Environmental Antidepressants Disrupt Metabolic Pathways in Spirostomum ambiguum and Daphnia magna: Insights from LC-MS-Based Metabolomics
Abstract
1. Introduction
2. Results
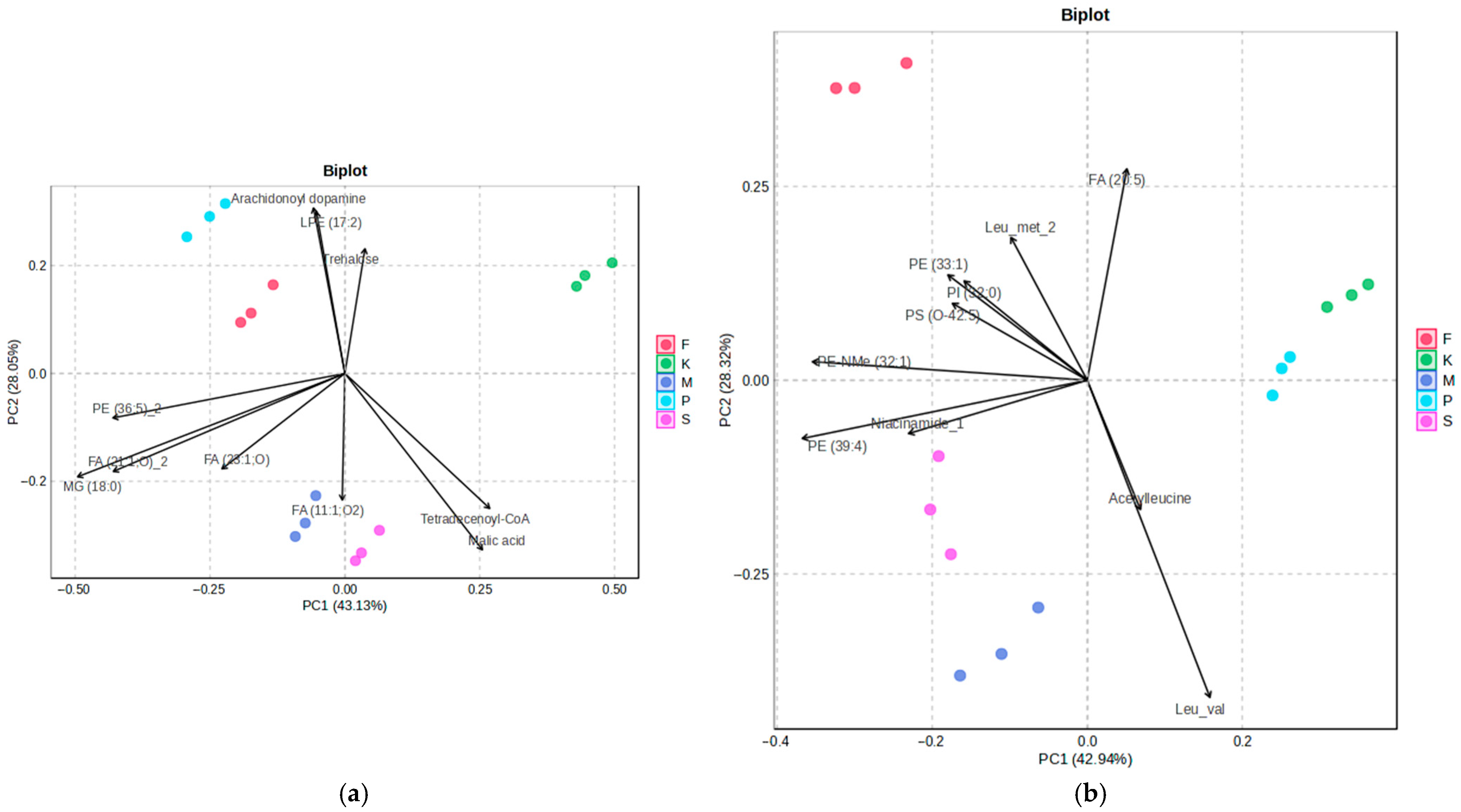
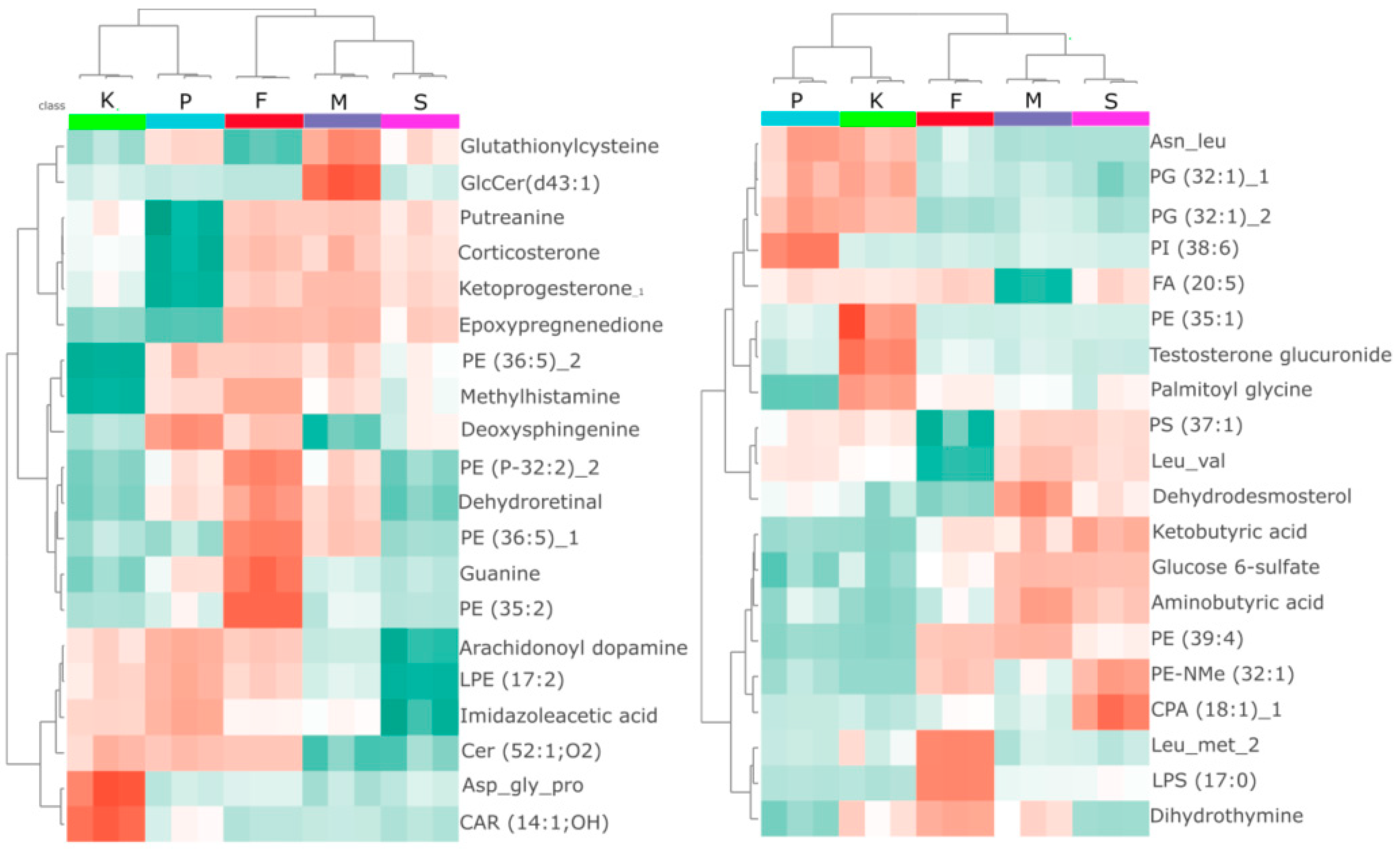
2.1. Multivariate Analysis Reveals Exposure Specific Metabolic Shifts
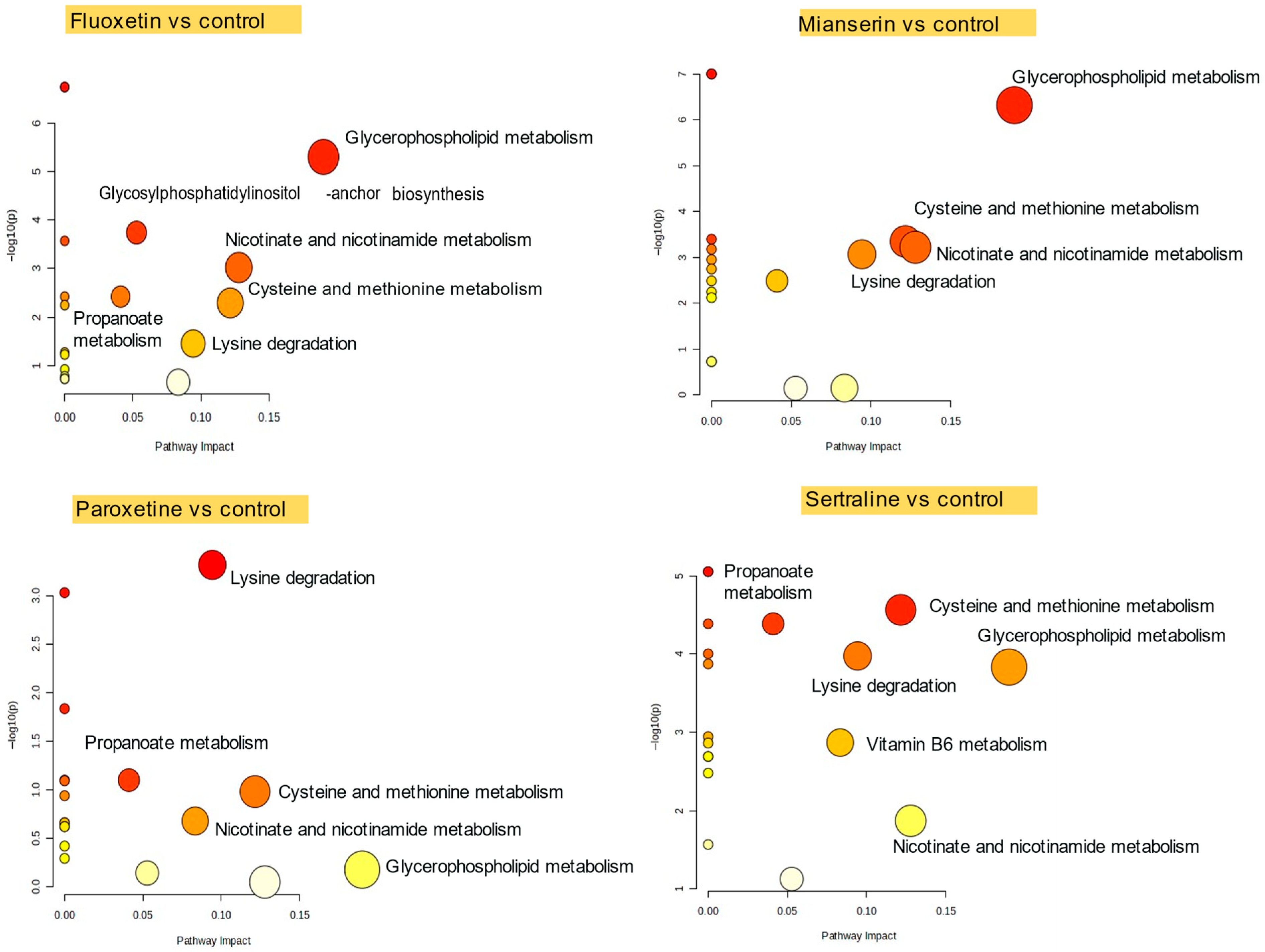
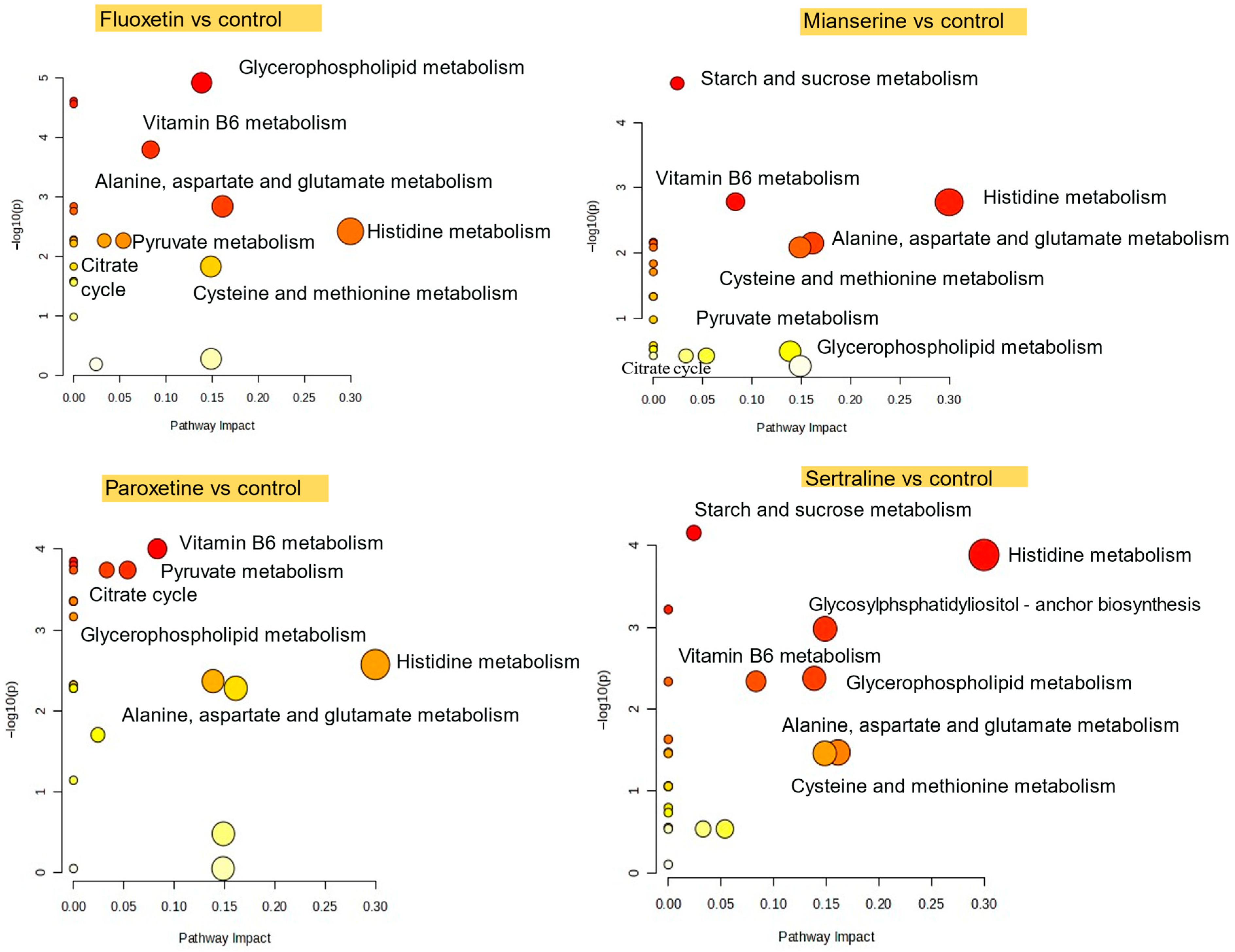
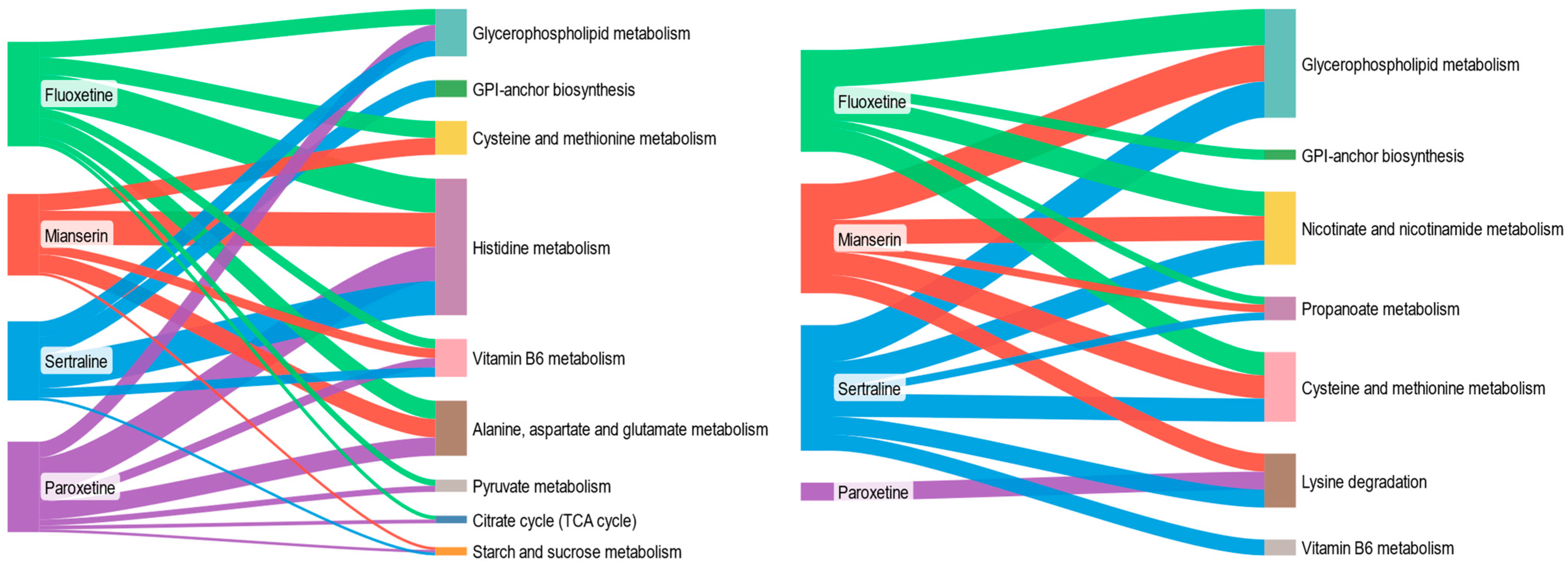
2.2. Pathway-Level Metabolic Alterations in D. magna Following Antidepressant Exposure
2.3. Pathway-Level Metabolic Alterations in S. ambiguum Following Antidepressant Exposure
3. Discussion
4. Materials and Methods
4.1. Culturing and Exposure Conditions for Test Organisms
4.2. Sample Preparation
4.3. Instrumental Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Miller, T.H.; Bury, N.R.; Owen, S.F.; MacRae, J.I.; Barron, L.P. A review of the pharmaceutical exposome in aquatic fauna. Environ. Pollut. 2018, 239, 129–146. [Google Scholar] [CrossRef]
- Aich, U.; Polverino, G.; Yazdan Parast, F.; Melo, G.C.; Tan, H.; Howells, J.; Nosrati, R.; Wong, B.B.M. Long-term effects of widespread pharmaceutical pollution on trade-offs between behavioural, life-history and reproductive traits in fish. J. Anim. Ecol. 2025, 94, 340–355. [Google Scholar] [CrossRef]
- Osorio, V.; Larranaga, A.; Acena, J.; Perez, S.; Barcelo, D. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers. Sci. Total Environ. 2016, 540, 267–277. [Google Scholar] [CrossRef]
- Sumpter, J.P.; Margiotta-Casaluci, L. Environmental Occurrence and Predicted Pharmacological Risk to Freshwater Fish of over 200 Neuroactive Pharmaceuticals in Widespread Use. Toxics 2022, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Rodriguez-Mozaz, S.; Barcelo, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar] [CrossRef]
