Advances in Nanotheranostic Systems for Concurrent Cancer Imaging and Therapy: An Overview of the Last 5 Years
Abstract
1. Introduction
2. Key Components of Nanotheranostic Systems
2.1. Characteristics of Ideal Nanoparticle-Based Carriers for Nanotheranostic Systems
2.2. Surface Modifiers of Nanoparticle-Based Carriers
2.3. Imaging and Therapeutic Agents in Nanotheranostic Systems
2.3.1. Imaging Agents in Nanotheranostic Systems
2.3.2. Therapeutic Agents in Nanotheranostic Systems
3. Conventional and Upcoming Imaging and Therapeutic Modalities Enabled by Nanotheranostic Systems
3.1. Conventional and Upcoming Imaging Modalities
3.2. Conventional and Upcoming Therapeutic Modalities
4. In Vitro and In Vivo Studies of Cancer Nanotheranostic Systems
4.1. Metal-Based Cancer Nanotheranostic Systems
4.1.1. Iron-Based Nanotheranostic Systems
4.1.2. Gold-Based Cancer Nanotheranostic Systems
4.2. Non-Metal-Based Cancer Nanotheranostic Systems
4.2.1. Polymer-Based Cancer Nanotheranostic Systems
4.2.2. Silica-Based Cancer Nanotheranostic Systems
4.2.3. Liposome-Based Cancer Nanotheranostic Systems
4.2.4. Dendrimer-Based Cancer Nanotheranostic Systems
4.2.5. Carbon-Based Cancer Nanotheranostic Systems
4.3. Core–Shell Nanoparticles and Nanocomposite Hybrids
5. Summary, Conclusions, and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Funkhouser, J. Reinventing pharma: The theranostic revolution. Curr. Drug Discov. 2002, 2, 17–19. [Google Scholar]
- Fahey, F.H.; Grant, F.D.; Thrall, J.H. Saul, Hertz, MD, and the birth of radionuclide therapy. EJNMMI Phys. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Królicki, L.; Kunikowska, J. Theranostics—Present and Future. Bio-Algorithms Med-Syst. 2021, 17, 213–220. [Google Scholar] [CrossRef]
- Marin, J.F.G.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-Emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Applications of Nanomaterials in Cancer Theranostics: Recent Advances and Challenges. Available online: https://www.researchgate.net/publication/321823702_Applications_of_Nanomaterials_in_Cancer_Theranostics_Recent_Advances_and_Challenges (accessed on 15 December 2017).
- Kumar, D.; Mutreja, I.; Kaushik, A. Recent Advances in Noble Metal Nanoparticles for Cancer Nanotheranostics. J. Nanotheranostics 2023, 4, 150–170. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mohammadnejad, J.; Salamat, S.; Beiram Zadeh, Z.; Tanhaei, M.; Ramakrishna, S. Theranostic Polymeric Nanoparticles as a New Approach in Cancer Therapy and Diagnosis: A Review. Mater. Today Chem. 2023, 29, 101400. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Mackeyev, Y.; Krishnan, S.; Bhandary, S. Mesoporous Silica Nanotechnology: Promising Advances in Augmenting Cancer Theranostics. Cancer Nanotechnol. 2024, 15, 9. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Khalbas, A.H.; Albayati, T.M.; Cata Saady, N.M.; Zendehboudi, S.; Salih, S.I.; Tofahe, M.L. Insights into drug loading techniques with mesoporous silica nanoparticles: Optimization of operating conditions and assessment of drug stability. J. Drug Deliv. Sci. Technol. 2024, 96, 105698. [Google Scholar] [CrossRef]
- Lou, H.; Hageman, M.J. Development of Drug Release Model for Suspensions in ESCAR (Emulator of SubCutaneous Absorption and Release). AAPS J. 2023, 25, 29. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, S.; Wan, J.; Wang, D.; Pang, X.; Gao, Y.; Ni, N.; Chen, D.; Sun, X. Disturbing cytoskeleton by engineered nanomaterials for enhanced cancer therapeutics. Bioact. Mater. 2023, 29, 50–71. [Google Scholar] [CrossRef]
- Thu, H.E.; Haider, M.; Khan, S.; Sohail, M.; Hussain, Z. Nanotoxicity induced by nanomaterials: A review of factors affecting nanotoxicity and possible adaptations. OpenNano 2023, 14, 100190. [Google Scholar] [CrossRef]
- Vega-Villa, K.R.; Takemoto, J.K.; Yáñez, J.A.; Remsberg, C.M.; Forrest, M.L.; Davies, N.M. Clinical toxicities of nanocarrier systems. Adv. Drug Deliv. Rev. 2008, 60, 929–938. [Google Scholar] [CrossRef]
