The Composition of Volatile Organic Compounds Correlates with the Genetic Variability Within the Calypogeia sphagnicola Species Complex (Marchantiophyta, Calypogeiaceae)
Abstract
1. Introduction
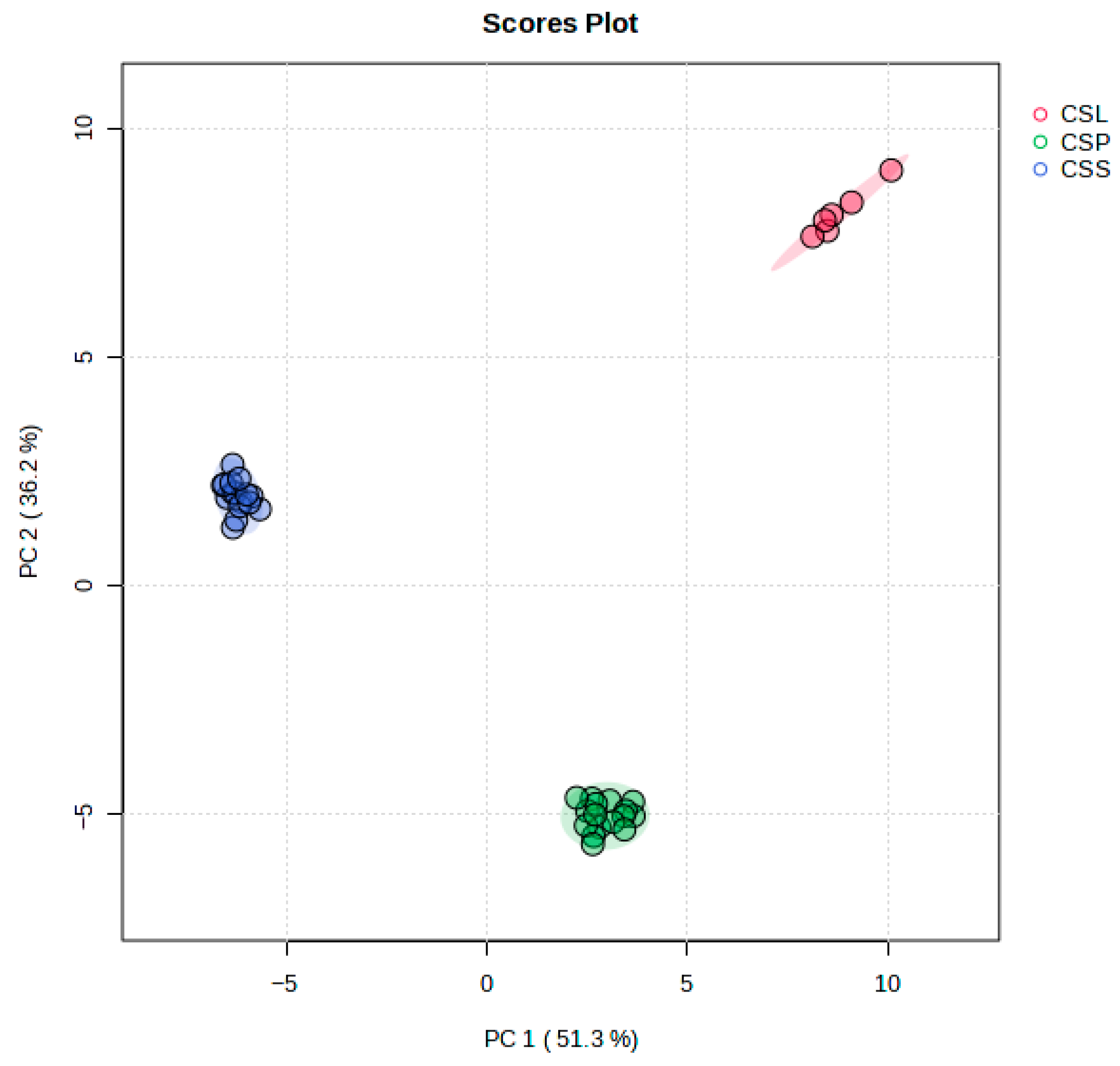
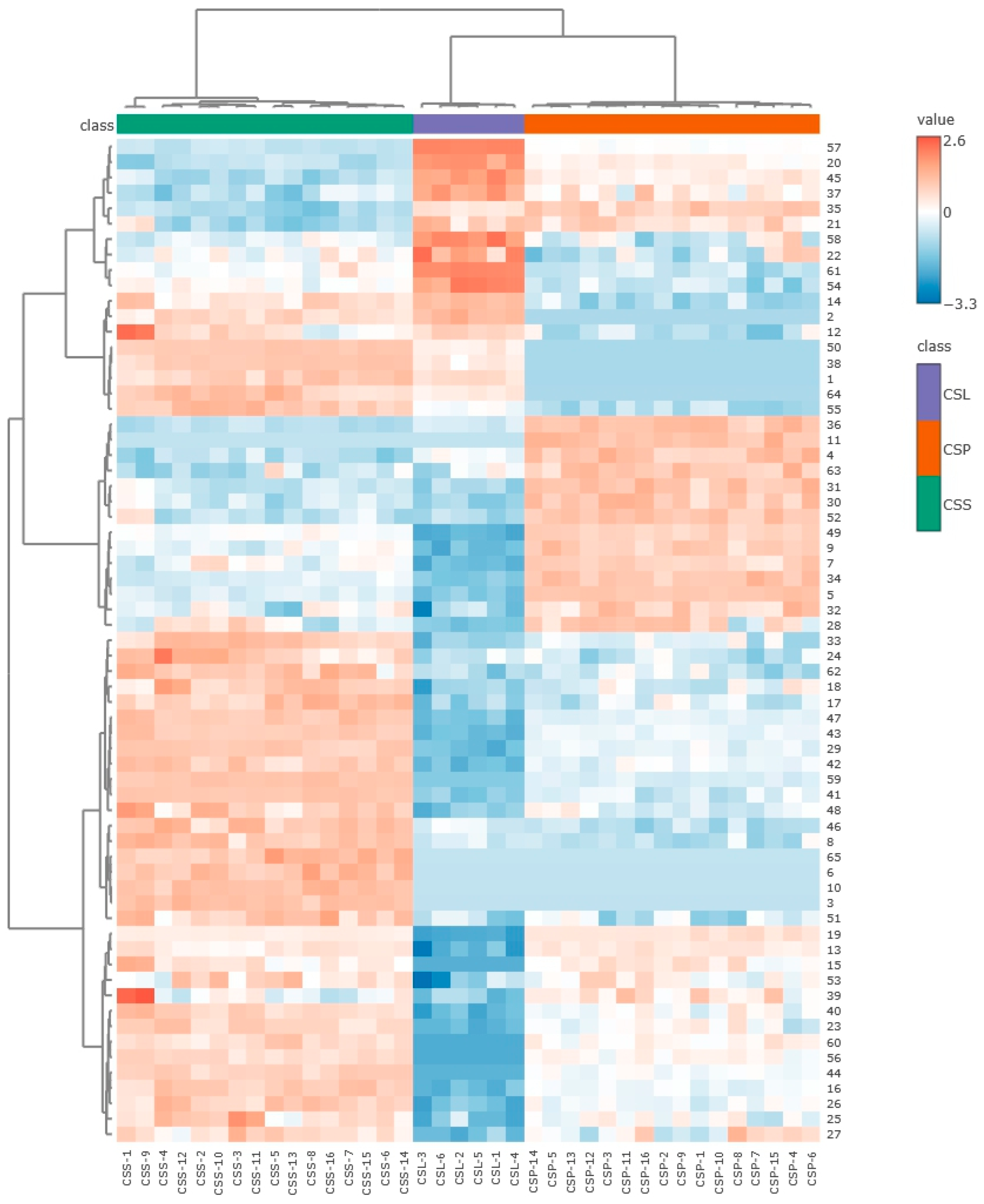
2. Results and Discussion
Volatiles Present in Calypogeia sphagnicola
3. Materials and Methods
3.1. Plant Material
3.2. HS-SPME Extraction
3.3. GC-MS Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crandall-Stotler, B.; Stotler, R.E.; Long, D.G. Morphology and classification of the Marchantiophyta. In Bryophyte Biology; Goffinet, B., Shaw, A.J., Eds.; Cambridge University Press: Cambridge, MA, USA, 2009; pp. 1–54. [Google Scholar]
- Wellman, C.H.; Osterloff, P.L.; Mohiuddin, U. Fragments of the earliest land plants. Nature 2003, 425, 282–285. [Google Scholar] [CrossRef]
- Rubinstein, C.V.; Gerrienne, P.; de la Puente, G.S.; Astini, R.A.; Steemans, P. Early Middle Ordovician evidence for land plants in Argentina (eastern Gondwana). New Phytol. 2010, 188, 365–369. [Google Scholar] [CrossRef]
- Clarke, J.T.; Warnock, R.C.M.; Donoghue, P.C.J. Establishing a time-scale for plant evolution. New Phytol. 2011, 192, 266–301. [Google Scholar] [CrossRef]
- Söderström, L.; Hagborg, A.; Konrat, M. World check list of hornworts and liverworts. PhytoKeys 2016, 59, 1–829. [Google Scholar] [CrossRef]
- Bakalin, V.; Vilnet, A.; Klimova, K.; Nguyen, V.S. Calypogeia vietnamica sp. nov. (Calypogeiaceae, Hepaticae) from North Vietnam and diversification in Calypogeia taxa with blue oil bodies. Herzogia 2019, 32, 219–229. [Google Scholar] [CrossRef]
- Konrat, M.; Hagborg, A.; Söderström, L.; Mutke, J.; Renner, M.; Gradstein, S.R.; Engel, J.; Zhu, R.L.; Pickering, J. Early Land Plants Today: Global Patterns of Liverwort Diversity, Distribution, and Floristic Knowledge. In Bryology in the New Millennium; Mohamed, H., Baki, B.B., Nasrulhaq-Boyce, A., Lee, P.K.Y., Eds.; University of Malaya: Kuala Lumpur, Malaya, 2008; pp. 425–438. [Google Scholar]
- He, X.; Sun, Y.; Zhu, R.L. The Oil Bodies of Liverworts: Unique and Important Organelles in Land Plants. Crit. Rev. Plant Sci. 2013, 32, 293–302. [Google Scholar] [CrossRef]
- Asakawa, Y. Chemical constituents of the bryophytes. In Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, W.B., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer: Vienna, Austria, 1995; Volume 65, pp. 1–618. [Google Scholar]
- Asakawa, Y. Chemosystematics of the Hepaticae. Phytochemistry 2004, 65, 623–669. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Bio- and Chemical Diversity, Biological Activity, and Chemosystematics. In Chemical Constituences of Bryophytes; Springer: Wien, Austria, 2013; pp. 1–665. [Google Scholar]
- Salimpour, F.; Mazooji, A.; Akhoondi, S. Chemotaxonomy of six Salvia species using essential oil composition markers. J. Med. Plants Res. 2011, 5, 1795–1805. [Google Scholar] [CrossRef]
