 equiv., 688 mmol, 180.46 g) and I
2 (2.0 equiv., 344 mmol, 87.31 g) were added. The resulting reaction mixture was heated for 16 h under reflux. The volatile components were evaporated in vacuo. The residue was dissolved in EtOAc (500 mL) and washed with Na
2SO
3 (aq. sat., 5 × 100 mL or until the reaction mixture changed color from dark violet to light yellow) and NaCl (aq. sat., 3 × 50 mL). The organic phase was dried under anhydrous Na
2SO
4, filtered, and the volatile components were evaporated in vacuo. The solid residue was suspended in petroleum ether (or hexane) (500 mL) and stirred for 12 h at room temperature. The solid residue was filtered, washed with petroleum ether (or hexane) (2 × 50 mL), and the volatile components (filtrate) were evaporated in vacuo to obtain 10-iodocamphor
2. Yield: 37.77 g (ω = 0.95, 129 mmol, 75%) of yellowish solid.
1H-NMR (500 MHz, CDCl
3):
δ 0.90 (
s, 3H), 1.08 (
s, 3H), 1.35–1.43 (
m, 1H), 1.57–1.64 (
m, 1H), 1.91 (
d,
J = 18.4 Hz, 1H), 1.95–2.05 (
m, 2H), 2.16 (
t,
J = 4.2 Hz, 1H), 2.40 (
dd,
J = 4.9, 18.4 Hz, 1H), 3.12 (
d,
J = 10.6 Hz, 1H), 3.31 (
d,
J = 10.6 Hz, 1H).
13C-NMR (126 MHz, CDCl
3):
δ 0.8, 20.3, 20.5, 26.9, 30.7, 43.1, 44.2, 48.4, 59.2, 215.1.
3.2. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetic Acid (3) [25]
Compound
3 was prepared according to the procedure described in the literature [
25]. To a solution of 10-iodocamphor (
2) (1.0 equiv., ω = 0.95, 42.9 mmol, 12.56 g) in a mixture of DMSO (120 mL) and H
2O (40 mL) was added KOH (5.0 equiv., 214.5 mmol, 12.04 g). The resulting reaction mixture was stirred at 65 °C for 2 h. NaCl (aq. sat., 100 mL) was added to the cooled reaction mixture (room temperature), followed by extraction with Et
2O (3 × 100 mL). The organic phase was discarded, and the aqueous phase was carefully acidified with HCl (6 M in H
2O, 180 mmol, 30 mL) to pH 1–2, followed by extraction with EtOAc (3 × 100 mL). The combined organic phase was dried under anhydrous Na
2SO
4, filtered, and the volatiles evaporated in vacuo to give acid
3. Product
3 was stored under argon in the absence of light. Yield: 5.85 g (34.75 mmol, 81%) of yellowish oil.
1H-NMR (500 MHz, CDCl
3):
δ 0.85 (
s, 3H), 1.08 (
s, 3H), 1.32–1.44 (
m, 1H), 1.91–2.05 (
m, 2H), 2.15 (
dd,
J = 10.4, 14.9 Hz, 1H), 2.28–2.39 (
m, 1H), 2.40–2.52 (
m, 2H), 4.78 (
s, 1H), 4.80 (
s, 1H), 11.34 (br
s, 1H).
13C-NMR (126 MHz, CDCl
3):
δ 23.5, 26.7, 28.4, 30.4, 35.2, 43.9, 46.4, 103.8, 161.0, 180.5.
3.3. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) [28]
A solution of (+)-isocampholenic acid (3) (1.0 equiv., 25.1 mmol, 4.22 g) in anhydrous THF (200 mL) was cooled to 0 °C under argon, followed by a careful addition of LiAlH4 (1 M in THF, 2.0 equiv., 50.2 mmol, 50.2 mL). The resulting reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 20 h. The reaction mixture was cooled to 0 °C, and then cooled H2O (0 °C, 100 mL) was carefully added dropwise to quench the excess LiAlH4. After stirring the reaction mixture at room temperature for 15 min, most of the THF was evaporated in vacuo. The residue was extracted with Et2O (100 mL). The organic phase was washed with NaCl (aq. sat., 3 × 100 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo to give alcohol 4. Yield: 3.255 g (21.1 mmol, 84%) of a white solid. [α]Dr.t. = +21.0 (0.30, CH2Cl2). EI-HRMS: m/z = 155.1429 (MH+); C10H19O: requires m/z = 155.1430 (MH+); νmax 3332, 3071, 2958, 2866, 1651, 1462, 1433, 1378, 1362, 1199, 1151, 1055, 1038, 999, 976, 921, 877, 850 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.83 (s, 3H), 1.06 (s, 3H), 1.24–1.32 (m, 1H), 1.34–1.42 (m, 1H), 1.54–1.62 (m, 1H), 1.67 (br s, 1H), 1.69–1.76 (m, 1H), 1.82–1.88 (m, 1H), 2.25–2.34 (m, 1H), 2.42–2.50 (m, 1H), 3.60–3.68 (m, 1H), 3.70–3.79 (m, 1H), 4.75–4.79 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.6, 28.3, 30.8, 33.2, 44.0, 46.8, 62.4, 103.1, 162.4.
3.4. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) [31]
To a solution of alcohol
4 (1.0 equiv., 5.5 mmol, 848 mg) in anhydrous CH
2Cl
2 (15 mL) was added freshly prepared 3-carboxypyridinium dichromate (NDC) [
29,
30] (1.857 g, 4 mmol, 0.727 equiv.). The resulting reaction mixture was stirred at room temperature for 20 h. The reaction mixture was filtered through a short plug of Silica gel 60, and the plug was washed with CH
2Cl
2 (2 × 15 mL). The volatiles were evaporated in vacuo, and the residue was purified by column chromatography (Silica gel 60, petroleum ether/EtOAc = 20:1). The fractions containing the pure product
5 were combined, and the volatile components were evaporated in vacuo. Yield: 630 mg (4.14 mmol, 75%) of a colorless oil.
1H-NMR (500 MHz, CDCl
3):
δ 0.85 (
s, 3H), 1.08 (
s, 3H), 1.28–1.39 (
m, 1H), 1.88–1.97 (
m, 1H), 2.01–2.10 (
m, 1H), 2.25 (
ddd,
J = 2.7, 10.3, 16.0 Hz, 1H), 2.31–2.41 (
m, 1H), 2.44–2.54 (
m, 2H), 4.79 (
t,
J = 2.5 Hz, 1H), 4.81 (
td,
J = 0.7, 2.2 Hz, 1H), 9.81 (
dd,
J = 1.8, 2.6 Hz, 1H).
13C-NMR (126 MHz, CDCl
3):
δ 23.5, 26.4, 28.7, 30.6, 43.8, 44.2, 44.9, 103.8, 160.7, 202.7.
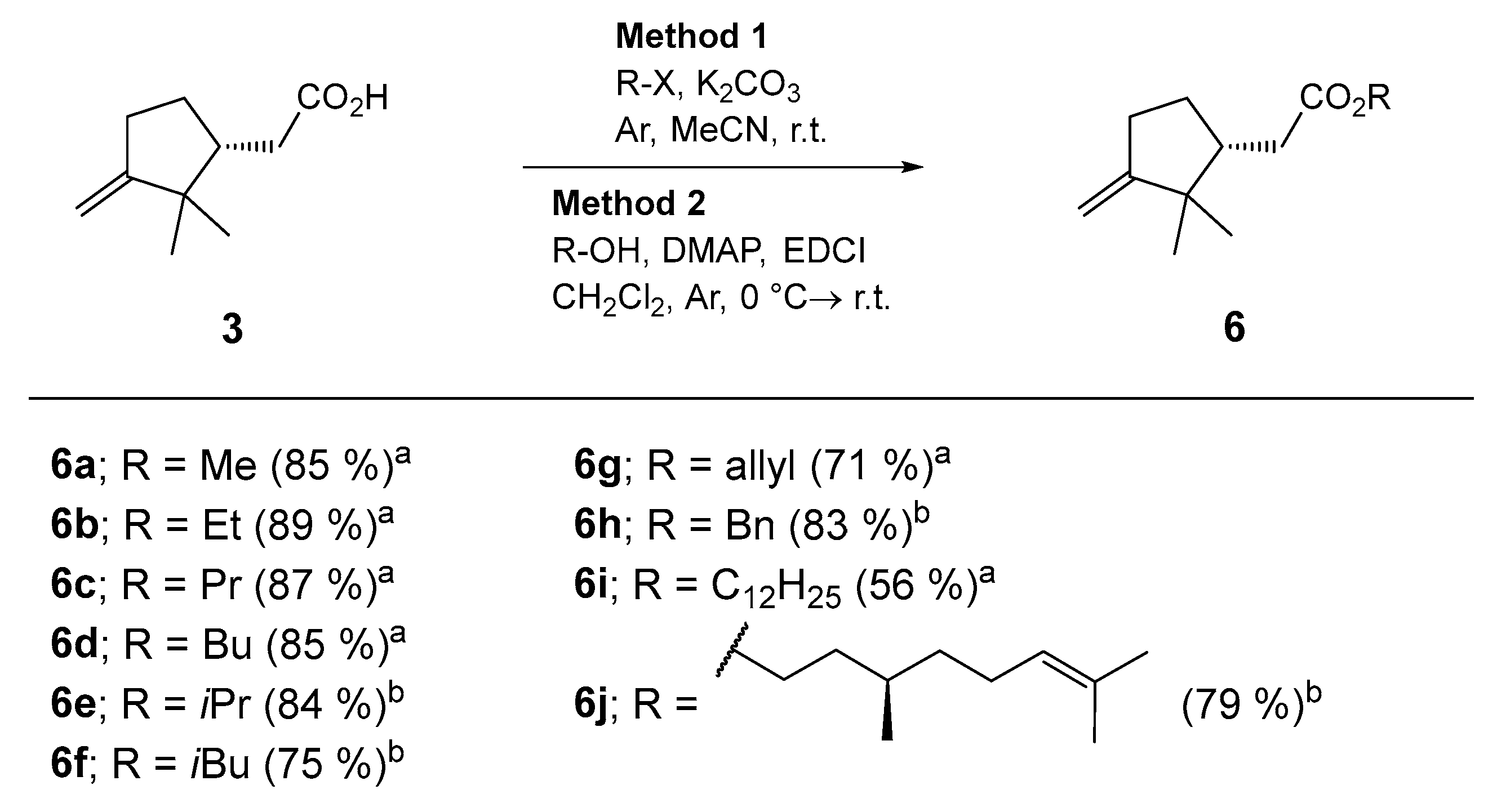
3.5. Synthesis of Esters 6 from (+)-Isocampholenic Acid—General Procedure 1 (GP1)
To a solution of (+)-isocampholenic acid (3) (1.0 equiv.) in anhydrous MeCN (2 mL) under argon, K2CO3 (2.0 equiv.) and the corresponding aliphatic halide were added. The resulting reaction mixture was stirred at room temperature for 20 h. The volatiles were evaporated in vacuo, and the residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product 6 were combined, and the volatiles were evaporated in vacuo. The isolated esters 6 were fully characterized.
3.6. Synthesis of Esters 6 from (+)-Isocampholenic Acid—General Procedure 2 (GP2)
To a solution of (+)-isocampholenic acid (3) (1.0 equiv.) in anhydrous CH2Cl2 (7 mL) under argon, the corresponding alcohol and DMAP (0.1 equiv.) were added. The reaction mixture was cooled to −5 °C, and then EDCI (2.0 equiv.) was added. The resulting reaction mixture was stirred at −5 °C for 1 h and then at room temperature for 20 h. The volatiles were evaporated in vacuo. The residue was dissolved in EtOAc (50 mL) and washed with NaHSO4 (aq., 1 M, 2 × 10 mL), NaHCO3 (aq. sat., 2 × 10 mL) and NaCl (aq. sat., 3 × 10 mL). The organic phase was dried under anhydrous Na2SO4, filtered, and the volatile components evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product 6 were combined, and the volatiles were evaporated in vacuo. The isolated esters 6 were fully characterized.
3.7. Synthesis of Methyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6a) [32]
Following GP1. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), K2CO3 (2.0 mmol, 276 mg), MeCN (2 mL) and iodomethane (1.5 mmol, 49 μL); column chromatography: EtOAc/petroleum ether = 1:10. Yield: 154 mg (0.85 mmol, 85%) of colorless oil. [α]Dr.t. = +21.0 (0.16, CH2Cl2). EI-HRMS: m/z = 183.1379 (MH+); C11H19O2: requires m/z = 183.1380 (MH+); νmax 2958, 1737, 1652, 1435, 1364, 1291, 1253, 1195, 1143, 1060, 999, 880 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 1.07 (s, 3H), 1.29–1.41 (m, 1H), 1.87–1.94 (m, 1H), 1.96–2.04 (m, 1H), 2.12 (dd, J = 10.5, 14.7 Hz, 1H), 2.28–2.50 (m, 3H), 3.68 (s, 3H), 4.77 (t, J = 2.5 Hz, 1H), 4.80 (td, J = 0.8, 2.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.7, 28.4, 30.5, 35.1, 43.9, 46.7, 51.6, 103.7, 161.3, 174.2.
3.8. Synthesis of Ethyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6b) [28]
Following GP1. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), K2CO3 (2.0 mmol, 276 mg), MeCN (2 mL) and bromoethane (1.5 mmol, 114 μL); column chromatography: EtOAc/petroleum ether = 1:10. Yield: 174 mg (0.89 mmol, 89%) of colorless oil. [α]Dr.t. = +7.0 (0.30, CH2Cl2). EI-HRMS: m/z = 197.1535 (MH+); C12H21O2: requires m/z = 197.1536 (MH+); νmax 2961, 1733, 1652, 1464, 1365, 1290, 1252, 1182, 1140, 1031, 879 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 1.07 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.30–1.41 (m, 1H), 1.87–1.94 (m, 1H), 1.96–2.04 (m, 1H), 2.11 (dd, J = 10.5, 14.6 Hz, 1H), 2.28–2.50 (m, 3H), 4.14 (q, J = 7.1 Hz, 2H), 4.77 (t, J = 2.5 Hz, 1H), 4.79 (td, J = 0.7, 2.1 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.4, 23.5, 26.7, 28.4, 30.5, 35.4, 43.9, 46.7, 60.4, 103.6, 161.4, 173.7.