- Kaymaz, S.V.; Nobar, H.M.; Sarıgül, H.; Soylukan, C.; Akyüz, L.; Yüce, M. Nanomaterial surface modification toolkit: Principles, components, recipes, and applications. Adv. Colloid Interface Sci. 2023, 322, 103035. [Google Scholar] [CrossRef]
- Prasad, R.; Selvaraj, K. Choice of Nanoparticles for Theranostics Engineering: Surface Coating to Nanovalves Approach. Nanotheranostics 2024, 8, 12–32. [Google Scholar] [CrossRef]
- Niculescu, V.C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold Nanoparticles (GNPs) in Biomedical and Clinical Applications: A Review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent Strategies towards the Surface Modification of Liposomes: An Innovative Approach for Different Clinical Applications. 3 Biotech. 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K. Surface Modification of Colloidal CdX-Based Quantum Dots for Biomedical Applications. J. Mater. Chem. 2010, 20, 8433–8445. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Z.; Li, Y.; Yang, J.; Zhang, X. Surface Modification of Iron Oxide-Based Magnetic Nanoparticles for Cerebral Theranostics: Application and Prospection. Nanomaterials 2020, 10, 1441. [Google Scholar] [CrossRef]
- Kawahara, D.; Nagata, Y. T1-Weighted and T2-Weighted MRI Image Synthesis with Convolutional Generative Adversarial Networks. Rep. Pract. Oncol. Radiother. 2021, 26, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI Contrast Agents: Classification and Application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Yeh, B.M.; FitzGerald, P.F.; Edic, P.M.; Lambert, J.W.; Colborn, R.E.; Marino, M.E.; Evans, P.M.; Roberts, J.C.; Wang, Z.J.; Wong, M.J.; et al. Opportunities for New CT Contrast Agents to Maximize the Diagnostic Potential of Emerging Spectral CT Technologies. Adv. Drug Deliv. Rev. 2017, 113, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Tekieli, Ł.; Szot, W.; Kwiecień, E.; Mazurek, A.; Borkowska, E.; Czyż, Ł.; Dąbrowski, M.; Kozynacka, A.; Skubera, M.; Podolec, P.; et al. Single-Photon Emission Computed Tomography as a Fundamental Tool in Evaluation of Myocardial Reparation and Regeneration Therapies. Postep. W Kardiol. Interwencyjnej 2022, 18, 326–339. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- Apriyanto, D.K.; Mitrayana; Setiawan, A.; Widyaningrum, R. Therapeutic and Contrast Agents for Photoacoustic Imaging-Guided Photothermal Therapy: A Narrative Review. Nanotheranostics 2024, 8, 506–520. [Google Scholar] [CrossRef]
- Qiu, T.; Lan, Y.; Gao, W.; Zhou, M.; Liu, S.; Huang, W.; Zeng, S.; Pathak, J.L.; Yang, B.; Zhang, J. Photoacoustic Imaging as a Highly Efficient and Precise Imaging Strategy for the Evaluation of Brain Diseases. Quant. Imaging Med. Surg. 2021, 11, 2169–2186. [Google Scholar] [CrossRef]
- Kenry; Duan, Y.; Liu, B. Recent Advances of Optical Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, 1802394. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, D.; He, L.; Yuan, L. Recent Progresses in NIR-I/II Fluorescence Imaging for Surgical Navigation. Front. Bioeng. Biotechnol. 2021, 9, 768698. [Google Scholar] [CrossRef]
- Sobhana, S.; Sarathy, N.P.; Karthikeyan, L.; Shanthi, K.; Vivek, R. Ultra-Small NIR-Responsive Nanotheranostic Agent for Targeted Photothermal Ablation Induced Damage-Associated Molecular Patterns (DAMPs) from Post-PTT of Tumor Cells Activate Immunogenic Cell Death. Nanotheranostics 2023, 7, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.G.; Chan, K.L.A. Applications of ATR-FTIR Spectroscopic Imaging to Biomedical Samples. Biochim. Biophys. Acta Biomembr. 2006, 1758, 858–867. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Padaga, S.G.; Ghosh, B.; Biswas, S. Nanotherapeutic Intervention in Photodynamic Therapy for Cancer. ACS Omega 2022, 7, 45882–45909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal Therapy and Photoacoustic Imaging: Via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Jyothi, V.G.S.S.; Kommineni, N. Peptide Conjugated Boron Neutron Capture Therapy for Enhanced Tumor Targeting. Nanotheranostics 2024, 8, 458–472. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shi, Y.; Du, P.; Zhang, Z.; Liu, T.; Zhang, R.; Liu, Z. On-Demand Biodegradable Boron Nitride Nanoparticles for Treating Triple Negative Breast Cancer with Boron Neutron Capture Therapy. ACS Nano 2019, 13, 13843–13852. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, A.; Abdullah, B.J.J.; Abu Kasim, N.H.; Abdullah, H.; Hamdi, M. A New Mechanism of Thermal Sensitivity for Rapid Drug Release and Low Systemic Toxicity in Hyperthermia and Thermal Ablation Temperature Ranges. Int. J. Hyperth. 2015, 31, 375–385. [Google Scholar] [CrossRef]
- Cheng, C.A.; Chen, W.; Zhang, L.; Wu, H.H.; Zink, J.I. A Responsive Mesoporous Silica Nanoparticle Platform for Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound-Stimulated Cargo Delivery with Controllable Location, Time, and Dose. J. Am. Chem. Soc. 2019, 141, 17670–17684. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Menon, J.U.; Takahashi, M.; Hsieh, J.T.; Yang, J.; Nguyen, K.T.; Wadajkar, A.S. Thermo-Responsive Fluorescent Nanoparticles for Multimodal Imaging and Treatment of Cancers. Nanotheranostics 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Shen, H.; Liu, E.; Xu, S.; Tang, W.; Sun, J.; Gao, Z.; Gong, J. Modular Assembly of Drug and Monodisperse SPIONs for Superior Magnetic and T2-Imaging Performance. Bioconjug Chem. 2021, 32, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Ngen, E.J.; Chen, Y.; Azad, B.B.; Boinapally, S.; Jacob, D.; Lisok, A.; Shen, C.; Hossain, M.S.; Jin, J.; Bhujwalla, Z.M.; et al. Prostate-Specific Membrane Antigen (Psma)-Targeted Photodynamic Therapy Enhances the Delivery of PSMA-Targeted Magnetic Nanoparticles to Psma-Expressing Prostate Tumors. Nanotheranostics 2021, 5, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, H.; Mao, Q.; Tao, Q.; Yuan, G.; Zeng, L.; Chen, Z.; Zhang, Y.; Cheng, L.; Zhang, J.; et al. Macrophage-Mediated Porous Magnetic Nanoparticles for Multimodal Imaging and Postoperative Photothermal Therapy of Gliomas. ACS Appl. Mater. Interfaces 2021, 13, 56825–56837. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, H.; Gholibegloo, E.; Mortezazadeh, T.; Yazdi, M.H.; Ashouri, F.; Malekzadeh, R.; Najafi, A.; Foroumadi, A.; Khoobi, M. A Biocompatible Theranostic Nanoplatform Based on Magnetic Gadolinium-Chelated Polycyclodextrin: In Vitro and in Vivo Studies. Carbohydr. Polym. 2021, 254, 117262. [Google Scholar] [CrossRef]
- Yang, C.; Che, X.; Zhang, Y.; Gu, D.; Dai, G.; Shu, J.; Yang, L. Hybrid FeWO4-Hyaluronic Acid Nanoparticles as a Targeted Nanotheranostic Agent for Multimodal Imaging-Guided Tumor Photothermal Therapy. Int. J. Nanomed. 2023, 18, 8023–8037. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, Z.; Liu, H.; Wang, X.; Hua, J.; Ling, Y.; Wei, L.; Xia, Y.; Sun, S.; Xiao, L. Cell Membrane Coated Smart Two-Dimensional Supraparticle for in Vivo Homotypic Cancer Targeting and Enhanced Combinational Theranostics. Nanotheranostics 2021, 5, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.; Borum, R.M.; Zhou, J.; Mantri, Y.; Wu, Z.; Zhou, J.; Jin, Z.; Creyer, M.; Jokerst, J.V. Ultrasmall Gold Nanorod-Polydopamine Hybrids for Enhanced Photoacoustic Imaging and Photothermal Therapy in Second near-Infrared Window. Nanotheranostics 2022, 6, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Mendes, C.; D’onofrio, A.; Campello, M.P.C.; Marques, F.; Pinheiro, T.; Gonçalves, K.; Figueiredo, S.; Gano, L.; Ravera, M.; et al. Image-Guided Nanodelivery of Pt(IV) Prodrugs to GRP-Receptor Positive Tumors. Nanotheranostics 2023, 7, 22–40. [Google Scholar] [CrossRef]