- Lopez-Serna, R.; Jurado, A.; Vazquez-Sune, E.; Carrera, J.; Petrovic, M.; Barcelo, D. Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Giebultowicz, J.; Nalecz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Arnnok, P.; Singh, R.R.; Burakham, R.; Perez-Fuentetaja, A.; Aga, D.S. Selective Uptake and Bioaccumulation of Antidepressants in Fish from Effluent-Impacted Niagara River. Environ. Sci. Technol. 2017, 51, 10652–10662. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T. Trace analysis of antidepressant pharmaceuticals and their select degradates in aquatic matrixes by LC/ESI/MS/MS. Anal. Chem. 2008, 80, 1756–1762. [Google Scholar] [CrossRef]
- Kleywegt, S.; Payne, M.; Ng, F.; Fletcher, T. Environmental loadings of Active Pharmaceutical Ingredients from manufacturing facilities in Canada. Sci. Total Environ. 2019, 646, 257–264. [Google Scholar] [CrossRef]
- Salgado, R.; Marques, R.; Noronha, J.P.; Mexia, J.T.; Carvalho, G.; Oehmen, A.; Reis, M.A. Assessing the diurnal variability of pharmaceutical and personal care products in a full-scale activated sludge plant. Environ. Pollut. 2011, 159, 2359–2367. [Google Scholar] [CrossRef]
- Kostich, M.S.; Batt, A.L.; Lazorchak, J.M. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut. 2014, 184, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauve, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Jiang, X.; Xia, X.; Zhang, H.; Zheng, S. Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere 2013, 90, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, K.; Giebultowicz, J.; Kamaszewski, M.; Adamski, A.; Szudrowicz, H.; Ostaszewska, T.; Solarska-Dzieciolowska, U.; Nalecz-Jawecki, G.; Wroczynski, P.; Drobniewska, A. Acute exposure of zebrafish (Danio rerio) larvae to environmental concentrations of selected antidepressants: Bioaccumulation, physiological and histological changes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 229, 108670. [Google Scholar] [CrossRef]
- Dumas, T.; Courant, F.; Fenet, H.; Gomez, E. Environmental Metabolomics Promises and Achievements in the Field of Aquatic Ecotoxicology: Viewed through the Pharmaceutical Lens. Metabolites 2022, 12, 186. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Pappalardo, C.; Suarez, E.G.P.; Salvatore, F.; Andolfi, A.; Gesuele, R.; Galdiero, E.; Libralato, G.; Guida, M.; Siciliano, A. Ecotoxicological and metabolomic investigation of chronic exposure of Daphnia magna (Straus, 1820) to yttrium environmental concentrations. Aquat. Toxicol. 2024, 276, 107117. [Google Scholar] [CrossRef]