- Celiński, K.; Bonikowski, R.; Wojnicka-Półtorak, A.; Chudzińska, E.; Maliński, T. Volatiles as chemosystematic markers for distinguishing closely related species within the Pinus mugo complex. Chem. Biodiv. 2015, 12, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, X.; Wang, K.; Lv, X.; Li, R.; Ma, W. GC–MS Untargeted Analysis of Volatile Compounds in Four Red Grape Varieties (Vitis vinifera L. cv) at Different Maturity Stages near Harvest. Foods 2022, 11, 2804. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ren, H.; Song, Y.; Liu, F.; Qian, L.; Zuo, F.; Li, M. Analysis of volatile compounds by GCMS reveals their rice cultivars. Sci. Rep. 2023, 13, 7973. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Asakawa, Y. Chemosystematics of selected liverworts collected in Borneo. Trop. Bryol. 2010, 31, 33–42. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Asakawa, Y. Fingerprinting of secondary metabolites of liverworts: Chemosystematic approach. J. AOAC Int. 2014, 97, 1234–1243. [Google Scholar] [CrossRef][Green Version]
- Ludwiczuk, A.; Asakawa, Y. GC/MS fingerprinting of solvent extracts and essential oils obtained from liverwort species. Nat. Prod. Commun. 2017, 12, 1301–1305. [Google Scholar] [CrossRef]
- Huang, W.J.; Wu, C.L.; Lin, C.W.; Chi, L.L.; Chen, P.Y.; Chiu, C.J.; Huang, C.Y.; Chen, C.N. Marchantin A, a cyclic bis(bibenzyl ether), isolated from the liverwort Marchantia emarginata subsp. tosana induces apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2010, 291, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Sun, D.F.; Sun, C.C.; Liu, H.P.; Yue, B.; Zhao, C.R.; Lou, H.X.; Qu, X.J. Inhibitory effect of riccardin D on growth of human non-small cell lung cancer: In vitro and in vivo studies. Lung Cancer 2012, 76, 300–308. [Google Scholar] [CrossRef]
- Yue, B.; Zhang, Y.S.; Xu, H.M.; Zhao, C.R.; Li, Y.Y.; Qin, Y.Z.; Wang, R.Q.; Sun, D.; Yuan, Y.; Lou, H.X.; et al. Riccardin D-26, a synthesized macrocyclic bisbibenzyl compound, inhibits human hepatocellular carcinoma growth through induction of apoptosis in p53-dependent way. Cancer Lett. 2013, 328, 104–113. [Google Scholar] [CrossRef]
- Iwai, Y.; Murakami, K.; Gomi, Y.; Hashimoto, T.; Asakawa, Y.; Okuno, Y.; Ishikawa, T.; Hatakeyama, D.; Echigo, N.; Kuzuhara, T. Anti-influenza activity of marchantins, macrocyclic bisbibenzyls contained in liverworts. PLoS ONE 2011, 6, e19825. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, W.; Sun, B.; Groh, M.; Speicher, A.; Lou, H. Bisbibenzyls, a new type of antifungal agent, inhibit morphogenesis switch and biofilm formation through upregulation of DPP3 in Candida albicans. PLoS ONE 2011, 6, e28953. [Google Scholar] [CrossRef]
- Andre, C.M.; Sansom, C.E.; Plunkett, B.J.; Hamiaux, C.; Massey, L.; Chan, A.; Caddie, M.; Espley, R.V.; Perry, N.B. Unique bibenzyl cannabinoids in the liverwort Radula marginata: Parallels with Cannabis chemistry. New Phytol. 2025, 246, 2666–2682. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.M. The Hepaticae and Anthocerotae of North America East of the Hundreth Meridian; Columbia University Press: New York, NY, USA, 1969; Volume 2. [Google Scholar]
- Paton, J.A. The Liverwort Flora of the British Isles; Harley Books Colchester: Colchester, UK, 1999. [Google Scholar]
- Stewart, N. Red Data Book of European Bryophytes; European Commottee for the Conservation of Bryophytes Press: Trondheim, Norway, 1995. [Google Scholar]