3.9. Synthesis of Propyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6c)
Following GP1. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), K2CO3 (2.0 mmol, 276 mg), MeCN (2 mL) and 1-bromopropane (1.2 mmol, 111 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 184 mg (0.87 mmol, 87%) of colorless oil. [α]Dr.t. = +8.0 (0.26, CH2Cl2). EI-HRMS: m/z = 211.1694 (MH+); C13H23O2: requires m/z = 211.1693 (MH+); νmax 2962, 1734, 1654, 1446, 1365, 1293, 1255, 1186, 1035, 879 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 0.95 (t, J = 7.4, 3H), 1.07 (s, 3H), 1.31–1.41 (m, 1H), 1.66 (h, J = 7.2 Hz, 2H), 1.86–1.94 (m, 1H), 1.96–2.04 (m, 1H), 2.12 (dd, J = 10.5, 14.6 Hz, 1H), 2.29–2.50 (m, 3H), 4.04 (t, J = 6.7 Hz, 2H), 4.78 (t, J = 2.5 Hz, 1H), 4.79–4.81 (m, 1H). 13C-NMR (126 MHz, CDCl3): δ 10.6, 22.1, 23.6, 26.7, 28.4, 30.5, 35.4, 44.0, 46.7, 66.1, 103.7, 161.5, 174.0.
3.10. Synthesis of Butyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6d)
Following GP1. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), K2CO3 (2.0 mmol, 276 mg), MeCN (2 mL) and 1-iodobutane (1.2 mmol, 138 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 192 mg (0.85 mmol, 85%) of colorless oil. [α]Dr.t. = +8.0 (0.29, CH2Cl2). EI-HRMS: m/z = 225.1847 (MH+); C14H25O2: requires m/z = 225.1849 (MH+); νmax 2959, 1734, 1652, 1464, 1364, 1289, 1249, 1179, 1141, 1063, 1020, 879 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 0.94 (t, J = 7.3 Hz, 3H), 1.07 (s, 3H), 1.33–1.44 (m, 3H), 1.58–1.66 (m, 2H), 1.86–1.94 (m, 1H), 1.96–2.04 (m, 1H), 2.11 (dd, J = 10.5, 14.6 Hz, 1H), 2.28–2.50 (m, 3H), 4.08 (t, J = 6.7 Hz, 2H), 4.77 (t, J = 2.3 Hz, 1H), 4.80 (t, J = 1.8 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 13.8, 19.3, 23.6, 26.7, 28.4, 30.5, 30.8, 35.4, 44.0, 46.7, 64.4, 103.7, 161.5, 173.9.
3.11. Synthesis of Isopropyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6e)
Following GP2. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), propan-2-ol (2.0 mmol, 121 μL), DMAP (0.1 mmol, 12.2 mg), CH2Cl2 (7 mL) in EDCI (2.0 mmol, ω = 98%, 391 mg); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 178 mg (0.84 mmol, 84%) of colorless oil. [α]Dr.t. = +11.0 (0.33, CH2Cl2). EI-HRMS: m/z = 211.1693 (MH+); C13H23O2: requires m/z = 211.1693 (MH+); νmax 2961, 1729, 1652, 1467, 1374, 1290, 1181, 1143, 1108, 959, 879 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.83 (s, 3H), 1.07 (s, 3H), 1.24 (dd, J = 1.4, 6.3 Hz, 6H), 1.30–1.41 (m, 1H), 1.86–1.93 (m, 1H), 1.96–2.03 (m, 1H), 2.08 (dd, J = 10.5, 14.3 Hz, 1H), 2.29–2.39 (m, 2H), 2.41–2.49 (m, 1H), 4.77 (t, J = 2.5 Hz, 1H), 4.78–4.80 (m, 1H), 4.98–5.06 (m, 1H). 13C-NMR (126 MHz, CDCl3): δ 22.0, 22.0, 23.6, 26.7, 28.3, 30.5, 35.8, 44.0, 46.8, 67.6, 103.6, 161.5, 173.3.
3.12. Synthesis of Isobutyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6f)
Following GP2. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), butan-2-ol (2.0 mmol, 187 μL), DMAP (0.1 mmol, 12.2 mg), CH2Cl2 (7 mL) in EDCI (2.0 mmol, ω = 98%, 391 mg); column chromatography: EtOAc/petroleum ether = 1:25. Yield: 169 mg (0.75 mmol, 75%) of colorless oil. [α]Dr.t. = +15.0 (0.34, CH2Cl2). EI-HRMS: m/z = 225.1849 (MH+); C14H25O2: requires m/z = 225.1849 (MH+); νmax 2960, 1734, 1652, 1468, 1379, 1289, 1250, 1177, 1139, 1001, 879, 671 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 0.94 (d, J = 6.8 Hz, 6H), 1.08 (s, 3H), 1.30–1.43 (m, 1H), 1.87–2.06 (m, 3H), 2.12 (dd, J = 10.5, 14.6 Hz, 1H), 2.28–2.51 (m, 3H), 3.86 (d, J = 6.7 Hz, 2H), 4.78 (t, J = 2.5 Hz, 1H), 4.80 (td, J = 0.7, 2.3 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 19.2, 23.5, 26.7, 27.8, 28.5, 30.5, 35.4, 44.0, 46.7, 70.6, 103.7, 161.4, 173.8.
3.13. Synthesis of Allyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6g) [33]
Following GP1. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), K2CO3 (2.0 mmol, 276 mg), MeCN (2 mL) and 3-bromopropene (1.2 mmol, 107 μL); column chromatography: EtOAc/petroleum ether = 1:10. Yield: 149 mg (0.71 mmol, 71%) of colorless oil. [α]Dr.t. = +10.0 (0.25, CH2Cl2). EI-HRMS: m/z = 209.1536 (MH+); C13H21O2: requires m/z = 209.1536 (MH+); νmax 2960, 1735, 1651, 1462, 1364, 1289, 1176, 1139, 1057, 987, 929, 879 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 1.07 (s, 3H), 1.31–1.41 (m, 1H), 1.87–1.96 (m, 1H), 1.97–2.07 (m, 1H), 2.15 (dd, J = 10.6, 14.7 Hz, 1H), 2.29–2.39 (m, 1H), 2.40–2.51 (m, 2H), 4.58 (t, J = 1.4 Hz, 1H), 4.59 (t, J = 1.4 Hz, 1H), 4.78 (t, J = 2.5 Hz, 1H), 4.80 (td, J = 0.7, 2.2 Hz, 1H), 5.24 (dq, J = 1.3, 10.4 Hz, 1H), 5.33 (dq, J = 1.5, 17.2 Hz, 1H), 5.93 (ddt, J = 5.8, 10.4, 17.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.6, 26.7, 28.4, 30.5, 35.3, 44.0, 46.7, 65.2, 103.7, 118.4, 132.4, 161.3, 173.4.
3.14. Synthesis of Benzyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6h)
Following GP2. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), benzyl alcohol (1.8 mmol, 189 μL), DMAP (0.1 mmol, 12.2 mg), CH2Cl2 (7 mL) in EDCI (2.0 mmol, ω = 98%, 391 mg); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 216 mg (0.83 mmol, 83%) of colorless oil. [α]Dr.t. = +9.0 (0.27, CH2Cl2). EI-HRMS: m/z = 259.1698 (MH+); C17H23O2: requires m/z = 259.1693 (MH+); νmax 2959, 1733, 1652, 1456, 1363, 1289, 1255, 1137, 971, 880, 735, 696 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.80 (s, 3H), 1.02 (s, 3H), 1.29–1.41 (m, 1H), 1.85–1.94 (m, 1H), 1.98–2.08 (m, 1H), 2.17 (dd, J = 10.6, 14.8 Hz, 1H), 2.30–2.37 (m, 1H), 2.39–2.50 (m, 2H), 4.77 (t, J = 2.3 Hz, 1H), 4.79 (t, J = 1.8 Hz, 1H), 5.12 (s, 2H), 7.30–7.40 (m, 5H). 13C-NMR (126 MHz, CDCl3): δ 23.6, 26.7, 28.4, 30.5, 35.4, 44.0, 46.7, 66.3, 103.7, 128.3, 128.4, 128.7, 136.1, 161.3, 173.6.
3.15. Synthesis of Dodecyl (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetate (6i)
Following GP1. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), K2CO3 (2.0 mmol, 276 mg), MeCN (2 mL) and 1-bromododecane (1.5 mmol, ω = 0.97, 371 μL); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 188 mg (0.56 mmol, 56%) of colorless oil. [α]Dr.t. = +6.2 (0.121, CH2Cl2). EI-HRMS: m/z = 337.3099 (MH+); C22H41O2: requires m/z = 337.3101 (MH+); νmax 2923, 2854, 1736, 1652, 1465, 1363, 1289, 1178, 1141, 880, 722 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 0.88 (t, J = 6.9 Hz, 3H), 1.07 (s, 3H), 1.20–1.41 (m, 19H), 1.58–1.67 (m, 2H), 1.85–1.94 (m, 1H), 1.95–2.06 (m, 1H), 2.11 (dd, J = 10.5 Hz, 14.6, 1H), 2.28–2.51 (m, 3H), 4.07 (t, J = 6.8 Hz, 2H), 4.77 (t, J = 2.6, 1H), 4.79–4.81 (m, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.3, 22.8, 23.6, 26.1, 26.7, 28.5, 28.8, 29.4, 29.5, 29.7, 29.7, 29.8, 29.8, 30.5, 32.1, 35.5, 44.0, 46.8, 64.7, 103.7, 161.5, 173.9.
3.16. Synthesis of (R)-3,7-dimethyloct-6-en-1-yl 2-((R)-2,2-dimethyl-3-methylenecyclopentyl)acetate (6j)
Following GP2. Prepared from (+)-isocampholenic acid (3) (1.0 mmol, 168 mg), (R)-(+)-β-citronellol (1.1 mmol, ω = 0.95, 211 μL), DMAP (0.1 mmol, 12.2 mg), CH2Cl2 (7 mL) in EDCI (2.0 mmol, ω = 98%, 391 mg); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 242 mg (0.79 mmol, 79%) of colorless oil. [α]Dr.t. = +6.8 (0.272, CH2Cl2). EI-HRMS: m/z = 307.2635 (MH+); C20H35O2: requires m/z = 307.2632 (MH+); νmax 2959, 1735, 1652, 1460, 1378, 1290, 1178, 1140, 1058, 982, 880 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 0.92 (d, J = 6.6 Hz, 3H), 1.07 (s, 3H), 1.14–1.23 (m, 1H), 1.31–1.40 (m, 2H), 1.41–1.48 (m, 1H), 1.51–1.59 (m, 1H), 1.60 (s, 3H), 1.63–1.72 (m, 1H), 1.68 (s, 3H), 1.86–2.05 (m, 4H), 2.10 (dd, J = 10.5, 14.6 Hz, 1H), 2.28–2.36 (m, 1H), 2.39 (dd, J = 4.2, 14.6 Hz, 1H), 2.42–2.50 (m, 1H), 4.05–4.18 (m, 2H), 4.77 (t, J = 2.5 Hz, 1H), 4.79 (t, J = 2.2 Hz, 1H), 5.05–5.13 (m, 1H). 13C-NMR (126 MHz, CDCl3): δ 17.8, 19.5, 23.6, 25.5, 25.9, 26.7, 28.4, 29.6, 30.5, 35.5, 35.6, 37.1, 44.0, 46.7, 63.0, 103.7, 124.7, 131.5, 161.4, 173.9.
3.17. Synthesis of Esters 7 from Alcohol 4—General Procedure 3 (GP3)
To a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 equiv.) in anhydrous CH2Cl2 (2 mL) under argon, pyridine (3.0 equiv.), DMAP (0.04 equiv.) and the corresponding acid anhydride/acid chloride were added. The resulting reaction mixture was stirred at room temperature for 20 h. The volatiles were evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product 7 were combined, and the volatiles were evaporated in vacuo. The isolated esters 7 were fully characterized.
3.18. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl Acetate (7a)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and acetic anhydride (1.5 mmol, 142 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 169 mg (0.86 mmol, 86%) of colorless oil. [α]Dr.t. = +10.0 (0.34, CH2Cl2). EI-HRMS: m/z = 197.1497 (MH+); C12H21O2: requires m/z = 197.1493 (MH+); νmax 2959, 2868, 1739, 1652, 1463, 1434, 1364, 1228, 1153, 1038, 968, 878, 636, 606 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.24–1.37 (m, 1H), 1.37–1.48 (m, 1H), 1.51–1.59 (m, 1H), 1.75–1.82 (m, 1H), 1.82–1.90 (m, 1H), 2.05 (s, 3H), 2.24–2.36 (m, 1H), 2.42–2.52 (m, 1H), 4.05–4.11 (m, 1H), 4.12–4.18 (m, 1H), 4.74–4.80 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 21.2, 23.5, 26.6, 28.2, 29.0, 30.8, 44.1, 47.1, 64.2, 103.3, 162.1, 171.3.
3.19. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl Propionate (7b)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and propionic anhydride (1.5 mmol, 194 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 203 mg (0.96 mmol, 96%) of colorless oil. [α]Dr.t. = +14.0 (0.35, CH2Cl2). EI-HRMS: m/z = 211.1689 (MH+); C13H23O2: requires m/z = 211.1693 (MH+); νmax 2959, 1736, 1652, 1463, 1384, 1362, 1348, 1274, 1179, 1082, 1022, 958, 878, 807 cm−1. 1H-NMR (300 MHz, CDCl3): δ 0.83 (s, 3H), 1.06 (s, 3H), 1.14 (t, J = 7.6 Hz, 3H), 1.19–1.58 (m, 3H), 1.70–1.95 (m, 2H), 2.20–2.37 (m, 3H), 2.39–2.54 (m, 1H), 4.01–4.23 (m, 2H), 4.73–4.82 (m, 2H). 13C-NMR (75 MHz, CDCl3): δ 9.3, 23.4, 26.6, 27.8, 28.3, 29.1, 30.8, 44.1, 47.2, 64.1, 103.3, 162.1, 174.6.