- O’Rourke, K.; Virgiliou, C.; Theodoridis, G.; Gika, H.; Grintzalis, K. The impact of pharmaceutical pollutants on daphnids—A metabolomic approach. Environ. Toxicol. Pharmacol. 2023, 100, 104157. [Google Scholar] [CrossRef]
- Michorowska, S.; Kucharski, D.; Chojnacka, J.; Nalecz-Jawecki, G.; Marek, D.; Giebultowicz, J. Metabolomic study on ostracods exposed to environmentally relevant concentrations of five pharmaceuticals selected via a novel approach. Sci. Total Environ. 2024, 946, 174036. [Google Scholar] [CrossRef]
- Bouly, L.; Courant, F.; Bonnafe, E.; Carayon, J.L.; Malgouyres, J.M.; Vignet, C.; Gomez, E.; Geret, F.; Fenet, H. Long-term exposure to environmental diclofenac concentrations impairs growth and induces molecular changes in Lymnaea stagnalis freshwater snails. Chemosphere 2022, 291, 133065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y.; Li, H.; Yang, Z.; Zuo, C. Responses in the crucian carp (Carassius auratus) exposed to environmentally relevant concentration of 17alpha-Ethinylestradiol based on metabolomics. Ecotoxicol. Environ. Saf. 2019, 183, 109501. [Google Scholar] [CrossRef] [PubMed]
- Cappello, T.; Fernandes, D.; Maisano, M.; Casano, A.; Bonastre, M.; Bebianno, M.J.; Mauceri, A.; Fasulo, S.; Porte, C. Sex steroids and metabolic responses in mussels Mytilus galloprovincialis exposed to drospirenone. Ecotoxicol. Environ. Saf. 2017, 143, 166–172. [Google Scholar] [CrossRef]
- Mishra, P.; Gong, Z.; Kelly, B.C. Assessing biological effects of fluoxetine in developing zebrafish embryos using gas chromatography-mass spectrometry based metabolomics. Chemosphere 2017, 188, 157–167. [Google Scholar] [CrossRef]
- Puga, A.; Moreira, M.M.; Sanroman, M.A.; Pazos, M.M.; Delerue-Matos, C. Antidepressants and COVID-19: Increased use, occurrence in water and effects and consequences on aquatic environment. A review. Sci. Total Environ. 2024, 953, 175993. [Google Scholar] [CrossRef] [PubMed]
- Wawryniuk, M.; Pietrzak, A.; Nałęcz-Jawecki, G. Evaluation of direct and indirect photodegradation of mianserin with high-performance liquid chromatography and short-term bioassays. Ecotoxicol. Environ. Saf. 2015, 115, 144–151. [Google Scholar] [CrossRef]