- Buczkowska, K.; Sawicki, J.; Szczecińska, M.; Klama, H.; Bączkiewicz, A. Allopoliploid speciation of Calypogeia sphagnicola (Jungermanniopsiada, Calypogeiaceae) based on isozyme and DNA markers. Plant Syst. Evol. 2012, 298, 549–560. [Google Scholar] [CrossRef]
- Buczkowska, K.; Sawicki, J.; Szczecińska, M.; Klama, H.; Bączkiewicz, A. Isozyme and DNA markers reveal a new genetically distinct taxon of Calypogeia sphagnicola (Jungermanniopsida, Calypogeiaceae). Pol. Bot. J. 2012, 57, 95–107. [Google Scholar]
- Damsholt, K. Illustrated Flora of Nordic Liverworts and Hornworts; Nordic Bryological Society: Lund, Sweden, 2002. [Google Scholar]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 2013, 91, 52–80. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Gradstein, S.R.; Nagashima, F.; Asakawa, Y. Chemosystematics of Porella (Marchantiophyta, Porellaceae). Nat Prod. Commun. 2011, 6, 315–321. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Odrzykoski, I.J.; Asakawa, Y. Identification of cryptic species with in liverwort Conocephalum conicum based on the volatile components. Phytochemistry 2013, 95, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Huneck, S. Campestryl behenate in liverworts. Phytochemistry 1974, 13, 1289. [Google Scholar] [CrossRef]
- Beneš, I.; Beizaee, N.; Vanek, T.; Vana, J. Campesteryl behenate, a chemical character of the liverwort genus Calypogeia. Phytochemistry 1981, 20, 2438–2439. [Google Scholar] [CrossRef]
- Warmers, U.; Wihstutz, K.; Bülow, N.; Fricke, C.; König, W.A. Sesquiterpene constituents of the liverwort Calypogeia muelleriana. Phytochemistry 1998, 49, 1723–1731. [Google Scholar] [CrossRef]
- Warmers, U.; König, W.A. Sesquiterpene constituents of the liverwort Calypogeia fissa. Phytochemistry 1999, 52, 695–704. [Google Scholar] [CrossRef]
- Warmers, U.; Rieck, A.; König, W.A.; Muhle, H. (+)-Bisabola-2,10-diene[1,9]oxide, a constituent of the liverwort Calypogeia suecica. Phytochemistry 1999, 51, 679–682. [Google Scholar] [CrossRef]
- Guzowska, M.; Wawrzyniak, R.; Wasiak, W. Seasonal Variability and Effect of Sample Storage on Volatile Components in Calypogeia azurea. Molecules 2022, 27, 2426. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, R.; Guzowska, M.; Wasiak, W.; Jasiewicz, B.; Bączkiewicz, A.; Buczkowska, K. Seasonal Variability of Volatile Components in Calypogeia integristipula. Molecules 2023, 28, 7276. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, R.; Wasiak, W.; Guzowska, M.; Bączkiewicz, A.; Buczkowska, K. The Content of Volatile Organic Compounds in Calypogeia suecica (Calypogeiaceae, Marchantiophyta) Confirms Genetic Differentiation of This Liverwort Species into Two Groups. Molecules 2024, 29, 4258. [Google Scholar] [CrossRef]
- Blatt-Janmaat, K.L.; Neumann, S.; Ziegler, J.; Peters, K. Host Tree and Geography Induce Metabolic Shifts in the Epiphytic Liverwort Radula complanata. Plants 2023, 12, 571. [Google Scholar] [CrossRef]
- Wawrzyniak, R.; Wasiak, W.; Jasiewicz, B.; Bączkiewicz, A.; Buczkowska, K. Chemical Fingerprinting of Cryptic Species and Genetic Lineages of Aneura pinguis (L.) Dumort. (Marchantiophyta, Metzgeriidae). Molecules 2021, 26, 1180. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.E. NIST/EPA/NIH Mass Spectral Database (NIST 11); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011.