3.20. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl Butyrate (7c)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and butyric anhydride (1.3 mmol, 215 μL); column chromatography: EtOAc/petroleum ether = 1:40. Yield: 198 mg (0.88 mmol, 88%) of colorless oil. [α]Dr.t. = +12.0 (0.34, CH2Cl2). EI-HRMS: m/z = 225.1854 (MH+); C14H25O2: requires m/z = 225.1849 (MH+); νmax 2960, 2871, 1735, 1652, 1461, 1362, 1252, 1174, 1091, 1048, 992, 975, 933, 878 cm−1. 1H-NMR (300 MHz, CDCl3): δ 0.82 (s, 3H), 0.95 (t, J = 7.4 Hz, 3H), 1.06 (s, 3H), 1.20–1.95 (m, 7H), 2.21–2.37 (m, 3H), 2.40–2.54 (m, 1H), 4.01–4.23 (m, 2H), 4.73–4.82 (m, 2H). 13C-NMR (75 MHz, CDCl3): δ 13.8, 18.6, 23.5, 26.6, 28.2, 29.2, 30.8, 36.4, 44.1, 47.2, 63.9, 103.3, 162.2, 173.9.
3.21. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl Isobutyrate (7d)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and isobutyric anhydride (1.5 mmol, 249 μL); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 181 mg (0.80 mmol, 80%) of colorless oil. [α]Dr.t. = +15.0 (0.30, CH2Cl2). EI-HRMS: m/z = 225.1854 (MH+); C14H25O2: requires m/z = 225.1849 (MH+); νmax 2960, 1733, 1652, 1469, 1388, 1363, 1344,1258, 1190, 1153, 1075, 990, 971, 879, 754 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.17 (d, J = 7.0, 6H), 1.26–1.36 (m, 1H), 1.39–1.47 (m, 1H), 1.51–1.60 (m, 1H), 1.74–1.82 (m, 1H), 1.83–1.92 (m, 1H), 2.24–2.36 (m, 1H), 2.42–2.50 (m, 1H), 2.54 (p, J = 7.0 Hz, 1H), 4.04–4.11 (m, 1H), 4.12–4.20 (m, 1H), 4.74–4.81 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 19.2, 23.5, 26.6, 28.3, 29.1, 30.8, 34.2, 44.1, 47.2, 64.0, 103.3, 162.2, 177.4.
3.22. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl 3,3-dimethylbutanoate (7e)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and 3,3-dimethylbutyryl chloride (1.1 mmol, ω = 0.98, 153 μL); column chromatography: EtOAc/petroleum ether = 1:40. Yield: 187 mg (0.74 mmol, 74%) of colorless oil. [α]Dr.t. = +10.3 (0.25, CH2Cl2). νmax 3071, 2957, 2869, 1733, 1652, 1466, 1434, 1365, 1322, 1226, 1197, 1129, 1049, 1020, 996, 978, 931, 879, 795, 729, 704, 620 cm−1. 1H-NMR (300 MHz, CDCl3): δ 0.83 (s, 3H), 1.03 (s, 9H), 1.06 (s, 3H), 1.22–1.48 (m, 2H), 1.50–1.64 (m, 1H), 1.70–1.95 (m, 2H), 2.19 (s, 2H), 2.22–2.37 (m, 1H), 2.39–2.55 (m, 1H), 3.99–4.23 (m, 2H), 4.72–4.82 (m, 2H). 13C-NMR (75 MHz, CDCl3): δ 23.5, 26.6, 28.2, 29.2, 29.6, 29.8, 30.8, 44.1, 47.2, 48.2, 63.6, 103.3, 162.2, 172.6.
3.23. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl pent-4-enoate (7f)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and 4-pentenoic anhydride (1.5 mmol, ω = 0.98, 280 μL); column chromatography: EtOAc/petroleum ether = 1:40. Yield: 194 mg (0.82 mmol, 82%) of colorless oil. [α]Dr.t. = +13.9 (0.28, CH2Cl2). EI-HRMS: m/z = 237.1849 (MH+); C15H25O2: requires m/z = 237.1849 (MH+); νmax 3073, 2959, 2868, 1735, 1651, 1463, 1435, 1362, 1235, 1167, 1102, 1050, 993, 914, 879 cm−1. 1H-NMR (300 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.20–1.65 (m, 3H), 1.70–1.94 (m, 2H), 2.20–2.54 (m, 6H), 4.02–4.24 (m, 2H), 4.72–4.82 (m, 2H), 4.95–5.13 (m, 2H), 5.73–5.92 (m, 1H). 13C-NMR (75 MHz, CDCl3): δ 23.5, 26.6, 28.2, 29.0, 29.1, 30.8, 33.8, 44.1, 47.2, 64.1, 103.3, 115.6, 136.9, 162.1, 173.2.
3.24. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl Benzoate (7g)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and benzoic anhydride (1.3 mmol, 297 mg); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 226 mg (0.87 mmol, 87%) of colorless oil. [α]Dr.t. = +8.0 (0.37, CH2Cl2). EI-HRMS: m/z = 259.1691 (MH+); C17H23O2: requires m/z = 259.1693 (MH+); νmax 2958, 1717, 1651, 1602, 1452, 1385, 1362, 1314, 1268, 1175, 1110, 1069, 1026, 958, 878, 708, 687, 675 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.86 (s, 3H), 1.10 (s, 3H), 1.31–1.44 (m, 1H), 1.52–1.61 (m, 1H), 1.62–1.70 (m, 1H), 1.88–2.00 (m, 2H), 2.26–2.38 (m, 1H), 2.44–2.54 (m, 1H), 4.30–4.44 (m, 2H), 4.76–4.82 (m, 2H), 7.40–7.48 (m, 2H), 7.52–7.59 (m, 1H), 8.01–8.08 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.6, 28.3, 29.2, 30.8, 44.2, 47.4, 64.8, 103.3, 128.5, 129.7, 130.6, 133.0, 162.1, 166.8.
3.25. Synthesis of (R)-4-(2-(2,2-dimethyl-3-methylenecyclopentyl)ethoxy)-4-oxobutanoic Acid (7h)
Following GP3. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), CH2Cl2 (2 mL), pyridine (3.0 mmol, 243 μL), DMAP (0.04 mmol, 4.9 mg) and benzoic anhydride (1.0 mmol, 100 mg). The residue (after evaporation of the volatiles) was dissolved in EtOAc (50 mL) and washed with NaHSO4 (aq., 1 M, 2 × 5 mL) and NaCl (aq. sat., 5 mL). The organic phase was dried under anhydrous Na2SO4, filtered, and the volatile components evaporated in vacuo. The residue was purified by column chromatography (EtOAc). Yield: 191 mg (0.75 mmol, 75%) of colorless oil. [α]Dr.t. = +4.8 (0.255, CH2Cl2). EI-HRMS: m/z = 255.1589 (MH+); C14H23O4: requires m/z = 255.1591 (MH+); νmax 2958, 1734, 1710, 1651, 1397, 1362, 1163, 1047, 993, 930, 878, 840 cm−1. 1H-NMR (300 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.20–1.64 (m, 3H), 1.71–1.93 (m, 2H), 2.21–2.37 (m, 1H), 2.39–2.55 (m, 1H), 2.56–2.74 (m, 4H), 4.04–4.26 (m, 2H), 4.72–4.82 (m, 2H), 10.33 (br s, 1H). 13C-NMR (75 MHz, CDCl3): δ 23.4, 26.6, 28.2, 29.0, 29.0, 29.1, 30.8, 44.1, 47.1, 64.6, 103.3, 162.0, 172.3, 178.3.
3.26. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl Methyl Succinate (7i)
Following GP2. Prepared from (R)-4-(2-(2,2-dimethyl-3-methylenecyclopentyl)ethoxy)-4-oxobutanoic acid (7h) (1.0 mmol, 254 mg), methanol (3.0 mmol, 122 μL), DMAP (0.1 mmol, 12.2 mg), and CH2Cl2 (7 mL) in EDCI (2.0 mmol, ω = 98%, 391 mg); column chromatography: EtOAc/petroleum ether = 1:10. Yield: 215 mg (0.80 mmol, 80%) of colorless oil. [α]Dr.t. = +10.6 (0.23, CH2Cl2). EI-HRMS: m/z = 269.1751 (MH+); C15H25O4: requires m/z = 269.1747 (MH+); νmax 2957, 1733, 1651, 1436, 1362, 1317, 1155, 996, 879, 846 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.24–1.36 (m, 1H), 1.37–1.48 (m, 1H), 1.49–1.59 (m, 1H), 1.73–1.90 (m, 2H), 2.24–2.36 (m, 1H), 2.41–2.52 (m, 1H), 2.64 (s, 4H), 3.70 (s, 3H), 4.06–4.22 (m, 2H), 4.74–4.80 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.6, 28.2, 29.0, 29.1, 29.3, 30.8, 44.1, 47.1, 52.0, 64.5, 103.3, 162.1, 172.5, 172.9.
3.27. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethyl Succinate (7j)
Following GP2. Prepared from (R)-4-(2-(2,2-dimethyl-3-methylenecyclopentyl)ethoxy)-4-oxobutanoic acid (7h) (1.0 mmol, 254 mg), ethanol (3.0 mmol, 175 μL), DMAP (0.1 mmol, 12.2 mg), and CH2Cl2 (7 mL) in EDCI (2.0 mmol, ω = 98%, 391 mg); column chromatography: EtOAc/petroleum ether = 1:10. Yield: 220 mg (0.78 mmol, 78%) of colorless oil. [α]Dr.t. = +11.6 (0.23, CH2Cl2). EI-HRMS: m/z = 283.1904 (MH+); C16H27O4: requires m/z = 283.1904 (MH+); νmax 2959, 1732, 1651, 1464, 1363, 1349, 1314, 1154, 1125, 967. 879 cm−1. 1H-NMR (300 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.22–1.32 (m, 1H), 1.36–1.61 (m, 2H), 1.70–1.94 (m, 2H), 2.21–2.37 (m, 1H), 2.38–2.57 (m, 1H), 2.62 (s, 4H), 4.03–4.26 (m, 4H), 4.73–4.82 (m, 2H). 13C-NMR (75 MHz, CDCl3): δ 14.3, 23.5, 26.6, 28.2, 29.1, 29.4, 29.4, 30.8, 44.1, 47.2, 60.8, 64.5, 103.3, 162.1, 172.4, 172.5.
3.28. Synthesis of Ethers 8 from Alcohol 4—General Procedure 4 (GP4)
To a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 equiv.) in anhydrous THF (3 mL) under argon at 0 °C was added NaH (1.2 equiv.). The resulting reaction mixture was stirred at 0 °C for 30 min, then the electrophile (aliphatic halide) was added. After stirring at room temperature for 20 h, H2O (10 mL) was carefully added. The resulting mixture was extracted with Et2O (30 mL). The organic phase was washed with NaCl (aq. sat., 3 × 10 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product 8 were combined, and the volatiles were evaporated in vacuo. The isolated ethers 8 were fully characterized with the exception of HRMS, as the products were not ionized.
3.29. Synthesis of (R)-2-(2-methoxyethyl)-1,1-dimethyl-5-methylenecyclopentane (8a)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and iodomethane (2.0 mmol, 129 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 156 mg (0.93 mmol, 93%) of colorless oil. [α]Dr.t. = +13.0 (0.20, CH2Cl2). νmax 2958, 2866, 1651, 1462, 1386, 1362, 1199, 1152, 1120, 1003, 957, 877, 836, 704 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.24–1.32 (m, 1H), 1.33–1.40 (m, 1H), 1.51–1.61 (m, 1H), 1.71–1.78 (m, 1H), 1.80–1.88 (m, 1H), 2.23–2.35 (m, 1H), 2.40–2.50 (m, 1H), 3.34 (s, 3H), 3.37–3.48 (m, 2H), 4.73–4.80 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.6, 28.3, 30.0, 30.9, 44.1, 47.1, 58.7, 72.4, 103.1, 162.6.
3.30. Synthesis of (R)-2-(2-ethoxyethyl)-1,1-dimethyl-5-methylenecyclopentane (8b)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and bromoethane (2.0 mmol, 153 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 125 mg (0.68 mmol, 68%) of colorless oil. [α]Dr.t. = +17.0 (0.22, CH2Cl2). νmax 2959, 2863, 1651, 1463, 1377, 1362, 1111, 877, 704 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.21 (t, J = 7.0 Hz, 3H), 1.25–1.35 (m, 1H), 1.36–1.43 (m, 1H), 1.50–1.58 (m, 1H), 1.73–1.79 (m, 1H), 1.79–1.87 (m, 1H), 2.23–2.37 (m, 1H), 2.40–2.50 (m, 1H), 3.37–3.54 (m, 4H), 4.73–4.79 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 15.4, 23.5, 26.6, 28.4, 30.2, 30.9, 44.1, 47.3, 66.2, 70.4, 103.0, 162.6.
3.31. Synthesis of (R)-1,1-dimethyl-2-methylene-5-(2-propoxyethyl)cyclopentane (8c)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and 1-bromopropane (1.2 mmol, 109 μL); column chromatography: EtOAc/petroleum ether = 1:25. Yield: 154 mg (0.78 mmol, 78%) of colorless oil. [α]Dr.t. = +13.0 (0.22, CH2Cl2). νmax 3071, 2959, 2935, 2860, 1651, 1462, 1435, 1362, 1249, 1115, 981, 956, 877, 703 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 0.92 (t, J = 7.5 Hz, 3H), 1.06 (s, 3H), 1.21–1.42 (m, 2H), 1.51–1.64 (m, 3H), 1.72–1.79 (m, 1H), 1.80–1.89 (m, 1H), 2.23–2.34 (m, 1H), 2.40–2.50 (m, 1H), 3.33–3.45 (m, 3H), 3.46–3.53 (m, 1H), 4.73–4.81 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 10.8, 23.1, 23.5, 26.6, 28.4, 30.1, 30.9, 44.1, 47.3, 70.5, 72.8, 103.0, 162.6.