- Castano-Ortiz, J.M.; Courant, F.; Gomez, E.; Garcia-Pimentel, M.M.; Leon, V.M.; Campillo, J.A.; Santos, L.; Barcelo, D.; Rodriguez-Mozaz, S. Combined exposure of the bivalve Mytilus galloprovincialis to polyethylene microplastics and two pharmaceuticals (citalopram and bezafibrate): Bioaccumulation and metabolomic studies. J. Hazard. Mater. 2023, 458, 131904. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, X.; Bao, X.; Ling, X.; Yang, H.; Liu, J.; Lu, G.; Ji, Y. Influence of dissolved organic matter on the accumulation, metabolite production and multi-biological effects of environmentally relevant fluoxetine in crucian carp (Carassius auratus). Aquat. Toxicol. 2020, 226, 105581. [Google Scholar] [CrossRef]
- Nalecz-Jawecki, G. Spirotox-Spirostomum ambiguum acute toxicity test-10 years of experience. Environ. Toxicol. 2004, 19, 359–364. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the testing of chemicals, section 2: Effects on biotic systems. In OECD Library; The Organisation for Economic Co-operation and Development: Paris, France, 2004. [Google Scholar] [CrossRef]
- Zhukov, A.; Popov, V. Eukaryotic Cell Membranes: Structure, Composition, Research Methods and Computational Modelling. Int. J. Mol. Sci. 2023, 24, 11226. [Google Scholar] [CrossRef]
- Minguez, L.; Farcy, E.; Ballandonne, C.; Lepailleur, A.; Serpentini, A.; Lebel, J.M.; Bureau, R.; Halm-Lemeille, M.P. Acute toxicity of 8 antidepressants: What are their modes of action? Chemosphere 2014, 108, 314–319. [Google Scholar] [CrossRef]
- Nalecz-Jawecki, G.; Wawryniuk, M.; Giebultowicz, J.; Olkowski, A.; Drobniewska, A. Influence of Selected Antidepressants on the Ciliated Protozoan Spirostomum ambiguum: Toxicity, Bioaccumulation, and Biotransformation Products. Molecules 2020, 25, 1476. [Google Scholar] [CrossRef] [PubMed]
- Atli, G.; Sevgiler, Y. Binary effects of fluoxetine and zinc on the biomarker responses of the non-target model organism Daphnia magna. Environ. Sci. Pollut. Res. 2024, 31, 27988–28006. [Google Scholar] [CrossRef] [PubMed]