- Stein, S.E. NIST Chemistry WebBook. In NIST Standard Reference Database 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. Available online: http://webbook.nist.gov/ (accessed on 24 June 2025).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4., 1st ed.; Diablo Analytical, Inc.: Antioch, CA, USA, 2017; Available online: https://diabloanalytical.com/ms-software/essentialoilcomponentsbygcms (accessed on 24 June 2025).
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com/ (accessed on 24 June 2025).
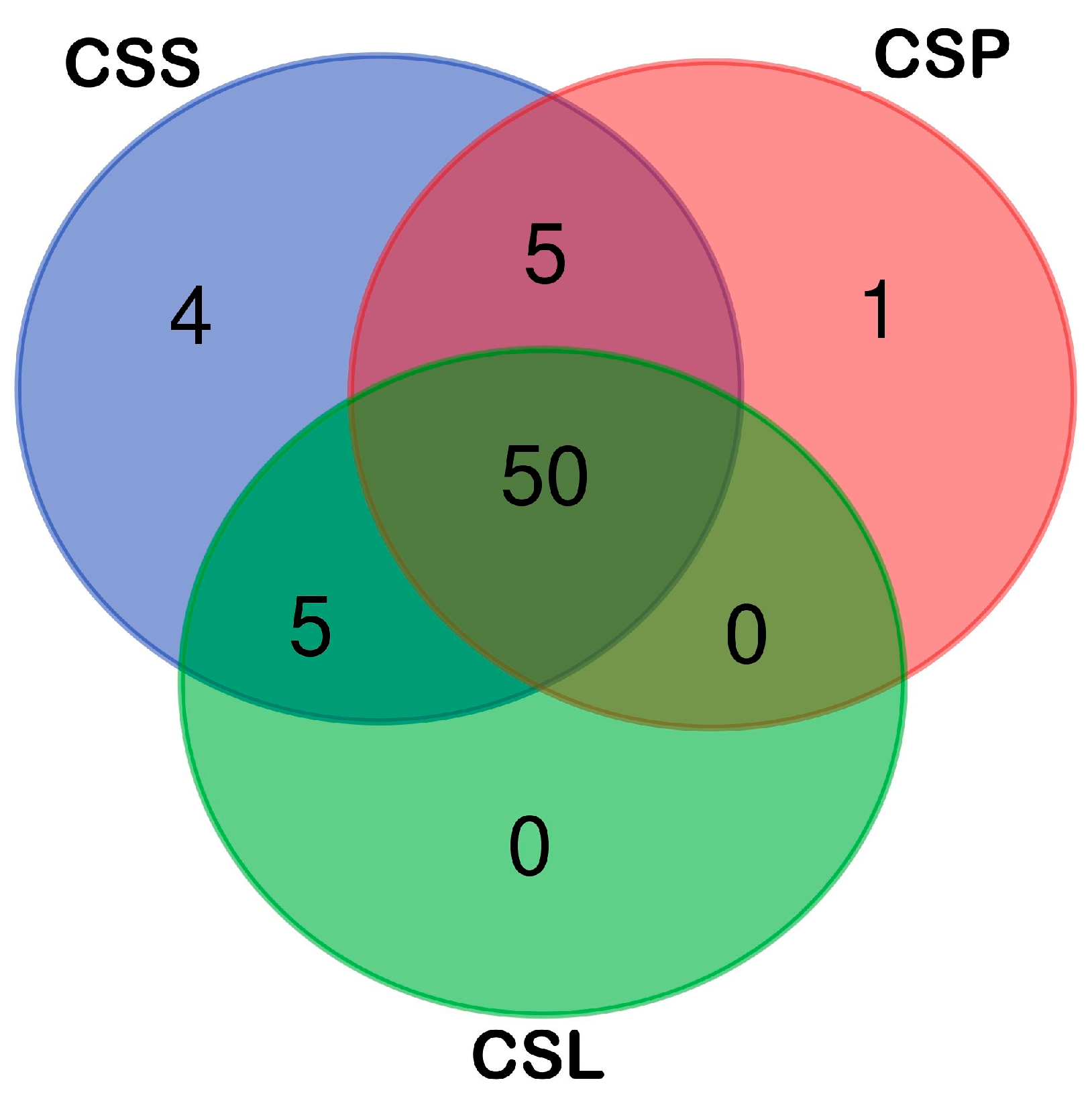
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Zhang, Z.; Murtagh, F.; Van Poucke, S.; Lin, S.; Lan, P. Hierarchical cluster analysis in clinical research with heterogeneous study population: Highlighting its visualization with R. Ann. Transl. Med. 2017, 5, 75. [Google Scholar] [CrossRef]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So you think you can PLS-DA? Bioinformatics 2020, 21 (Suppl. S1), 2. [Google Scholar] [CrossRef]
- Haarman, B.C.M.; Riemersma-Vander Lek, R.F.; Burger, H.; Nolen, W.A.; Mendes, R.; Drexhage, H.A.; Burger, H. Feature expression heatmaps—A new visual method to explore complex associations between two variable sets. J. Biomed. Inform. 2015, 53, 156–161. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
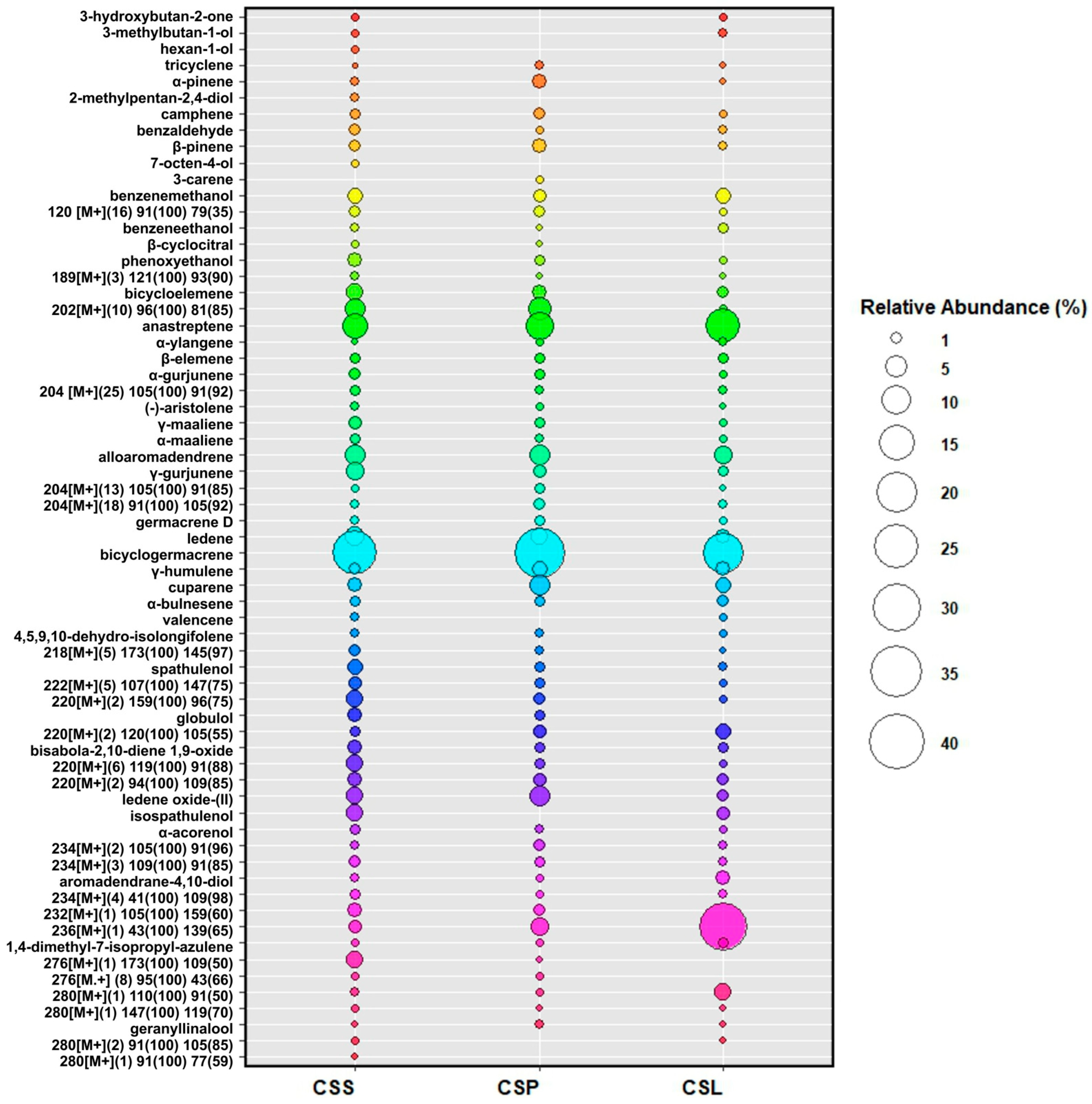
| No. | Compounds | RI a | Mean of Group | |||||
|---|---|---|---|---|---|---|---|---|
| CSS | CSP | CSL | ||||||
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | |||
| 1 | 3-hydroxybutan-2-one | <700 | 0.32 (0.02) | 0.30 (0.02) | - | - | 0.23 (0.01) | 0.23 (0.01) |
| 2 | 3-methylbutan-1-ol | 706 | 0.25 (0.02) | 0.21 (0.01) | - | - | 0.54 (0.02) | 0.51 (0.03) |
| 3 | hexan-1-ol | 867 | 0.28 (0.03) | 0.30 (0.02) | - | - | - | - |
| 4 | tricyclene | 926 | 0.05 (0.01) | 0.06 (0.01) | 0.34 (0.03) | 0.35 (0.02) | 0.11 (0.01) | 0.12 (0.01) |
| 5 | α-pinene | 936 | 0.39 (0.03) | 0.36 (0.02) | 1.56 (0.05) | 1.54 (0.05) | 0.14 (0.01) | 0.12 (0.01) |
| 6 | 2-methylpentan-2,4-diol | 938 | 0.41 (0.03) | 0.38 (0.02) | - | - | - | - |
| 7 | camphene | 953 | 0.56 (0.04) | 0.56 (0.03) | 0.85 (0.04) | 0.84 (0.03) | 0.23 (0.01) | 0.24 (0.01) |