3.32. Synthesis of (R)-2-(2-butoxyethyl)-1,1-dimethyl-5-methylenecyclopentane (8d)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and 1-iodobutane (1.0 mmol, 114 μL); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 177 mg (0.84 mmol, 84%) of colorless oil. [α]Dr.t. = +14.0 (0.20, CH2Cl2). νmax 3071, 2957, 2928, 2857, 2796, 1652, 1463, 1434, 1375, 1362, 1302, 1234, 1114, 998, 962, 940, 878, 738, 704 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 0.92 (t, J = 7.4 Hz, 3H), 1.06 (s, 3H), 1.19–1.48 (m, 4H), 1.48–1.62 (m, 3H), 1.70–1.88 (m, 2H), 2.23–2.37 (m, 1H), 2.40–2.50 (m, 1H), 3.36–3.52 (m, 4H), 4.73–4.79 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 14.1, 19.6, 23.6, 26.6, 28.4, 30.1, 30.9, 32.0, 44.1, 47.3, 70.6, 70.9, 103.0, 162.6.
3.33. Synthesis of (R)-2-(2-(allyloxy)ethyl)-1,1-dimethyl-5-methylenecyclopentane (8e)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and allyl bromide (1.1 mmol, ω = 0.97, 98 μL); column chromatography: EtOAc/petroleum ether = 1:15. Yield: 161 mg (0.83 mmol, 83%) of colorless oil. [α]Dr.t. = +23.0 (0.17, CH2Cl2). νmax 3071, 2958, 2934, 2865, 1651, 1463, 1433, 1362, 1145, 1106, 993, 920, 878, 704, 634 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.23–1.32 (m, 1H), 1.35–1.45 (m, 1H), 1.52–1.62 (m, 1H), 1.72–1.89 (m, 2H), 2.23–2.33 (m, 1H), 2.40–2.50 (m, 1H), 3.41–3.47 (m, 1H), 3.51 (td, J = 5.2, 8.8 Hz, 1H), 3.92–4.03 (m, 2H), 4.73–4.79 (m, 2H), 5.17 (dq, J = 1.4, 10.3 Hz, 1H), 5.28 (dq, J = 1.7, 17.2 Hz, 1H), 5.93 (ddt, J = 5.6, 10.4, 17.2, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.6, 28.4, 30.1, 30.9, 44.1, 47.2, 70.0, 72.0, 103.0, 116.9, 135.1, 162.6.
3.34. Synthesis of (R)-1,1-dimethyl-2-(2-((3-methylbut-2-en-1-yl)oxy)ethyl)-5-methylenecyclopentane (8f)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and prenyl bromide (1.0 mmol, ω = 0.95, 121 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 171 mg (0.77 mmol, 77%) of colorless oil. [α]Dr.t. = +2.5 (0.24, CH2Cl2). EI-HRMS: m/z = 223.2056 (MH+); C15H27O: requires m/z = 223.2056 (MH+); νmax 2929, 2862, 1445, 1377, 1360, 1081, 1014, 799 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.81 (s, 3H), 1.06 (s, 3H), 1.24–1.34 (m, 1H), 1.34–1.43 (m, 1H), 1.49–1.61 (m, 1H), 1.68 (s, 3H), 1.75 (s, 3H), 1.76–1.87 (m, 2H), 2.23–2.34 (m, 1H), 2.40–2.50 (m, 1H), 3.42 (ddd, J = 6.9, 8.1, 9.1 Hz, 1H), 3.49 (td, J = 5.1, 8.9 Hz, 1H), 3.90–4.01 (m, 2H), 4.73–4.79 (m, 2H), 5.32–5.40 (m, 1H). 13C-NMR (75 MHz, CDCl3): δ 18.1, 23.5, 25.9, 26.7, 28.4, 30.2, 30.9, 44.1, 47.3, 67.4, 69.9, 103.0, 121.5, 136.7, 162.6.
3.35. Synthesis of (R)-1,1-dimethyl-2-methylene-5-(2-(prop-2-yn-1-yloxy)ethyl)cyclopentane (8g)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and propargyl bromide (1.1 mmol, ω = 0.80, 118 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 125 mg (0.65 mmol, 65%) of colorless oil. [α]Dr.t. = +17.9 (0.19, CH2Cl2). νmax 3306, 2958, 2866, 1651, 1463, 1361, 1093, 1012, 878, 626 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.26–1.34 (m, 1H), 1.37–1.45 (m, 1H), 1.52–1.63 (m, 1H), 1.74–1.81 (m, 1H), 1.82–1.89 (m, 1H), 2.23–2.36 (m, 1H), 2.43 (t, J = 2.4 Hz, 1H), 2.43–2.51 (m, 1H), 3.53 (dt, J = 7.4, 8.9 Hz, 1H), 3.60 (ddd, J = 5.1, 8.1, 9.0 Hz, 1H), 4.10–4.21 (m, 2H), 4.73–4.81 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.6, 28.3, 29.9, 30.8, 44.1, 47.1, 58.2, 69.8, 74.3, 80.1, 103.1, 162.5.
3.36. Synthesis of (R)-((2-(2,2-dimethyl-3-methylenecyclopentyl)ethoxy)methyl)benzene (8h)
Following GP4. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (1.0 mmol, 154 mg), THF (3 mL), NaH (1.2 mmol; ω = 60%, 48.0 mg) and benzyl bromide (1.1 mmol, ω = 0.98, 134 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 209 mg (0.85 mmol, 85%) of colorless oil. [α]Dr.t. = +14.0 (0.23, CH2Cl2). EI-HRMS: m/z = 245.1898 (MH+); C17H25O: requires m/z = 245.1900 (MH+); νmax 3067, 2958, 2864, 1651, 1496, 1454, 1361, 1202, 1100, 1028, 1000, 877, 733, 696, 611 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 1.06 (s, 3H), 1.20–1.34 (m, 1H), 1.36–1.47 (m, 1H), 1.59–1.60 (m, 1H), 1.75–1.86 (m, 2H), 2.27–2.28 (m, 1H), 2.39–2.50 (m, 1H), 3.45–3.51 (m, 1H), 3.52–3.60 (m, 1H), 4.49 (d, J = 11.8 Hz, 1H), 4.53 (d, J = 11.9 Hz, 1H), 4.73–4.79 (m, 2H), 7.25–7.31 (m, 1H), 7.32–7.39 (m, 4H). 13C-NMR (126 MHz, CDCl3): δ 23.6, 26.6, 28.4, 30.1, 30.9, 44.1, 47.2, 70.1, 73.1, 103.0, 127.6, 127.8, 128.5, 138.7, 162.6.
3.37. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9)
To a solution of (+)-isocampholenic acid (3) (10 mmol, 1.68 g) in anhydrous CH2Cl2 (50 mL) under argon at room temperature was added CDI (12 mmol, ω = 0.95, 2.05 g). The resulting reaction mixture was stirred at room temperature for 2 h, then N,O-dimethylhydroxylamine hydrochloride (30 mmol, 2.93 g) and Et3N (30 mmol, 4.18 mL) were added. After stirring the reaction mixture at room temperature for another 20 h, the volatile components were evaporated in vacuo. The residue was dissolved in EtOAc (150 mL) and washed with NaHSO4 (aq., 1 M, 2 × 50 mL) and NaCl (aq. sat., 2 × 50 mL). The organic phase was dried under anhydrous Na2SO4, filtered, and the volatile components evaporated in vacuo. Yield: 1.80 g (8.5 mmol, 85%) of white solid. [α]Dr.t. = +21.0 (0.30, CH2Cl2). EI-HRMS: m/z = 212.1606 (MH+); C12H22NO2: requires m/z = 212.1612 (MH+); νmax 2959, 1662, 1462, 1436, 1413, 1384, 1362, 1178, 1114, 1002, 935, 878, 785, 969 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.87 (s, 3H), 1.08 (s, 3H), 1.29–1.40 (m, 1H), 1.90–1.98 (m, 1H), 2.02–2.10 (m, 1H), 2.21–2.38 (m, 2H), 2.41–2.52 (m, 2H), 3.19 (s, 3H), 3.69 (s, 3H), 4.78 (t, J = 2.5 Hz, 1H), 4.79 (t, J = 2.0 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.7, 26.7, 28.6, 30.6, 32.3, 32.6, 44.0, 46.2, 61.3, 103.5, 161.8, 174.6.
3.38. Synthesis of Ketones 11 from Weinreb Amide 10—General Procedure 5 (GP5)
To a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (1.0 equiv.) in anhydrous Et2O (7 mL) under argon at 0 °C, the corresponding Grignard reagent (1.5 equiv.) was added slowly. The resulting reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 20 h. The excess Grignard reagent was quenched with NaCl (aq. sat., 3 mL), and the resulting mixture was extracted with Et2O (3 × 10 mL). The combined organic phase was washed with NaCl (aq. sat., 3 × 10 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product 10 were combined, and the volatiles evaporated in vacuo. The isolated ketones 10 were fully characterized.
3.39. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)propan-2-one (10a)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and methylmagnesium bromide (2.0 M in Et2O, 3.0 mmol, 1.5 mL); column chromatography: EtOAc/petroleum ether = 1:10. Yield: 278 mg (1.67 mmol, 83%) of colorless oil. [α]Dr.t. = +15.0 (0.22, CH2Cl2). EI-HRMS: m/z = 167.1430 (MH+); C11H19O: requires m/z = 167.1430 (MH+); νmax 3071, 2960, 1712, 1651, 1463, 1434, 1362, 1285, 1241, 1170, 1148, 962, 878, 704 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.83 (s, 3H), 1.06 (s, 3H), 1.21–1.30 (m, 1H), 1.86–1.94 (m, 1H), 1.97–2.04 (m, 1H), 2.17 (s, 3H), 2.25 (dd, J = 10.5, 15.9 Hz, 1H), 2.29–2.38 (m, 1H), 2.40–2.48 (m, 1H), 2.50 (dd, J = 3.6, 15.9 Hz, 1H), 4.77 (t, J = 2.5 Hz, 1H), 4.79 (td, J = 0.8, 2.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.7, 26.6, 28.4, 30.5, 30.6, 43.9, 44.6, 45.6, 103.6, 161.3, 209.2.
3.40. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)butan-2-one (10b) [34]
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and ethylmagnesium bromide (3.0 M in Et2O, 3.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:10. Yield: 314 mg (1.74 mmol, 87%) of colorless oil. [α]Dr.t. = +14.0 (0.23, CH2Cl2). EI-HRMS: m/z = 181.1588 (MH+); C12H21O: requires m/z = 181.1587 (MH+); νmax 3071, 2960, 1712, 1651, 1460, 1413, 1376, 1363, 1283, 1151, 1114, 1021, 986, 878, 806, 705, 635 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.83 (s, 3H), 1.02–1.10 (m, 6H), 1.19–1.30 (m, 1H), 1.83–1.91 (m, 1H), 1.97–2.05 (m, 1H), 2.24 (dd, J = 10.6, 15.7 Hz, 1H), 2.29–2.38 (m, 1H), 2.40–2.54 (m, 4H), 4.77 (t, J = 2.5 Hz, 1H), 4.79 (t, J = 2.3 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 8.0, 23.7, 26.6, 28.5, 30.6, 36.5, 43.2, 43.9, 45.7, 103.6, 161.4, 211.8.
3.41. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)pentan-2-one (10c)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and propylmagnesium bromide (2.0 M in Et2O, 3.0 mmol, 1.5 mL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 315 mg (1.62 mmol, 81%) of colorless oil. [α]Dr.t. = +9.0 (0.26, CH2Cl2). EI-HRMS: m/z = 195.1743 (MH+); C13H23O: requires m/z = 195.1743 (MH+); νmax 3071, 2960, 2873, 1711, 1651, 1462, 1409, 1363, 1285, 1198, 1125, 1026, 878, 740, 63 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 0.92 (t, J = 7.4 Hz, 3H), 1.06 (s, 3H), 1.19–1.30 (m, 1H), 1.61 (h, J = 7.3 Hz, 2H), 1.84–1.92 (m, 1H), 1.96–2.05 (m, 1H), 2.23 (dd, J = 10.6, 15.7 Hz, 1H), 2.28–2.49 (m, 5H), 4.77 (t, J = 2.5 Hz, 1H), 4.79 (t, J = 2.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 13.9, 17.4, 23.8, 26.6, 28.5, 30.6, 43.6, 43.9, 45.4, 45.6, 103.6, 161.4, 211.4.
3.42. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)hexan-2-one (10d)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and butylmagnesium chloride (2.0 M in Et2O, 3.0 mmol, 1.5 mL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 369 mg (1.77 mmol, 88%) of colorless oil. [α]Dr.t. = +9.0 (0.21, CH2Cl2). EI-HRMS: m/z = 209.1897 (MH+); C14H25O: requires m/z = 209.1900 (MH+); νmax 2958, 2871, 1712, 1651, 1463, 1435, 1409, 1377, 1363, 1285, 1198, 1126, 1035, 878, 732, 634 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 3H), 0.91 (t, J = 7.4 Hz, 3H), 1.05 (s, 3H), 1.18–1.36 (m, 3H), 1.52–1.60 (m, 2H), 1.84–1.91 (m, 1H), 1.97–2.05 (m, 1H), 2.23 (dd, J = 10.6, 15.7 Hz, 1H), 2.28–2.49 (m, 5H), 4.77 (t, J = 2.6 Hz, 1H), 4.79 (t, J = 2.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.0, 22.5, 23.8, 26.1, 26.6, 28.5, 30.6, 43.2, 43.6, 43.9, 45.6, 103.6, 161.4, 211.5.