- Jordão, R.; Garreta, E.; Campos, B.; Lemos, M.F.L.; Soares, A.M.V.M.; Tauler, R.; Barata, C. Compounds altering fat storage in Daphnia magna. Sci. Total Environ. 2016, 545–546, 127–136. [Google Scholar] [CrossRef]
- Fuertes, I.; Barata, C. Characterization of neurotransmitters and related metabolites in Daphnia magna juveniles deficient in serotonin and exposed to neuroactive chemicals that affect its behavior: A targeted LC-MS/MS method. Chemosphere 2021, 263, 127814. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, L.; Barulin, N.; Alava, J.J.; Liu, S.; Xiong, D. Maternal Daphnia magna exposure to the antidepressant sertraline causes molting disorder, multi-generational reproductive and serotonergic dysfunction. Aquat. Toxicol. 2025, 278, 107161. [Google Scholar] [CrossRef]
- McCoole, M.D.; Atkinson, N.J.; Graham, D.I.; Grasser, E.B.; Joselow, A.L.; McCall, N.M.; Welker, A.M.; Wilsterman, E.J., Jr.; Baer, K.N.; Tilden, A.R.; et al. Genomic analyses of aminergic signaling systems (dopamine, octopamine and serotonin) in Daphnia pulex. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2012, 7, 35–58. [Google Scholar] [CrossRef]
- Campos, B.; Rivetti, C.; Kress, T.; Barata, C.; Dircksen, H. Depressing Antidepressant: Fluoxetine Affects Serotonin Neurons Causing Adverse Reproductive Responses in Daphnia magna. Environ. Sci. Technol. 2016, 50, 6000–6007. [Google Scholar] [CrossRef]
- Ding, J.; Zou, H.; Liu, Q.; Zhang, S.; Mamitiana Razanajatovo, R. Bioconcentration of the antidepressant fluoxetine and its effects on the physiological and biochemical status in Daphnia magna. Ecotoxicol. Environ. Saf. 2017, 142, 102–109. [Google Scholar] [CrossRef]
- Över, S.B.; Güven, C.; Taskin, E.; Sevgiler, Y. Oxidative and apoptotic effects of fluoxetine and its metabolite norfluoxetine in Daphnia magna. Arh. Hig. Rada Toksikol. 2020, 71, 211–222. [Google Scholar] [CrossRef]
- Campos, B.; Piña, B.; Barata, C.C. Mechanisms of Action of Selective Serotonin Reuptake Inhibitors in Daphnia magna. Environ. Sci. Technol. 2012, 46, 2943–2950. [Google Scholar] [CrossRef]