| 8 | benzaldehyde | 960 | 1.01 (0.05) | 1.05 (0.04) | 0.31 (0.02) | 0.28 (0.02) | 0.32 (0.02) | 0.38 (0.02) |
| 9 | β-pinene | 978 | 0.97 (0.05) | 1.00 (0.04) | 1.58 (0.06) | 1.58 (0.05) | 0.52 (0.03) | 0.47 (0.03) |
| 10 | 7-octen-4-ol | 982 | 0.30 (0.02) | 0.32 (0.02) | - | - | - | - |
| 11 | 3-carene | 1009 | - | - | 0.24 (0.01) | 0.28 (0.02) | - | - |
| 12 | benzenemethanol | 1023 | 1.95 (0.08) | 1.98 (0.06) | 1.30 (0.05) | 1.30 (0.06) | 2.02 (0.05) | 2.04 (0.06) |
| 13 | 120[M+](16) 91(100) 79(35) | 1041 | 1.04 (0.06) | 1.00 (0.04) | 1.04 (0.04) | 1.04 (0.04) | 0.22 (0.01) | 0.18 (0.01) |
| 14 | benzeneethanol | 1114 | 0.45 (0.03) | 0.43 (0.03) | 0.15 (0.01) | 0.11 (0.01) | 0.69 (0.03) | 0.68 (0.04) |
| 15 | β-cyclocitral | 1222 | 0.17 (0.02) | 0.19 (0.01) | 0.15 (0.01) | 0.15 (0.01) | - | - |
| 16 | phenoxyethanol | 1226 | 1.65 (0.06) | 1.67 (0.05) | 0.72 (0.03) | 0.75 (0.04) | 0.22 (0.01) | 0.23 (0.01) |
| 17 | 189[M+](3) 121(100) 93(90) | 1322 | 0.43 (0.03) | 0.42 (0.02) | 0.16 (0.01) | 0.14 (0.01) | 0.08 (0.01) | 0.09 (0.01) |
| 18 | bicycloelemene | 1325 | 2.40 (0.07) | 2.37 (0.05) | 1.62 (0.06) | 1.35 (0.06) | 1.00 (0.05) | 1.06 (0.05) |
| 19 | 202[M+](10) 96(100) 81(85) | 1350 | 4.50 (0.08) | 4.49 (0.06) | 5.85 (0.08) | 5.12 (0.09) | 0.34 (0.02) | 0.32 (0.02) |
| 20 | anastreptene | 1370 | 6.77 (0.10) | 6.75 (0.07) | 9.27 (0.11) | 9.33 (0.11) | 14.13 (0.12) | 14.95 (0.13) |
| 21 | α-ylangene | 1373 | 0.14 (0.01) | 0.14 (0.01) | 0.29 (0.02) | 0.35 (0.03) | 0.35 (0.02) | 0.43 (0.03) |
| 22 | β-elemene | 1391 | 0.58 (0.03) | 0.59 (0.02) | 0.55 (0.03) | 0.54 (0.03) | 0.73 (0.04) | 0.74 (0.05) |
| 23 | α-gurjunene | 1419 | 0.90 (0.04) | 0.90 (0.03) | 0.63 (0.04) | 0.65 (0.04) | 0.31 (0.02) | 0.30 (0.02) |
| 24 | 204[M+](25) 105(100) 91(92) | 1423 | 0.78 (0.04) | 0.73 (0.03) | 0.39 (0.03) | 0.40 (0.03) | 0.39 (0.02) | 0.39 (0.03) |
| 25 | (-)-aristolene | 1427 | 0.49 (0.03) | 0.43 (0.02) | 0.29 (0.02) | 0.31 (0.02) | 0.16 (0.01) | 0.12 (0.01) |
| 26 | γ-maaliene | 1430 | 1.45 (0.06) | 1.30 (0.05) | 0.72 (0.04) | 0.75 (0.04) | 0.28 (0.01) | 0.28 (0.02) |
| 27 | α-maaliene | 1438 | 0.65 (0.04) | 0.58 (0.03) | 0.56 (0.03) | 0.51 (0.04) | 0.32 (0.02) | 0.30 (0.02) |