3.43. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)-3-methylbutan-2-one (10e)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and isopropylmagnesium bromide (2.0 M in Et2O, 3.0 mmol, 1.5 mL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 327 mg (1.68 mmol, 84%) of colorless oil. [α]Dr.t. = +8.0 (0.24, CH2Cl2). EI-HRMS: m/z = 195.1740 (MH+); C13H23O: requires m/z = 195.1743 (MH+); νmax 2961, 2871, 1710, 1652, 1464, 1382, 1363, 1286, 1198, 1152, 1089, 1028, 878, 793, 703 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 1.06 (s, 3H), 1.10 (d, J = 7.0 Hz, 6H), 1.17–1.27 (m, 1H), 1.83–1.91 (m, 1H), 1.99–2.07 (m, 1H), 2.26–2.38 (m, 2H), 2.39–2.45 (m, 1H), 2.48 (dd, J = 3.5, 16.1 Hz, 1H), 2.63 (hept, J = 6.9 Hz, 1H), 4.77 (d, J = 2.5 Hz, 1H), 4.79 (td, J = 0.8, 2.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 18.3, 18.4, 23.8, 26.6, 28.6, 30.6, 41.2, 41.3, 43.9, 45.4, 103.5, 161.5, 214.9.
3.44. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)pent-4-en-2-one (10f)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and allylmagnesium bromide (1.0 M in THF, 3.0 mmol, 3.0 mL); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 319 mg (1.66 mmol, 83%) of colorless oil. [α]Dr.t. = +10.0 (0.25, CH2Cl2). EI-HRMS: m/z = 193.1563 (MH+); C13H21O: requires m/z = 193.1587 (MH+); νmax 3073, 2960, 1714, 1651, 1463, 1434, 1363, 1332, 1290, 1198, 1113, 1039, 992, 918, 878, 706, 663 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.83 (s, 3H), 1.05 (s, 3H), 1.18–1.30 (m, 1H), 1.83–1.94 (m, 1H), 1.97–2.06 (m, 1H), 2.27 (dd, J = 10.5, 16.0 Hz, 1H), 2.31–2.39 (m, 1H), 2.40–2.48 (m, 1H), 2.51 (dd, J = 3.5, 16.0 Hz, 1H), 3.14–3.25 (m, 2H), 4.77 (t, J = 2.5 Hz, 1H), 4.79 (td, J = 0.8, 2.2 Hz, 1H), 5.11–5.23 (m, 2H), 5.93 (ddt, J = 7.0, 10.2, 17.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.7, 26.6, 28.5, 30.6, 43.2, 43.9, 45.4, 48.3, 103.6, 118.9, 130.8, 161.3, 208.8.
3.45. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)-3-phenylpropan-2-one (10g)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and benzylmagnesium bromide (1.0 M in THF, 3.0 mmol, 3.0 mL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 378 mg (1.56 mmol, 78%) of colorless oil. [α]Dr.t. = +4.0 (0.26, CH2Cl2). EI-HRMS: m/z = 243.1745 (MH+); C17H23O: requires m/z = 243.1743 (MH+); νmax 3066, 3029, 2959, 1712, 1651, 1602, 1495, 1454, 1434, 1363, 1286, 1200, 1108, 1031, 879, 748, 697 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.77 (s, 3H), 1.01 (s, 3H), 1.09–1.22 (m, 1H), 1.79–1.89 (m, 1H), 1.95–2.06 (m, 1H), 2.24–2.34 (m, 2H), 2.36–2.44 (m, 1H), 2.51 (dd, J = 3.6, 16.0 Hz, 1H), 3.71 (s, 2H), 4.75 (t, J = 2.5 Hz, 1H), 4.77 (t, J = 2.2 Hz, 1H), 7.17–7.23 (m, 2H), 7.25–7.28 (m, 1H), 7.30–7.36 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.7, 26.6, 28.4, 30.6, 42.8, 43.9, 45.5, 50.6, 103.6, 127.1, 128.8, 129.6, 134.3, 161.3, 208.4.
3.46. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)but-3-en-2-one (10h)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and vinylmagnesium bromide (1.0 M in THF, 3.0 mmol, 3.0 mL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 267 mg (1.50 mmol, 75%) of colorless oil. [α]Dr.t. = +5.0 (0.24, CH2Cl2). EI-HRMS: m/z = 179.1424 (MH+); C12H19O: requires m/z = 179.1430 (MH+); νmax 3071, 2959, 1681, 1651, 1614, 1463, 1434, 1399, 1363, 1332, 1292, 1194, 1149, 1112, 1068, 985, 961, 915, 878, 809, 634 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.86 (s, 3H), 1.08 (s, 3H), 1.22–1.34 (m, 1H), 1.83–1.93 (m, 1H), 1.99–2.09 (m, 1H), 2.28–2.50 (m, 3H), 2.65 (dd, J = 3.6, 15.5 Hz, 1H), 4.78 (t, J = 2.5 Hz, 1H), 4.80 (td, J = 0.8, 2.2 Hz, 1H), 5.83 (dd, J = 1.1, 10.6 Hz, 1H), 6.23 (dd, J = 1.1, 17.6 Hz, 1H), 6.38 (dd, J = 10.6, 17.6 Hz, 1H). 13C-NMR (75 MHz, CDCl3): δ 23.8, 26.7, 28.5, 30.6, 40.7, 44.1, 46.0, 103.7, 128.1, 136.9, 161.4, 201.0.
3.47. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)but-3-yn-2-one (10i)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and ethynylmagnesium bromide (0.5 M in THF, 3.0 mmol, 6.0 mL); column chromatography: EtOAc/petroleum ether = 1:40. Yield: 282 mg (1.60 mmol, 80%) of colorless oil. [α]Dr.t. = +12.0 (0.297, CH2Cl2). νmax 3258, 2960, 2092, 1678, 1652, 1463, 1435, 1364, 1285, 1237, 1198, 1112, 1070, 880, 807, 694, 649 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.85 (s, 3H), 1.09 (s, 3H), 1.20–1.38 (m, 1H), 1.88–1.98 (m, 1H), 2.08–2.18 (m, 1H), 2.30–2.51 (m, 3H), 2.67 (dd, J = 3.9, 15.6 Hz, 1H), 3.23 (s, 1H), 4.79 (t, J = 2.5 Hz, 1H), 4.80–4.82 (m, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.7, 26.7, 28.2, 30.5, 44.0, 45.7, 46.6, 78.6, 81.8, 103.9, 160.8, 187.5.
3.48. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)pent-3-yn-2-one (10j)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and 1-propynylmagnesium bromide (0.5 M in THF, 3.0 mmol, 6.0 mL); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 263 mg (1.38 mmol, 69%) of colorless oil. [α]Dr.t. = +6.0 (0.243, CH2Cl2). EI-HRMS: m/z = 191.1430 (MH+); C13H19O: requires m/z = 191.1430 (MH+); νmax 2960, 2218, 1669, 1463, 1435, 1364, 1330, 1286, 1258, 1175, 1143, 1021, 972, 879, 783, 680 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 3H), 1.08 (s, 3H), 1.18–1.41 (m, 1H), 1.85–1.98 (m, 1H), 2.01–2.03 (m, 3H), 2.05–2.18 (m, 1H), 2.24–2.53 (m, 3H), 2.61 (dd, J = 3.7, 15.2 Hz, 1H), 4.74–4.84 (m, 2H). 13C-NMR (75 MHz, CDCl3): δ 4.2, 23.7, 26.7, 28.3, 30.6, 44.0, 45.9, 46.6, 80.6, 90.0, 103.7, 161.2, 188.2.
3.49. Synthesis of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-1-phenylethan-1-one (10k)
Following GP5. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (2.0 mmol, 422 mg), Et2O (7 mL), and phenylmagnesium bromide (3.0 M in THF, 3.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:30. Yield: 374 mg (1.64 mmol, 82%) of colorless oil. [α]Dr.t. = +6.0 (0.25, CH2Cl2). EI-HRMS: m/z = 229.1594 (MH+); C16H21O: requires m/z = 229.1587 (MH+); νmax 3068, 2959, 1682, 1651, 1597, 1580, 1462, 1448, 1363, 1333, 1286, 1205, 1180, 1147, 1075, 1001, 878, 750, 688, 646, 616 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.93 (s, 3H), 1.13 (s, 3H), 1.28–1.39 (m, 1H), 1.87–1.97 (m, 1H), 2.21–2.21 (m, 1H), 2.28–2.40 (m, 1H), 2.41–2.51 (m, 1H), 2.78 (dd, J = 10.5, 15.7 Hz, 1H), 3.06 (dd, J = 3.5, 15.7 Hz, 1H), 4.77–4.83 (m, 2H), 7.46–7.46 (m, 2H), 7.53–7.60 (m, 1H), 7.93–8.00 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.9, 26.8, 28.6, 30.6, 39.4, 44.2, 46.2, 103.6, 128.3, 128.7, 133.1, 137.4, 161.5, 200.6.
3.50. Synthesis of Secondary Alcohols 11 from Aldehyde 5—General Procedure 6 (GP6)
To a solution of aldehyde 5 (1.0 equiv.) in anhydrous Et2O (3 mL) under argon at 0 °C, the corresponding Grignard reagent (1.4 equiv.) was added slowly. The resulting reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 20 h. The excess Grignard reagent was quenched with NaCl (aq. sat., 3 mL), and the resulting mixture was extracted with Et2O (3 × 10 mL). The combined organic phase was washed with NaCl (aq. sat., 2 × 5 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified/separated by column chromatography (Silica gel 60). The fractions containing the pure product 11 were combined, and the volatile components were evaporated in vacuo. The isolated secondary alcohols 11 were fully characterized. The two diastereomers formed could, in most cases, be partially separated by column chromatography.
3.51. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)propan-2-ol (11a/11a′)
Following GP6. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (91.3 mg, 0.6 mmol), Et2O (3 mL), and methylmagnesium bromide (3.0 M in Et2O, 0.84 mmol, 0.28 mL); column chromatography: EtOAc/petroleum ether = 1:5. The two diastereomers formed could be partially separated by column chromatography. Diastereomeric ratio: 11a/11a′ = 54:46. Diastereomer 11a (major): Elutes first from the column. Yield: 20 mg (0.119 mmol, 20%) of colorless oil. [α]Dr.t. = +37.1 (0.11, CH2Cl2). EI-HRMS: m/z = 169.1586 (MH+); C11H21O: requires m/z = 169.1587 (MH+); νmax 3351, 2960, 2929, 1711, 1461, 1375, 1363, 1068, 877 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.74 (s, 3H), 0.99 (s, 3H), 1.16 (d, J = 6.2 Hz, 3H), 1.13–1.24 (m, 2H), 1.32 (br s, 1H), 1.38–1.47 (m, 1H), 1.60–1.70 (m, 1H), 1.79–1.89 (m, 1H), 2.18–2.30 (m, 1H), 2.34–2.44 (m, 1H), 3.75–3.84 (m, 1H), 4.67–4.73 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 24.9, 26.5, 28.2, 30.8, 39.7, 44.0, 46.4, 66.7, 103.0, 162.5. Diastereomer 11a′ (minor): Elutes secondly from the column. Yield: 15 mg (0.089 mmol, 15%; contains 24% of 11a) of colorless oil. 1H-NMR (500 MHz, CDCl3): δ 0.74 (s, 3H), 1.16 (d, J = 6.1 Hz, 3H) (the remaining signals overlap with the signals of diastereomer 11a).
3.52. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)pent-4-en-2-ol (11b/11b′)
Following GP6. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (91.3 mg, 0.6 mmol), Et2O (3 mL), and allylmagnesium bromide (1.0 M in THF, 0.84 mmol, 0.84 mL); column chromatography: EtOAc/petroleum ether = 1:10. The two diastereomers formed could be partially separated by column chromatography. Diastereomeric ratio: 11b/11b′ = 48:53. Diastereomer 11b (minor): Elutes first from the column. Yield: 32 mg (0.1647 mmol, 27%) of colorless oil. [α]Dr.t. = +21.0 (0.215, CH2Cl2). EI-HRMS: m/z = 195.1745 (MH+); C13H23O: requires m/z = 195.1743 (MH+); νmax 3366, 3073, 2958, 1710, 1462, 1434, 1362, 1067, 1016, 913, 877 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.74 (s, 3H), 1.00 (s, 3H), 1.13–1.26 (m, 2H), 1.39–1.53 (m, 2H), 1.67–1.77 (m, 1H), 1.80–1.89 (m, 1H), 2.07–2.17 (m, 1H), 2.19–2.31 (m, 2H), 2.34–2.44 (m, 1H), 3.60–3.68 (m, 1H), 4.67–4.73 (m, 2H), 5.04–5.12 (m, 2H), 5.72–5.84 (m, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 26.5, 28.1, 30.8, 37.2, 43.3, 44.0, 46.2, 69.2, 103.1, 118.4, 135.0, 162.6. Diastereomer 11b′ (major): Elutes secondly from the column. Yield: 30 mg (0.154 mmol, 25%; contains 19% of 11b) of colorless oil. 1H-NMR (500 MHz, CDCl3): δ 0.74 (s, 3H), 0.99 (s, 3H), 1.24–1.37 (m, 2H), 1.41–1.54 (m, 2H), 1.60 (s, 1H), 1.79–1.87 (m, 1H), 1.99–2.07 (m, 1H), 2.19–2.27 (m, 1H), 2.27–2.35 (m, 1H), 2.36–2.44 (m, 1H), 3.60–3.70 (m, 1H), 4.66–4.73 (m, 2H), 5.03–5.12 (m, 2H), 5.71–5.83 (m, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.4, 26.5, 29.0, 30.9, 37.2, 41.7, 44.3, 47.5, 70.4, 103.1, 118.6, 134.8, 162.2.