- Campos, B.; Garcia-Reyero, N.; Rivetti, C.; Escalon, L.; Habib, T.; Tauler, R.; Tsakovski, S.; Piña, B.; Barata, C. Identification of Metabolic Pathways in Daphnia magna Explaining Hormetic Effects of Selective Serotonin Reuptake Inhibitors and 4-Nonylphenol Using Transcriptomic and Phenotypic Responses. Environ. Sci. Technol. 2013, 47, 9434–9443. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R. Fluoxetine and the mitochondria: A review of the toxicological aspects. Toxicol. Lett. 2016, 258, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Orlowska, K.; Fling, R.R.; Nault, R.; Schilmiller, A.L.; Zacharewski, T.R. Cystine/Glutamate Xc(-) Antiporter Induction Compensates for Transsulfuration Pathway Repression by 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) to Ensure Cysteine for Hepatic Glutathione Biosynthesis. Chem. Res. Toxicol. 2023, 36, 900–915. [Google Scholar] [CrossRef]
- Jordão, R.; Casas, J.; Fabrias, G.; Campos, B.; Piña, B.; Lemos, M.F.; Soares, A.M.; Tauler, R.; Barata, C. Obesogens beyond Vertebrates: Lipid Perturbation by Tributyltin in the Crustacean Daphnia magna. Environ. Health Perspect. 2015, 123, 813–819. [Google Scholar] [CrossRef]
- Fuertes, I.; Piña, B.; Barata, C. Changes in lipid profiles in Daphnia magna individuals exposed to low environmental levels of neuroactive pharmaceuticals. Sci. Total Environ. 2020, 733, 139029. [Google Scholar] [CrossRef]
- Garreta-Lara, E.; Checa, A.; Fuchs, D.; Tauler, R.; Lacorte, S.; Wheelock, C.E.; Barata, C. Effect of psychiatric drugs on Daphnia magna oxylipin profiles. Sci. Total Environ. 2018, 644, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xing, H.; Chen, Z.; Kong, L.; Jiang, H.; Zhu, T. Comparative Toxicokinetics and Biomarker Responses of Typical Psychiatric Pharmaceuticals in Daphnia magna. Toxics 2025, 13, 481. [Google Scholar] [CrossRef]
- Chojnacka, J.; Drobniewska, A.; Lenga, W.; Misztal, J.; Wawryniuk, M.; Nałęcz-Jawecki, G. The Mutual Effect of Microparticles and Antidepressants on the Protozoan Spirostomum ambiguum (Müller, 1786) Ehrenberg, 1835. Water 2023, 15, 552. [Google Scholar] [CrossRef]