| 28 | alloaromadendrene | 1457 | 4.06 (0.08) | 3.92 (0.06) | 4.21 (0.07) | 4.86 (0.08) | 3.01 (0.07) | 3.02 (0.09) |
| 29 | γ-gurjunene | 1463 | 2.79 (0.06) | 2.76 (0.05) | 1.39 (0.05) | 1.41 (0.05) | 0.61 (0.04) | 0.65 (0.05) |
| 30 | 204[M+](13) 105(100) 91(85) | 1469 | 0.19 (0.02) | 0.20 (0.01) | 0.55 (0.04) | 0.65 (0.03) | 0.11 (0.01) | 0.11 (0.01) |
| 31 | 204[M+](18) 91(100) 105(92) | 1471 | 0.48 (0.03) | 0.45 (0.02) | 1.01 (0.05) | 1.04 (0.05) | 0.36 (0.02) | 0.37 (0.02) |
| 32 | germacrene D | 1474 | 0.43 (0.03) | 0.42 (0.02) | 0.56 (0.03) | 0.55 (0.03) | 0.29 (0.02) | 0.33 (0.02) |
| 33 | ledene | 1476 | 3.75 (0.06) | 3.70 (0.06) | 2.26 (0.07) | 2.48 (0.06) | 1.69 (0.05) | 1.85 (0.05) |
| 34 | bicyclogermacrene | 1488 | 25.78 (0.15) | 25.44 (0.22) | 33.60 (0.22) | 33.23 (0.25) | 21.48 (0.16) | 20.92 (0.15) |
| 35 | γ-humulene | 1493 | 0.83 (0.04) | 0.80 (0.03) | 2.07 (0.06) | 2.16 (0.06) | 1.68 (0.05) | 1.73 (0.05) |
| 36 | cuparene | 1502 | 1.51 (0.05) | 1.57 (0.05) | 4.11 (0.06) | 4.30 (0.08) | 1.81 (0.05) | 1.87 (0.06) |
| 37 | α-bulnesene | 1505 | 0.54 (0.04) | 0.55 (0.03) | 0.65 (0.04) | 0.68 (0.04) | 0.83 (0.03) | 0.83 (0.04) |
| 38 | valencene | 1510 | 0.53 (0.03) | 0.53 (0.03) | - | - | 0.19 (0.01) | 0.23 (0.02) |
| 39 | 4,5,9,10-dehydro-isolongifolene | 1544 | 0.39 (0.03) | 0.42 (0.02) | 0.39 (0.03) | 0.45 (0.04) | 0.22 (0.01) | 0.24 (0.02) |
| 40 | 218[M+](5) 173(100) 145(97) | 1555 | 0.82 (0.05) | 0.80 (0.03) | 0.46 (0.03) | 0.48 (0.03) | 0.14 (0.01) | 0.14 (0.01) |
| 41 | spathulenol | 1570 | 1.97 (0.06) | 1.94 (0.06) | 0.77 (0.04) | 0.69 (0.05) | 0.38 (0.02) | 0.39 (0.03) |
| 42 | 222[M+](5) 107(100) 147(75) | 1573 | 1.32 (0.06) | 1.22 (0.05) | 0.70 (0.04) | 0.70 (0.04) | 0.30 (0.01) | 0.33 (0.02) |
| 43 | 220[M+](2) 159(100) 96(75) | 1576 | 2.40 (0.07) | 2.36 (0.07) | 0.86 (0.04) | 0.88 (0.05) | 0.24 (0.02) | 0.23 (0.01) |
| 44 | globulol | 1590 | 1.51 (0.06) | 1.57 (0.05) | 0.62 (0.04) | 0.62 (0.04) | - | - |
| 45 | 220[M+](2) 120(100) 105(55) | 1593 | 0.71 (0.04) | 0.70 (0.03) | 1.21 (0.06) | 1.22 (0.06) | 2.40 (0.05) | 2.14 (0.09) |
| 46 | bisabola-2,10-diene 1,9-oxide | 1602 | 1.53 (0.06) | 1.53 (0.04) | 0.63 (0.04) | 0.61 (0.03) | 0.75 (0.04) | 0.81 (0.05) |