3.53. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)-3-methylbutan-2-ol (11c/11c′)
Following GP6. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (91.3 mg, 0.6 mmol), Et2O (3 mL), and isopropylmagnesium bromide (2.0 M in Et2O, 1.4 mmol, 0.7 mL); column chromatography: EtOAc/petroleum ether = 1:10. The two diastereomers formed could not be separated by column chromatography. Diastereomeric ratio: 11c/11c′ = 53:47. Yield: 104 mg (0.1647 mmol, 88%) of colorless oil. [α]Dr.t. = +25.1 (0.28, CH2Cl2). EI-HRMS: m/z = 197.1904 (MH+); C13H25O: requires m/z = 197.1900 (MH+); νmax 3376, 2958, 2870, 1651, 1464, 1362, 1065, 994, 976, 877 cm−1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.82 (d, J = 3.8 Hz, 3H), 0.87–1.00 (m, 6H), 1.07 (d, J = 3.8 Hz, 3H), 1.18–1.31 (m, 2H), 1.34–1.47 (m, 1H), 1.51–1.58 (m, 1H), 1.60–1.81 (m, 2H), 1.86–1.96 (m, 1H), 2.23–2.37 (m, 1H), 2.47–2.47 (m, 1H), 3.37–3.55 (m, 1H), 4.74–4.80 (m, 2H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 15.8, 17.6, 18.9, 19.4, 23.5, 23.6, 26.5, 26.7, 28.0, 29.4, 30.7, 31.0, 32.8, 34.3, 34.6, 34.9, 44.0, 44.4, 46.3, 48.0, 75.1, 76.5, 103.0, 103.1, 162.5, 162.7.
3.54. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)but-3-yn-2-ol (11d/11d′)
Following GP6. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (91.3 mg, 0.6 mmol), Et2O (3 mL), and ethynylmagnesium bromide (0.5 M in THF, 0.84 mmol, 1.68 mL); column chromatography: EtOAc/petroleum ether = 1:7. The two diastereomers formed could be partially separated by column chromatography. Diastereomeric ratio: 11d/11d′ = 54:46. Diastereomer 11d (major): Elutes first from the column. Yield: 26 mg (0.146 mmol, 24%) of colorless oil. [α]Dr.t. = +19.7 (0.14, CH2Cl2). EI-HRMS: m/z = 179.1432 (MH+); C12H19O: requires m/z = 179.143 (MH+); νmax 3348, 3073, 2965, 1732, 1327, 1181, 1045, 892 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.76 (s, 3H), 1.01 (s, 3H), 1.21–1.33 (m, 1H), 1.45–1.56 (m, 1H), 1.68–1.83 (m, 3H), 1.84–1.94 (m, 1H), 2.19–2.31 (m, 1H), 2.35–2.46 (m, 2H), 4.33–4.39 (m, 1H), 4.68–4.74 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.57, 26.47, 28.17, 30.79, 38.57, 44.03, 45.97, 61.35, 72.92, 85.64, 103.29, 162.11. Diastereomer 11d′ (minor): Elutes secondly from the column. Yield: 46 mg (0.258 mmol, 43%; contains 17% of 11d) of colorless oil. 1H-NMR (500 MHz, CDCl3): δ 0.76 (s, 3H), 1.02 (s, 3H), 1.22–1.32 (m, 1H), 1.50–1.58 (m, 1H), 1.61–1.68 (m, 1H), 1.70–1.77 (m, 1H), 1.79–1.88 (m, 1H), 1.96 (s, 1H), 2.19–2.31 (m, 1H), 2.35–2.48 (m, 2H), 4.32–4.39 (m, 1H), 4.68–4.76 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.6, 26.3, 28.3, 30.8, 38.4, 44.1, 46.9, 62.4, 73.4, 84.9, 103.3, 161.9.
3.55. Synthesis of Tertiary Alcohols 12 from Ketones 10—General Procedure 7 (GP7)
To a solution of ketone 10 (1.0 equiv.) in anhydrous Et2O (4 mL) under argon at 0 °C, Grignard reagent (2.0 equiv.) was added slowly. The resulting reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 2 h. The excess Grignard reagent was quenched with NaCl (aq. sat., 3 mL), and the resulting mixture was extracted with Et2O (3 × 10 mL). The combined organic phase was washed with NaCl (aq. sat., 3 × 10 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product 12 were combined, and the volatiles evaporated in vacuo. The isolated tertiary alcohols 12 were fully characterized (as mixtures of two diastereomers).
3.56. Synthesis of (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)-2-methylpropan-2-ol (12a)
Following GP7. Prepared from (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)propan-2-one (10a) (1.0 mmol, 166 mg), Et2O (4 mL), and methylmagnesium bromide (2.0 M in Et2O, 2.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:2. Yield: 144 mg (0.79 mmol, 79%) of colorless oil. [α]Dr.t. = +21.0 (0.13, CH2Cl2). EI-HRMS: m/z = 165.1637 (M-OH)H+; C12H21: requires m/z = 165.1638 (M-OH)H+; νmax 3380, 3071, 2961, 1652, 1465, 1377, 1362, 1189, 1141, 1047, 955, 905, 877, 770 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.78 (s, 3H), 1.04 (s, 3H), 1.24 (s, 3H), 1.25 (s, 3H), 1.31–1.41 (m, 2H), 1.52–1.63 (m, 2H), 1.97–2.06 (m, 1H), 2.24–2.37 (m, 1H), 2.43–2.53 (m, 1H), 4.74–4.79 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 23.2, 25.7, 29.7, 30.4, 30.6, 31.0, 44.0, 44.7, 46.1, 71.5, 103.0, 161.9.
3.57. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)-2-methylbutan-2-ol (12b/12b′)
Following GP7. Prepared from (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)butan-2-one (10b) (1.0 mmol, 180 mg), Et2O (4 mL), and methylmagnesium bromide (2.0 M in Et2O, 2.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:1. The two diastereomers formed could not be separated by column chromatography. Diastereomeric ratio: 60:40. Yield: 159 mg (0.81 mmol, 81%) of colorless oil. [α]Dr.t. = +26.0 (0.13, CH2Cl2). EI-HRMS: m/z = 179.1796 (M-OH)H+; C13H23: requires m/z = 179.1794 (M-OH)H+; νmax 3394, 3070, 2961, 2927, 1652, 1461, 1377, 1362, 1297, 1138, 1054, 996, 920, 877, 849, 787, 756 cm−1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.78 (s, 3H), 0.91 (q, J = 7.6 Hz, 3H), 1.04 (s, 3H), 1.17 (d, J = 4.4 Hz, 3H), 1.30–1.45 (m, 2H), 1.50–1.62 (m, 4H), 1.96–2.06 (m, 1H), 2.24–2.35 (m, 1H), 2.43–2.53 (m, 1H), 4.74–4.80 (m, 2H) (one signal missing). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 8.3, 8.6, 23.2, 23.2, 25.8, 25.8, 26.7, 27.6, 30.3, 30.4, 31.0, 34.5, 35.5, 41.5, 41.6, 44.8, 66.8, 45.5, 45.6, 73.3, 73.4, 103.0, 161.9, 162.0.
3.58. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)-2-methylpentan-2-ol (12c/12c′)
Following GP7. Prepared from (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)pentan-2-one (10c) (1.0 mmol, 194 mg), Et2O (4 mL), and methylmagnesium bromide (2.0 M in Et2O, 2.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:5. The two diastereomers formed could not be separated by column chromatography. Diastereomeric ratio: 60:40. Yield: 162 mg (0.77 mmol, 77%) of colorless oil. [α]Dr.t. = +13.0 (0.13, CH2Cl2). EI-HRMS: m/z = 193.1952 (M-OH)H+; C14H25: requires m/z = 193.1951 (M-OH)H+; νmax 3411, 3070, 2958, 2932, 2872, 1745, 1652, 1464, 1376, 1362, 1291, 1239, 1139, 1079, 1050, 1005, 929, 877, 775, 743 cm−1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.78 (s, 3H), 0.93 (t, J = 7.2 Hz, 3H), 1.03 (s, 3H), 1.15 (d, J = 5.0 Hz, 1H), 1.18 (d, J = 4.6 Hz, 3H), 1.29–1.59 (m, 8H), 1.95–2.04 (m, 1H), 2.24–2.36 (m, 1H), 2.43–2.53 (m, 1H), 4.74–4.79 (m, 2H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 14.8, 14.8, 17.2, 17.5, 23.2, 23.2, 25.7, 25.8, 27.3, 28.1, 30.3, 30.4, 31.0, 42.0, 42.1, 44.6, 44.8, 44.8, 45.5, 45.6, 45.6, 73.2, 73.3, 103.0, 161.9, 162.0.
3.59. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)-2-methylhexan-2-ol (12d/12d′)
Following GP7. Prepared from (R)-1-(2,2-Dimethyl-3-methylenecyclopentyl)hexan-2-one (10d) (1.0 mmol, 208 mg), Et2O (4 mL), and methylmagnesium bromide (2.0 M in Et2O, 2.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:5. The two diastereomers formed could not be separated by column chromatography. Diastereomeric ratio: 59:41. Yield: 153 mg (0.68 mmol, 68%) of colorless oil. [α]Dr.t. = +20.0 (0.25, CH2Cl2). EI-HRMS: m/z = 207.2105 (M-OH)H+; C15H27: requires m/z = 207.2107 (M-OH)H+; νmax 3404, 2958, 2931, 2869, 1652, 1462, 1376, 1362, 1297, 1139, 1085, 1025, 936, 904, 877, 776, 730 cm−1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.78 (s, 3H), 0.89–0.95 (m, 3H), 1.04 (s, 3H), 1.14 (d, J = 6.0 Hz, 1H), 1.18 (d, J = 4.6 Hz, 3H), 1.28–1.60 (m, 10H), 1.96–2.04 (m, 1H), 2.24–2.37 (m, 1H), 2.43–2.53 (m, 1H), 4.74–4.79 (m, 2H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 14.3, 23.2, 23.2, 23.4, 25.8, 25.8, 26.2, 26.4, 27.3, 28.2, 30.4, 30.4, 31.0, 41.9, 42.0, 42.0, 43.0, 44.8, 44.8, 45.5, 45.6, 73.2, 73.2, 103.0, 161.9, 162.0.
3.60. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)-2-methylpent-4-en-2-ol (12e/12e′)
Following GP7. Prepared from (R)-1-(2,2-dimethyl-3-methylenecyclopentyl)pent-4-en-2-one (10f) (1.0 mmol, 192 mg), Et2O (4 mL), and methylmagnesium bromide (2.0 M in Et2O, 2.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:10. The two diastereomers formed could not be separated by column chromatography. Diastereomeric ratio: 54:46. Yield: 148 mg (0.71 mmol, 71%) of colorless oil. [α]Dr.t. = +4.5 (0.105, CH2Cl2). EI-HRMS: m/z = 191.1791 (M-OH)H+; C14H24O: requires m/z = 191.1794 (M-OH)H+; νmax 3428, 3073, 2960, 2927, 1651, 1461, 1434, 1376, 1362, 1293, 1114, 998, 913, 877, 781, 709, 632 cm−1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.78 (s, 3H), 1.04 (s, 3H), 1.20 (d, J = 5.3 Hz, 3H), 1.29–1.42 (m, 3H), 1.54–1.65 (m, 2H), 1.96–2.08 (m, 1H), 2.23–2.35 (m, 3H), 2.42–2.54 (m, 1H), 4.74–4.79 (m, 2H), 5.09–5.20 (m, 2H), 5.80–5.95 (m, 1H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 23.2, 23.2, 25.8, 25.8, 27.0, 28.0, 30.3, 30.4, 31.0, 42.1, 42.1, 44.8, 44.9, 45.5, 45.7, 46.7, 47.6, 72.5, 72.6, 103.0, 103.0, 119.0, 119.1, 134.1, 134.2, 161.8, 161.9.
3.61. Synthesis of 1-((R)-2,2-dimethyl-3-methylenecyclopentyl)-2-phenylpropan-2-ol (12f/12f′)
Following GP7. Prepared from (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-1-phenylethan-1-one (10k) (1.0 mmol, 228 mg), Et2O (4 mL), and methylmagnesium bromide (2.0 M in Et2O, 2.0 mmol, 1.0 mL); column chromatography: EtOAc/petroleum ether = 1:10. The two diastereomers formed could not be separated by column chromatography. Diastereomeric ratio: 57:43. Yield: 178 mg (0.73 mmol, 73%) of colorless oil. [α]Dr.t. = +12.0 (0.11, CH2Cl2). EI-HRMS: m/z = 227.1798 (M-OH)H+; C17H23: requires m/z = 227.1794 (M-OH)H+; νmax 3439, 3065, 2959, 1651, 1494, 1446, 1362, 1111, 1068, 1028, 936, 910, 877, 848, 765, 723, 699 cm−1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.75 (s, 1.5H), 0.76 (s, 1.5H), 0.88 (s, 1.5H), 0.98 (s, 1.5H), 1.02–1.13 (m, 0.5H), 1.23–1.39 (m, 1.5H), 1.48–1.55 (m, 0.5H), 1.59 (d, J = 3.5 Hz, 3H), 1.63–1.75 (m, 2.5H), 1.90 (dd, J = 1.8, 14.0 Hz, 0.5H), 1.98 (dd, J = 1.3, 14.4 Hz, 0.5H), 2.07–2.20 (m, 1H), 2.27–2.43 (m, 1H), 4.66–4.74 (m, 2H), 7.19–7.26 (m, 1H), 7.30–7.36 (m, 2H), 7.39–7.47 (m, 2H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 23.3, 23.3, 25.4, 25.6, 29.7, 29.9, 30.8, 30.9, 30.9, 31.6, 44.6, 44.7, 44.7, 44.9, 45.5, 45.8, 74.7, 75.4, 102.8, 102.9, 124.9, 125.0, 126.6, 126.7, 128.2, 128.2, 148.0, 148.6, 161.8, 161.9.