- Twagilimana, L.; Bohatier, J.; Groliere, C.A.; Bonnemoy, F.; Sargos, D. A New Low-Cost Microbiotest with the ProtozoanSpirostomum teres: Culture Conditions and Assessment of Sensitivity of the Ciliate to 14 Pure Chemicals. Ecotoxicol. Environ. Saf. 1998, 41, 231–244. [Google Scholar] [CrossRef]
- Renaud, F.L.; Chiesa, R.; De Jesus, J.M.; Lopez, A.; Miranda, J.; Tomassini, N. Hormones and signal transduction in protozoa. Comp. Biochem. Physiol. A Comp. Physiol. 1991, 100, 41–45. [Google Scholar] [CrossRef]
- Csaba, G.; Kovács, P.; Pállinger, E. How does the unicellular Tetrahymena utilise the hormones that it produces? Paying a visit to the realm of atto-and zeptomolar concentrations. Cell Tissue Res. 2007, 327, 199–203. [Google Scholar] [CrossRef]
- Ferreira, D.D.; Mesquita, J.T.; da Costa Silva, T.A.; Romanelli, M.M.; da Gama Jaen Batista, D.; da Silva, C.F.; da Gama, A.N.S.; Neves, B.J.; Melo-Filho, C.C.; Correia Soeiro, M.N.; et al. Efficacy of sertraline against Trypanosoma cruzi: An in vitro and in silico study. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 30. [Google Scholar] [CrossRef] [PubMed]
- El-Bassat, R.A.; Touliabah, H.E.; Harisa, G.I.; Sayegh, F.A. Aquatic toxicity of various pharmaceuticals on some isolated plankton species. Int. J. Med. Sci. 2012, 3, 170–180. [Google Scholar]
- Servillo, L.; Castaldo, D.; Giovane, A.; Casale, R.; D’Onofrio, N.; Cautela, D.; Balestrieri, M.L. Ophthalmic acid is a marker of oxidative stress in plants as in animals. Biochim. Biophys. Acta (BBA)—General. Subj. 2018, 1862, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, N.; Kaur, P.K.; Swamy, K.K.; Singh, S. Mianserin, an antidepressant kills Leishmania donovani by depleting ergosterol levels. Exp. Parasitol. 2014, 144, 84–90. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Rovira García, X.; Martínez-Jerónimo, F.; Marcé, R.M.; Barata, C. Analysis of neurotransmitters in Daphnia magna affected by neuroactive pharmaceuticals using liquid chromatography-high resolution mass spectrometry. Environ. Pollut. 2019, 254, 113029. [Google Scholar] [CrossRef]
- Daphtoxkit, F. Daphnia magna. Crustacean Toxicity Screening Test for Freshwater. Standard Operational Procedure. Creasel: Deinze, Belgium. 1996. Available online: http://www.microbiotests.be/SOPs/Daphtoxkit%20magna%20F%20SOP%20-%20A5.pdf (accessed on 15 April 2025).