| 47 | 220[M+](6) 119(100) 91(88) | 1606 | 2.62 (0.07) | 2.51 (0.05) | 0.77 (0.05) | 0.75 (0.04) | 0.26 (0.01) | 0.23 (0.02) |
| 48 | 220[M+](2) 94(100) 109(85) | 1613 | 1.76 (0.06) | 1.73 (0.04) | 1.25 (0.06) | 1.28 (0.05) | 0.91 (0.03) | 0.96 (0.04) |
| 49 | ledene oxide-(II) | 1629 | 2.71 (0.08) | 2.73 (0.06) | 4.42 (0.08) | 4.47 (0.09) | 0.90 (0.03) | 0.86 (0.04) |
| 50 | isospathulenol | 1631 | 2.59 (0.07) | 2.70 (0.05) | - | - | 1.20 (0.05) | 1.05 (0.06) |
| 51 | α-acorenol | 1633 | 0.52 (0.04) | 0.57 (0.03) | 0.33 (0.02) | 0.36 (0.02) | 0.31 (0.02) | 0.31 (0.02) |
| 52 | 234[M+](2) 105(100) 91(96) | 1664 | 0.42 (0.03) | 0.44 (0.02) | 1.01 (0.04) | 1.07 (0.05) | 0.31 (0.01) | 0.36 (0.03) |
| 53 | 234[M+](3) 109(100) 91(85) | 1672 | 0.75 (0.04) | 0.87 (0.03) | 0.71 (0.04) | 0.75 (0.04) | 0.48 (0.04) | 0.46 (0.04) |
| 54 | aromadendrane-4,10-diol | 1683 | 0.33 (0.03) | 0.39 (0.02) | 0.17 (0.01) | 0.23 (0.01) | 1.50 (0.05) | 1.41 (0.05) |
| 55 | 234[M+](4) 41(100) 109(98) | 1686 | 0.71 (0.05) | 0.78 (0.03) | 0.24 (0.01) | 0.25 (0.02) | 0.40 (0.02) | 0.39 (0.03) |
| 56 | 232[M+](1) 105(100) 159(60) | 1691 | 1.40 (0.06) | 1.53 (0.05) | 0.85 (0.04) | 0.98 (0.05) | - | - |
| 57 | 236[M+](1) 43(100) 139(65) | 1694 | 1.19 (0.05) | 1.23 (0.04) | 2.80 (0.06) | 2.95 (0.08) | 29.24 (0.19) | 29.05 (0.21) |
| 58 | 1,4-dimethyl-7-isopropyl-azulene | 1772 | 0.31 (0.03) | 0.31 (0.01) | 0.32 (0.02) | 0.31 (0.02) | 0.81 (0.04) | 0.74 (0.04) |
| 59 | 276[M+](1) 173(100) 109(50) | 1805 | 2.28 (0.07) | 2.29 (0.06) | 0.11 (0.01) | 0.12 (0.01) | - | - |
| 60 | 276[M+] (8) 95(100) 43(66) | 1818 | 0.31 (0.03) | 0.35 (0.02) | 0.21 (0.01) | 0.23 (0.01) | - | - |
| 61 | 280[M+](1) 110(100) 91(50) | 1849 | 0.41 (0.03) | 0.40 (0.02) | 0.20 (0.02) | 0.21 (0.02) | 2.50 (0.05) | 2.45 (0.06) |
| 62 | 280[M+](1) 147(100) 119(70) | 1924 | 0.29 (0.03) | 0.32 (0.02) | 0.13 (0.01) | 0.11 (0.01) | 0.09 (0.01) | 0.10 (0.01) |
| 63 | geranyllinalool | 2034 | 0.14 (0.01) | 0.14 (0.01) | 0.33 (0.02) | 0.36 (0.02) | 0.18 (0.01) | 0.16 (0.01) |
| 64 | 280[M+](2) 91(100) 105(85) | 2041 | 0.29 (0.02) | 0.28 (0.02) | - | - | 0.11 (0.01) | 0.14 (0.01) |