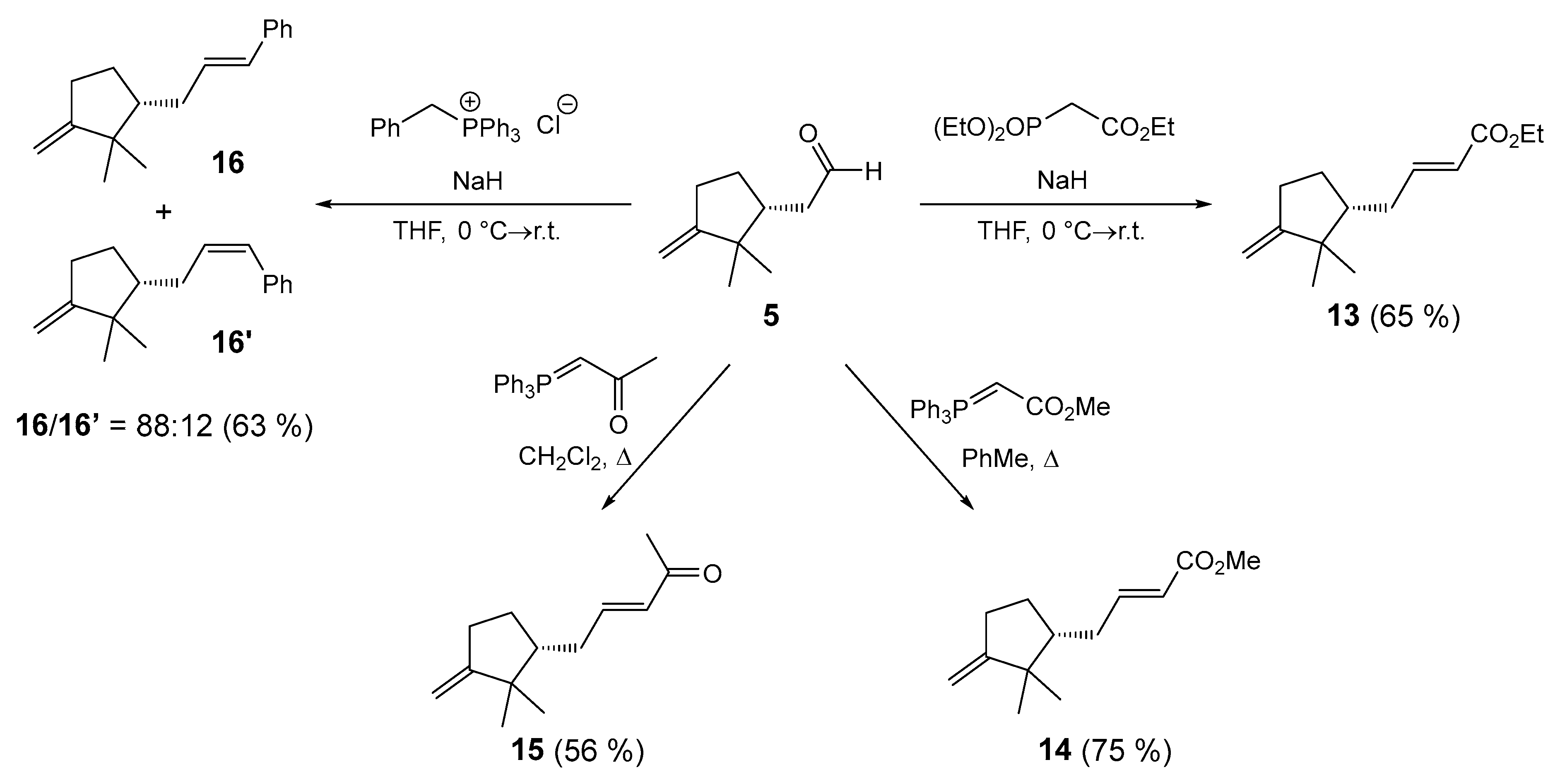
3.62. Synthesis of Ethyl-(R,E)-4-(2,2-dimethyl-3-methylenecyclopentyl)but-2-enoate (13)
To a suspension of NaH (0.825 mmol, ω = 0.60, 33 mg) in anhydrous THF (2 mL) under argon at 0 °C, triethyl phosphonoacetate (0.55 mmol, 110 μL) was added. The resulting reaction mixture was stirred at 0 °C for 45 min, then a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (84 mg, 0.55 mmol) in anhydrous THF (1.5 mL) was added. The reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 20 h. The excess NaH was quenched with NaCl (aq. sat., 3 mL), and the mixture was extracted with EtOAc (3 × 10 mL). The combined organic phase was washed with NaCl (aq. sat., 2 × 5 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60; EtOAc/petroleum ether = 1:30). The fractions containing the pure product 13 were combined, and the volatiles were evaporated in vacuo. Yield: 80 mg (0.360 mmol, 65%) of colorless oil. [α]Dr.t. = +8.8 (0.13, CH2Cl2). EI-HRMS: m/z = 223.1691 (MH+); C14H23O2: requires m/z = 223.1693 (MH+); νmax 2958, 1719, 1653, 1463, 1184, 1145, 1043, 978, 878 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.78 (s, 3H), 1.01 (s, 3H), 1.14–1.28 (m, 1H), 1.22 (t, J = 7.2 Hz, 3H), 1.52–1.62 (m, 1H), 1.72–1.82 (m, 1H), 1.87–1.97 (m, 1H), 2.17–2.32 (m, 2H), 2.33–2.43 (m, 1H), 4.12 (q, J = 7.1 Hz, 2H), 4.70 (t, J = 2.5 Hz, 1H), 4.72 (dt, J = 1.1, 2.1 Hz, 1H), 5.77 (dt, J = 1.5, 15.6 Hz, 1H), 6.90 (ddd, J = 6.9, 7.9, 15.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.4, 23.5, 27.0, 28.2, 30.6, 33.2, 44.2, 49.5, 60.3, 103.6, 122.0, 149.1, 161.9, 166.8.
3.63. Synthesis of Methyl (R,E)-4-(2,2-dimethyl-3-methylenecyclopentyl)but-2-enoate (14)
To a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (45.7 mg, 0.3 mmol) in anhydrous toluene (2 mL) under argon at room temperature, methyl (triphenylphosphoranylidene)acetate (0.45 mmol, 150 mg) was added. The resulting reaction mixture was heated under reflux for 24 h. The volatile components were evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60; EtOAc/petroleum ether = 1:20). The fractions containing the pure product 14 were combined, and the volatiles were evaporated in vacuo. Yield: 47 mg (0.2256 mmol, 75%) of colorless oil. [α]Dr.t. = +5.8 (0.135, CH2Cl2). EI-HRMS: m/z = 209.1536 (MH+); C13H21O2: requires m/z = 209.1536 (MH+); νmax 2956, 1723, 1655, 1435, 1269 1194, 1041, 978, 878 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.78 (s, 3H), 1.01 (s, 3H), 1.17–1.29 (m, 1H), 1.52–1.62 (m, 1H), 1.72–1.81 (m, 1H), 1.87–1.98 (m, 1H), 2.17–2.31 (m, 2H), 2.33–2.43 (m, 1H), 3.66 (s, 3H), 4.70 (t, J = 2.5 Hz, 1H), 4.71–4.74 (m, 1H), 5.78 (dt, J = 1.5, 15.6 Hz, 1H), 6.91 (ddd, J = 7.0, 7.9, 15.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 27.0, 28.2, 30.6, 33.2, 44.2, 49.5, 51.6, 103.6, 121.6, 149.4, 161.9, 167.2.
3.64. Synthesis of (R,E)-5-(2,2-dimethyl-3-methylenecyclopentyl)pent-3-en-2-one (15)
To a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (45.7 mg, 0.3 mmol) in anhydrous CH2Cl2 (2 mL) under argon at room temperature, 1-triphenylphosphoranyliden-2-propanon (0.45 mmol, 143 mg) was added. The resulting reaction mixture was heated under reflux for 24 h. The volatile components were evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60; EtOAc/petroleum ether = 1:30). The fractions containing the pure product 15 were combined, and the volatiles were evaporated in vacuo. Yield: 32 mg (0.166 mmol, 56%) of colorless oil. [α]Dr.t. = +2.2 (0.275, CH2Cl2). EI-HRMS: m/z = 193.1586 (MH+); C13H21O: requires m/z = 193.1587 (MH+); νmax 2958, 1698, 1627, 1361, 1251, 977, 878 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.80 (s, 3H), 1.02 (s, 3H), 1.19–1.30 (m, 1H), 1.55–1.63 (m, 1H), 1.71–1.80 (m, 1H), 1.90–2.01 (m, 1H), 2.18 (s, 3H), 2.21–2.32 (m, 2H), 2.35–2.43 (m, 1H), 4.71 (t, J = 2.5 Hz, 1H), 4.73 (dt, J = 1.1, 2.2 Hz, 1H), 6.03 (dt, J = 1.4, 15.8 Hz, 1H), 6.74 (ddd, J = 6.8, 7.7, 15.9 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 23.5, 27.0, 27.0, 28.3, 30.5, 33.5, 44.2, 49.6, 103.6, 132.0, 148.2, 161.7, 198.7.
3.65. Synthesis of (R,E)-(3-(2,2-dimethyl-3-methylenecyclopentyl)prop-1-en-1-yl)benzene (16) and (R,Z)-(3-(2,2-dimethyl-3-methylenecyclopentyl)prop-1-en-1-yl)benzene (16′)
To a suspension of NaH (0.45 mmol, ω = 0.60, 18 mg) in anhydrous THF (0.5 mL) under argon at 0 °C, benzyltriphenylphosphonium chloride (0.3 mmol, 117 mg) was added. The resulting reaction mixture was stirred at 0 °C for 30 min, then a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)acetaldehyde (5) (0.3 mmol, 45.6 mg) in anhydrous THF (1.0 mL) was added. The reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 20 h. The excess NaH was quenched with NaCl (aq. sat., 3 mL), and the mixture was extracted with EtOAc (3 × 10 mL). The combined organic phase was washed with NaCl (aq. sat., 2 × 5 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60; petroleum ether). The fractions containing the pure products 16/16′ were combined, and the volatiles were evaporated in vacuo. Diastereomeric ratio: 16/16′ = 88:12. The two diastereomers formed could not be separated by column chromatography. Yield: 43 mg (0.190 mmol, 63%) of colorless oil. [α]Dr.t. = +38.8 (0.22, CH2Cl2). EI-HRMS: the product was not ionized; νmax 2957, 1651, 1495, 1384, 878, 742, 692 cm−1. 1H-NMR (500 MHz, CDCl3) for 16: δ 0.82 (s, 3H), 1.04 (s, 3H), 1.23–1.34 (m, 1H), 1.53–1.63 (m, 1H), 1.77–1.84 (m, 1H), 1.89–1.99 (m, 1H), 2.18–2.32 (m, 2H), 2.34–2.43 (m, 1H), 4.69–4.73 (m, 2H), 6.15 (ddd, J = 6.8, 7.5, 15.7 Hz, 1H), 6.33 (dt, J = 1.5, 15.7 Hz, 1H), 7.10–7.14 (m, 1H), 7.20–7.24 (m, 2H), 7.26–7.29 (m, 2H). 13C-NMR (126 MHz, CDCl3) for 16: δ 23.5, 27.1, 28.4, 30.7, 33.9, 44.2, 50.6, 103.2, 126.1, 127.0, 128.6, 130.4, 130.7, 138.0, 162.7. 1H-NMR (500 MHz, CDCl3) for 16′: δ 0.74 (s, 3H), 1.02 (s, 3H), 2.02–2.09 (m, 1H), 4.67–4.69 (m, 1H), 5.64 (dt, J = 7.3, 11.7 Hz, 1H) (the remaining signals overlap with the signals of diastereomer 16).
3.66. Cyclopropanation of the Methylene Group—General Procedure 8 (GP8)
Zinc dust (84.0 mg, 1.285 mmol, 2.57 equiv.) and copper(I) chloride (127 mg, 1.285 mmol, 2.57 equiv.) were introduced into a flame-dried flask under argon, followed by the addition of Et2O (3 mL). The resulting mixture was refluxed under argon for 30 min. Diiodomethane (53 μL, 0.65 mmol, 1.3 equiv.) was then added. The reaction mixture turned dark in color, and bubbles began to form. After 5–10 min, alkene (8a or 9) (0.5 mmol) and further diiodomethane (243 μL, 3.0 mmol, 6 equiv.) were added. The resulting reaction mixture was refluxed under argon for 20 h. The cooled reaction mixture (room temperature) was filtered through a plague of Celite® and washed with Et2O (30 mL) to remove the solid particles. The organic phase was washed with HCl (aq., 1 M, 2 × 15 mL), H2O (2 × 15 mL) and NaCl (aq. sat., 3 × 15 mL). The organic phase was dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product were combined, and the volatile components were evaporated in vacuo. The isolated cyclopropanated products 17 and 18 were fully characterized.
3.67. Synthesis of (R)-5-(2-methoxyethyl)-4,4-dimethylspiro[2.4]heptane (17)
Following GP8. Prepared from (R)-2-(2-methoxyethyl)-1,1-dimethyl-5-methylenecyclopentane (8a) (0.5 mmol, 84 mg); column chromatography: EtOAc/petroleum ether = 1:50. Yield: 67 mg (0.367 mmol, 73%) of colorless oil. The product contains 9% of the starting alkene 8a. [α]Dr.t. = +43.3 (0.155, CH2Cl2). EI-HRMS: the product was not ionized; νmax 2935, 2867, 1468, 1386, 1117, 1070, 841 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.05–0.13 (m, 1H), 0.25–0.34 (m, 2H), 0.49–0.56 (m, 1H), 0.57 (s, 3H), 0.70 (s, 3H), 1.29–1.41 (m, 3H), 1.63–1.82 (m, 2H), 1.83–1.96 (m, 2H), 3.34 (s, 3H), 3.36–3.47 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 6.5, 12.3, 20.2, 22.9, 28.3, 30.7, 30.8, 34.0, 40.9, 47.3, 58.7, 72.6.