- Mole, R.A.; Brooks, B.W. Global scanning of selective serotonin reuptake inhibitors: Occurrence, wastewater treatment and hazards in aquatic systems. Environ. Pollut. 2019, 250, 1019–1031. [Google Scholar] [CrossRef]
- Davis, J.M.; Ekman, D.R.; Skelton, D.M.; LaLone, C.A.; Ankley, G.T.; Cavallin, J.E.; Villeneuve, D.L.; Collette, T.W. Metabolomics for informing adverse outcome pathways: Androgen receptor activation and the pharmaceutical spironolactone. Aquat. Toxicol. 2017, 184, 103–115. [Google Scholar] [CrossRef]
- Bedia, C. Metabolomics in environmental toxicology: Applications and challenges. Trends Environ. Anal. Chem. 2022, 34, e00161. [Google Scholar] [CrossRef]
- Olesti, E.; González-Ruiz, V.; Wilks, M.F.; Boccard, J.; Rudaz, S. Approaches in metabolomics for regulatory toxicology applications. Analyst 2021, 146, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędreas, A.; Michorowska, S.; Drobniewska, A.; Giebułtowicz, J. Environmental Antidepressants Disrupt Metabolic Pathways in Spirostomum ambiguum and Daphnia magna: Insights from LC-MS-Based Metabolomics. Molecules 2025, 30, 2952. https://doi.org/10.3390/molecules30142952
Jędreas A, Michorowska S, Drobniewska A, Giebułtowicz J. Environmental Antidepressants Disrupt Metabolic Pathways in Spirostomum ambiguum and Daphnia magna: Insights from LC-MS-Based Metabolomics. Molecules. 2025; 30(14):2952. https://doi.org/10.3390/molecules30142952
Chicago/Turabian StyleJędreas, Artur, Sylwia Michorowska, Agata Drobniewska, and Joanna Giebułtowicz. 2025. "Environmental Antidepressants Disrupt Metabolic Pathways in Spirostomum ambiguum and Daphnia magna: Insights from LC-MS-Based Metabolomics" Molecules 30, no. 14: 2952. https://doi.org/10.3390/molecules30142952
APA StyleJędreas, A., Michorowska, S., Drobniewska, A., & Giebułtowicz, J. (2025). Environmental Antidepressants Disrupt Metabolic Pathways in Spirostomum ambiguum and Daphnia magna: Insights from LC-MS-Based Metabolomics. Molecules, 30(14), 2952. https://doi.org/10.3390/molecules30142952


.png)