| 65 | 280[M+](1) 91(100) 77(59) | 2063 | 0.15 (0.02) | 0.16 (0.01) | - | - | - | - |
| Total | 99.57(2.89) | 99.08 (2.34) | 98.42 (2.33) | 99.05 (2.43) | 99.07 (1.81) | 99.05 (2.10) | ||
| % Identified | 74.34 (1.88) | 73.84 (1.56) | 77.94 (1.57) | 78.70 (1.64) | 60.17 (1.24) | 60.61 (1.42) | ||
| Including the following: | ||||||||
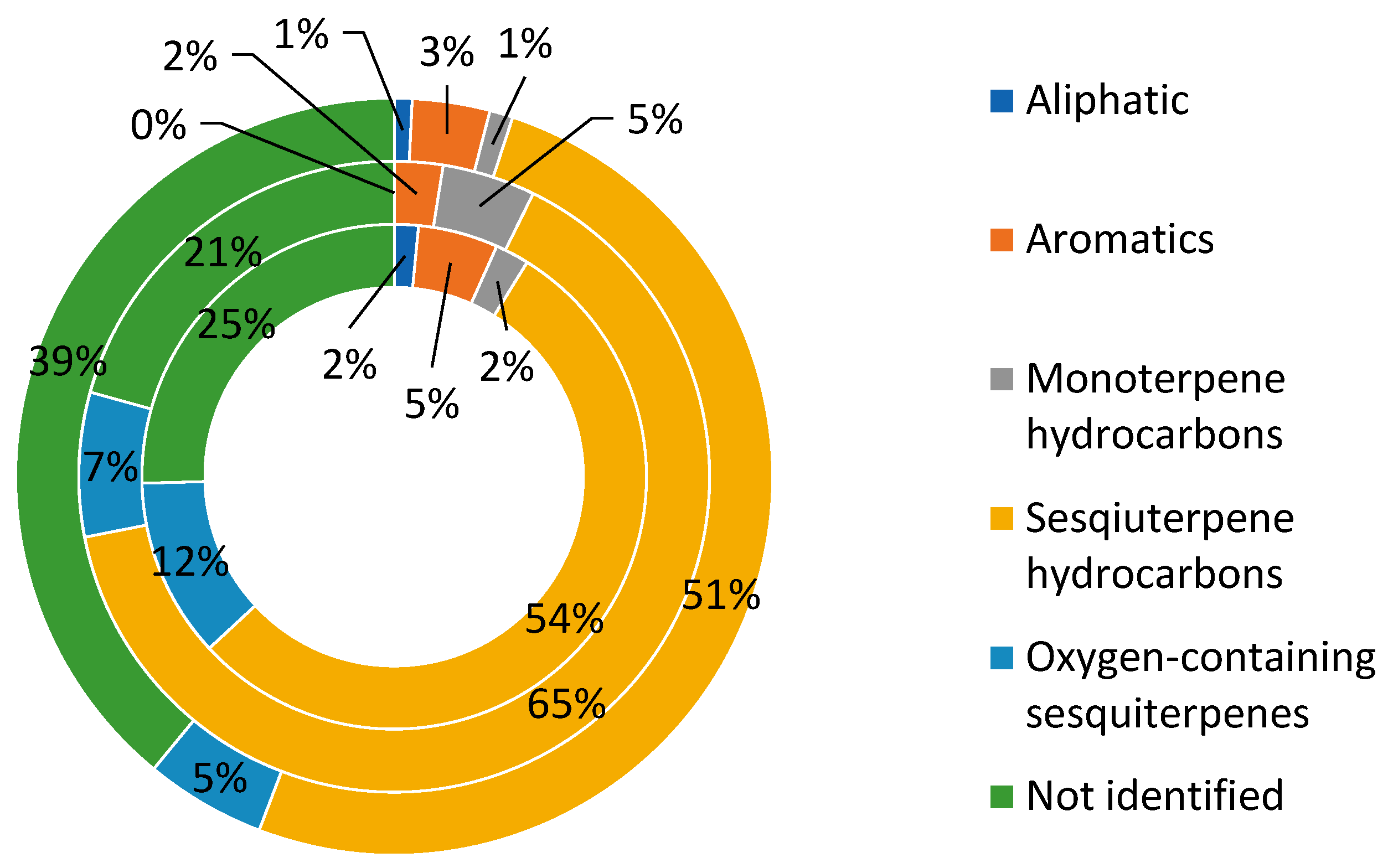
| Aliphatics | 1.55 (0.12) | 1.52 (0.08) | - | - | 0.77 (0.04) | 0.74 (0.04) | ||
| Aromatics | 5.05 (0.22) | 5.12 (0.18) | 2.48 (012) | 2.43 (0.12) | 3.25 (0.11) | 3.33 (0.14) | ||
| Monoterpene hydrocarbons | 2.14 (0.15) | 2.17 (0.11) | 4.71 (0.20) | 4.73 (0.19) | 1.01 (0.06) | 0.95 (0.06) | ||
| Sesquiterpene hydrocarbons | 54.31 (0.99) | 53.47 (0.88) | 63.49 (1.00) | 64.21 (1.07) | 49.91 (0.81) | 50.59 (0.91) | ||
| Sesquiterpenoide hydrocarbons | 11.28 (0.40) | 11.56 (0.31) | 7.26 (0.25) | 7.34 (0.27) | 5.22 (0.23) | 4.99 (0.27) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak, R.; Guzowska, M.; Buczkowska, K.; Bączkiewicz, A. The Composition of Volatile Organic Compounds Correlates with the Genetic Variability Within the Calypogeia sphagnicola Species Complex (Marchantiophyta, Calypogeiaceae). Molecules 2025, 30, 3642. https://doi.org/10.3390/molecules30173642
Wawrzyniak R, Guzowska M, Buczkowska K, Bączkiewicz A. The Composition of Volatile Organic Compounds Correlates with the Genetic Variability Within the Calypogeia sphagnicola Species Complex (Marchantiophyta, Calypogeiaceae). Molecules. 2025; 30(17):3642. https://doi.org/10.3390/molecules30173642
Chicago/Turabian StyleWawrzyniak, Rafał, Małgorzata Guzowska, Katarzyna Buczkowska, and Alina Bączkiewicz. 2025. "The Composition of Volatile Organic Compounds Correlates with the Genetic Variability Within the Calypogeia sphagnicola Species Complex (Marchantiophyta, Calypogeiaceae)" Molecules 30, no. 17: 3642. https://doi.org/10.3390/molecules30173642
APA StyleWawrzyniak, R., Guzowska, M., Buczkowska, K., & Bączkiewicz, A. (2025). The Composition of Volatile Organic Compounds Correlates with the Genetic Variability Within the Calypogeia sphagnicola Species Complex (Marchantiophyta, Calypogeiaceae). Molecules, 30(17), 3642. https://doi.org/10.3390/molecules30173642