3.68. Synthesis of (R)-2-(4,4-dimethylspiro[2.4]heptan-5-yl)-N-methoxy-N-methylacetamide (18)
Following GP8. Product 18 was prepared on a 3 mmol scale. Prepared from zinc dust (504 mg, 7.71 mmol, 2.57 equiv.), copper(I) chloride (763 mg, 7.71 mmol, 2.57 equiv.), Et2O (10 mL), diiodomethane (316 μL, 3.9 mmol, 1.3 equiv.), (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)-N-methoxy-N-methylacetamide (9) (3.0 mmol, 634 mg), and diiodomethane (1.461 mL, 18 mmol, 6 equiv.) and washed with Et2O (60 mL); column chromatography: EtOAc/petroleum ether = 1:5. Yield: 460 mg (2.04 mmol, 68%) of colorless oil. The product contains 5% of the starting alkene 9. [α]Dr.t. = +36.2 (0.175, CH2Cl2). EI-HRMS: m/z = 226.1800 (MH+); C13H24NO2: requires m/z = 226.1802 (MH+); νmax 2957, 2869, 1663, 1464, 1412, 1176, 1111, 1004 cm–1. 1H-NMR (500 MHz, CDCl3): δ 0.09–0.17 (m, 1H), 0.31 (dd, J = 7.0, 8.4 Hz, 2H), 0.47–0.58 (m, 1H), 0.62 (s, 3H), 0.73 (s, 3H), 1.30–1.47 (m, 2H), 1.80–1.91 (m, 1H), 1.96–2.07 (m, 1H), 2.14–2.24 (m, 1H), 2.26–2.34 (m, 1H), 2.49 (dd, J = 3.6, 14.6 Hz, 1H), 3.19 (s, 3H), 3.70 (s, 3H). 13C-NMR (126 MHz, CDCl3): δ 6.9, 12.0, 20.4, 22.9, 28.6, 30.4, 32.4, 33.4, 33.8, 40.9, 46.4, 61.3, 174.9.
3.69. Synthesis of Ketones 19 from Weinreb Amide 18—General Procedure 9 (GP9)
To a solution of (R)-2-(4,4-dimethylspiro[2.4]heptan-5-yl)-N-methoxy-N-methylacetamide (18) (1.0 equiv.; contains 5% of alkene 9) in anhydrous Et2O (5 mL) under argon at 0 °C, the corresponding Grignard reagent (1.6 equiv.) was added slowly. The resulting reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 20 h. The excess Grignard reagent was quenched with NaCl (aq. sat., 3 mL), and the resulting mixture was extracted with Et2O (3 × 15 mL). The combined organic phase was washed with NaCl (aq. sat., 3 × 5 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60). The fractions containing the pure product 19 were combined, and the volatiles evaporated in vacuo. The isolated ketones 19 were fully characterized.
3.70. Synthesis of (R)-1-(4,4-dimethylspiro[2.4]heptan-5-yl)propan-2-one (19a)
Following GP9. Prepared from (R)-2-(4,4-dimethylspiro[2.4]heptan-5-yl)-N-methoxy-N-methylacetamide (18) (0.5 mmol, 113 mg), Et2O (5 mL), and methylmagnesium bromide (3 M in Et2O, 0.8 mmol, 267 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 80 mg (0.444 mmol, 88%) of colorless oil. The product contains 4% of the alkene 9. [α]Dr.t. = +16.2 (0.28, CH2Cl2). EI-HRMS: m/z = 181.1586 (MH+); C12H21O: requires m/z = 181.1587 (MH+); νmax 2958, 2869, 1714, 1468, 1365, 1287, 1163, 1145, 1011 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.09–0.17 (m, 1H), 0.27–0.36 (m, 2H), 0.50–0.57 (m, 1H), 0.59 (s, 3H), 0.70 (s, 3H), 1.22–1.34 (m, 1H), 1.37–1.46 (m, 1H), 1.83–1.93 (m, 1H), 1.91–2.03 (m, 1H), 2.08–2.15 (m, 1H), 2.17 (s, 3H), 2.28 (dd, J = 10.7, 15.5 Hz, 1H), 2.51 (dd, J = 3.5, 15.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 6.8, 12.0, 20.4, 23.0, 28.5, 30.3, 30.4, 33.7, 40.9, 45.6, 46.0, 209.7.
3.71. Synthesis of (R)-1-(4,4-dimethylspiro[2.4]heptan-5-yl)pent-4-en-2-one (19b)
Following GP9. Prepared from (R)-2-(4,4-dimethylspiro[2.4]heptan-5-yl)-N-methoxy-N-methylacetamide (18) (0.5 mmol, 113 mg), Et2O (5 mL), and allylmagnesium bromide (1.0 M in Et2O, 0.8 mmol, 0.8 mL); column chromatography: EtOAc/petroleum ether = 1:50. Yield: 73 mg (0.354 mmol, 70%) of colorless oil. The product contains 3% of the alkene 9. [α]Dr.t. = +21.1 (0.25, CH2Cl2). EI-HRMS: m/z = 207.1784 (MH+); C14H23O: requires m/z = 207.1785 (MH+); νmax 2958, 2869, 1714, 1638, 1468, 1385, 1365, 1012, 991, 917 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.09–0.16 (m, 1H), 0.26–0.36 (m, 2H), 0.50–0.57 (m, 1H), 0.58 (s, 3H), 0.69 (s, 3H), 1.20–1.34 (m, 1H), 1.37–1.46 (m, 1H), 1.82–1.91 (m, 1H), 1.92–2.03 (m, 1H), 2.09–2.19 (m, 1H), 2.30 (dd, J = 10.7, 15.8 Hz, 1H), 2.52 (dd, J = 3.5, 15.8 Hz, 1H), 3.14–3.26 (m, 2H), 5.11–5.22 (m, 2H), 5.94 (ddt, J = 7.0, 10.2, 17.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 6.8, 12.0, 20.4, 23.0, 28.5, 30.3, 33.7, 40.9, 44.2, 45.8, 48.2, 118.8, 130.9, 209.2.
3.72. Synthesis of (R)-1-(4,4-dimethylspiro[2.4]heptan-5-yl)but-3-en-2-one (19c)
Following GP9. Prepared from (R)-2-(4,4-dimethylspiro[2.4]heptan-5-yl)-N-methoxy-N-methylacetamide (18) (0.5 mmol, 113 mg), Et2O (5 mL), and vinylmagnesium bromide (1.0 M in THF, 0.8 mmol, 0.8 mL); column chromatography: EtOAc/petroleum ether = 1:40. Yield: 65 mg (0.338 mmol, 67%) of colorless oil. [α]Dr.t. = +36.6 (0.155, CH2Cl2). EI-HRMS: m/z = 193.1584 (MH+); C13H21O: requires m/z = 193.1587 (MH+); νmax 2958, 2869, 1680, 1615, 1400, 1365, 1011, 985, 958 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.10–0.17 (m, 1H), 0.28–0.36 (m, 2H), 0.51–0.58 (m, 1H), 0.61 (s, 3H), 0.73 (s, 3H), 1.24–1.36 (m, 1H), 1.37–1.46 (m, 1H), 1.83–1.90 (m, 1H), 1.91–2.01 (m, 1H), 2.12–2.22 (m, 1H), 2.44 (dd, J = 10.7, 15.2 Hz, 1H), 2.67 (dd, J = 3.7, 15.2 Hz, 1H), 5.81 (dd, J = 1.2, 10.6 Hz, 1H), 6.23 (dd, J = 1.2, 17.6 Hz, 1H), 6.39 (dd, J = 10.6, 17.6 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 6.9, 12.1, 20.4, 23.0, 28.5, 30.3, 33.7, 41.0, 41.5, 46.2, 129.0, 136.9, 201.4.
3.73. Synthesis of (R)-2-(4,4-dimethylspiro[2.4]heptan-5-yl)-1-phenylethan-1-one (19d)
Following GP9. Prepared from (R)-2-(4,4-dimethylspiro[2.4]heptan-5-yl)-N-methoxy-N-methylacetamide (18) (0.5 mmol, 113 mg), Et2O (5 mL), and phenylmagnesium bromide (3.0 M in THF, 0.8 mmol, 267 μL); column chromatography: EtOAc/petroleum ether = 1:20. Yield: 76 mg (0.315 mmol, 63%) of colorless oil. The product contains 3% of the alkene 9. [α]Dr.t. = +40.1 (0.235, CH2Cl2). EI-HRMS: m/z = 243.1744 (MH+); C17H23O: requires m/z = 243.1743 (MH+); νmax 2957, 2868, 1682, 1448, 1365, 1286, 1213, 1045, 750, 689 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.10–0.18 (m, 1H), 0.29–0.37 (m, 2H), 0.51–0.59 (m, 1H), 0.66 (s, 3H), 0.80 (s, 3H), 1.29–1.46 (m, 2H), 1.83–1.94 (m, 1H), 2.02–2.04 (m, 1H), 2.23–2.34 (m, 1H), 2.80 (dd, J = 10.5, 15.4 Hz, 1H), 3.08 (dd, J = 3.5, 15.5 Hz, 1H), 7.41–7.51 (m, 2H), 7.52–7.62 (m, 1H), 7.93–8.00 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 6.9, 12.1, 20.6, 23.1, 28.6, 30.4, 33.8, 40.3, 41.2, 46.5, 128.3, 128.7, 133.0, 137.5, 200.9.
3.74. Synthesis of (3R)-1-methoxy-3-(2-methoxyethyl)-1,2,2-trimethylcyclopentane (20)
To a solution/suspension of Hg(OAc)2 (0.65 mmol, 207 mg) in anhydrous methanol (1.5 mL) at 0 °C under argon was added (R)-2-(2-methoxyethyl)-1,1-dimethyl-5-methylenecyclopentane (8a) (0.5 mmol, 84 mg). After stirring at 0 °C for 15 min, NaOH (aq., 3 M, 0.5 mL) was added. The reaction mixture turned from colorless to yellow. Then, a solution/suspension of NaBH4 (5.25 mmol, 199 mg) in NaOH (aq., 3 M, 0.5 mL) was added. The reaction mixture changed color from yellow to dark blue (black). The reaction mixture was stirred at room temperature under argon for 20 h. The reaction mixture was filtered through a pad of Celite® and washed with EtOAc (20 mL). The filtrate was washed with H2O (2 × 5 mL) and NaCl (aq. sat., 2 × 5 mL), dried under anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60, EtOAc/petroleum ether = 1:10). The fractions containing the pure product 20 were combined, and the volatile components were evaporated in vacuo. Yield: 66 mg (0.330 mmol, 66%) of colorless oil. [α]Dr.t. = +25.2 (0.15, CH2Cl2). EI-HRMS: the product was not ionized; νmax 2935, 2867, 1468, 1386, 1117, 1070, 841 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.67 (s, 3H), 0.87 (s, 3H), 1.06 (s, 3H), 1.15–1.24 (m, 1H), 1.28–1.37 (m, 1H), 1.37–1.46 (m, 1H), 1.64–1.88 (m, 2H), 1.90–2.06 (m, 2H), 3.13 (s, 3H), 3.33 (s, 3H), 3.35–3.43 (m, 2H). 13C-NMR (126 MHz, CDCl3): δ 16.0, 19.6, 19.7, 27.4, 30.0, 31.1, 43.1, 47.6, 49.4, 58.6, 73.0, 87.7.
3.75. Synthesis of (3aR,6aS)-6,6,6a-trimethylhexahydro-2H-cyclopenta[b]furan (21) [40]
To a solution of (R)-2-(2,2-dimethyl-3-methylenecyclopentyl)ethan-1-ol (4) (2.0 mmol, 308 mg) in anhydrous Et2O (4 mL) under argon at 0 °C was added TFA (4 mL). The resulting reaction mixture was stirred at 0 °C for 1 h and at room temperature for 1 h. The volatiles were thoroughly evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60, EtOAc/petroleum ether = 1:20). The fractions containing the pure product 21 were combined, and the volatile components were evaporated in vacuo. The isolated ether 21 was fully characterized with the exception of HRMS, as the product was not ionized. Yield: 118 mg (0.76 mmol, 38%) of colorless oil. [α]Dr.t. = +5.0 (0.23, CH2Cl2). νmax 2955, 2864, 1785, 1460, 1398, 1348, 1218, 1141, 943, 821, 776, 731 cm−1. 1H-NMR (500 MHz, CDCl3): δ 0.85 (s, 3H), 1.01 (s, 3H), 1.08 (s, 3H), 1.15–1.25 (m, 1H), 1.28–1.36 (m, 1H), 1.61–1.70 (m, 2H), 1.91–2.03 (m, 1H), 2.12–2.22 (m, 1H), 2.32–2.40 (m, 1H), 3.70–3.79 (m, 1H), 3.89 (td, J = 3.6, 8.2 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 19.1, 22.2, 25.5, 29.7, 34.9, 39.8, 46.0, 47.2, 68.0, 95.1.
3.76. DFT Calculations
Theoretical studies were carried out using AMS 2025.1, SCM, Theoretical Chemistry program [
66]. Geometry optimizations of relevant equilibrium structures were performed using the M06-2X [
67,
68] density functional with the TZ2P basis set as implemented in ADF–AMS 2025.1. Normal mode vibrational analysis on the stationary points allowed us to confirm they are minima (zero imaginary frequencies). The M06-2X functional was chosen, as it is better suited to handling kinetics, thermodynamics, and noncovalent interactions in organic molecular systems [
67,
69,
70,
71,
72,
73,
74]. Solvation effect (in dichloromethane) was considered using the COSMO model. An ultrafine grid was employed for all DFT calculations. Unless otherwise stated, relative Gibbs energies (ΔG) reported correspond to the M06-2X/TZ2P level at 298.13 K in dichloromethane. The 3D geometries were generated using GUI of AMS 2025.1.