- Vogel, S.; O’Keefe, A.; Seban, L.; Valceski, M.; Engels, E.; Khochaiche, A.; Hollis, C.; Lerch, M.; Corde, S.; Massard, C.; et al. Fluorescent Gold Nanoparticles in Suspension as an Efficient Theranostic Agent for Highly Radio-Resistant Cancer Cells. J. Nanotheranostics 2023, 4, 37–54. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Zhang, Y.; Xin, X.; Li, R.; Xie, C.; Fan, Q. Iodine-Rich Semiconducting Polymer Nanoparticles for CT/Fluorescence Dual-Modal Imaging-Guided Enhanced Photodynamic Therapy. Small 2020, 16, e1905641. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.; Ramesh, A.; Nandi, D.; Nguyen, A.; Brouillard, A.; Kulkarni, A. Supramolecular Polysaccharide Nanotheranostics That Inhibit Cancer Cells Growth and Monitor Targeted Therapy Response. Nanotheranostics 2020, 4, 156–172. [Google Scholar] [CrossRef]
- McCabe-Lankford, E.; McCarthy, B.; Berwick, M.A.P.; Salafian, K.; Galarza-Paez, L.; Sarkar, S.; Sloop, J.; Donati, G.; Brown, A.J.; Levi-Polyachenko, N. Binding of Targeted Semiconducting Photothermal Polymer Nanoparticles for Intraperitoneal Detection and Treatment of Colorectal Cancer. Nanotheranostics 2020, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Meher, N.; Seo, K.; Wang, S.; Bidkar, A.P.; Fogarty, M.; Dhrona, S.; Huang, X.; Tang, R.; Blaha, C.; Evans, M.J.; et al. Synthesis and Preliminary Biological Assessment of Carborane-Loaded Theranostic Nanoparticles to Target Prostate-Specific Membrane Antigen. ACS Appl. Mater. Interfaces 2021, 13, 54739–54752. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, J.; Hou, M.; Yang, M.; Yi, C. Gadolinium-Porphyrin Based Polymer Nanotheranostics for Fluorescence/Magnetic Resonance Imaging Guided Photodynamic Therapy. Nanoscale 2021, 13, 16197–16206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Shen, Y.; Yu, Q.S.; Gan, Z.H. Paclitaxel Prodrug Nanomedicine for Potential CT-Imaging Guided Breast Cancer Therapy. Chin. J. Polym. Sci. 2023, 41, 1747–1759. [Google Scholar] [CrossRef]
- Tian, Y.; Carrillo-Malani, N.; Feng, K.; Miller, J.; Busch, T.M.; Sundaram, K.M.; Cheng, Z.; Amirshaghaghi, A.; Tsourkas, A. Theranostic Phthalocyanine and Naphthalocyanine Nanoparticles for Photoacoustic Imaging and Photothermal Therapy of Tumors. Nanotheranostics 2024, 8, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Mehata, A.K.; Singh, V.; Vikas; Srivastava, P.; Koch, B.; Kumar, M.; Muthu, M.S. Chitosan Nanoplatform for the Co-Delivery of Palbociclib and Ultra-Small Magnesium Nanoclusters: Dual Receptor Targeting, Therapy and Imaging. Nanotheranostics 2024, 8, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.M.; Lu, Y.F.; Zhou, J.P.; Yang, X.Y.; Wang, X.J.; Yu, J.N.; Du, Y.Z.; Yu, R.S. Self-Amplification of Oxidative Stress with Tumour Microenvironment-Activatable Iron-Doped Nanoplatform for Targeting Hepatocellular Carcinoma Synergistic Cascade Therapy and Diagnosis. J. Nanobiotechnology 2021, 19, 361. [Google Scholar] [CrossRef]
- Fan, N.; Li, P.; Wu, C.; Wang, X.; Zhou, Y.; Tang, B. ALP-Activated Chemiluminescence PDT Nano-Platform for Liver Cancer-Specific Theranostics. ACS Appl. Bio Mater. 2021, 4, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Jain, N.K.; Yadav, A.S.; Jadhav, M.; Radharani, N.N.V.; Gorain, M.; Kundu, G.C.; Conde, J.; Srivastava, R. Ultrahigh Penetration and Retention of Graphene Quantum Dot Mesoporous Silica Nanohybrids for Image Guided Tumor Regression. ACS Appl. Bio Mater. 2021, 4, 1693–1703. [Google Scholar] [CrossRef]
- Ovejero-Paredes, K.; Díaz-García, D.; Mena-Palomo, I.; Marciello, M.; Lozano-Chamizo, L.; Morato, Y.L.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Synthesis of a Theranostic Platform Based on Fibrous Silica Nanoparticles for the Enhanced Treatment of Triple-Negative Breast Cancer Promoted by a Combination of Chemotherapeutic Agents. Biomater. Adv. 2022, 137, 212823. [Google Scholar] [CrossRef]
- Liu, S.; Hou, X.; Zhu, W.; Zhang, F.; Chen, W.; Yang, B.; Luo, X.; Wu, D.; Cao, Z. Lipid Perfluorohexane Nanoemulsion Hybrid for MRI-Guided High-Intensity Focused Ultrasound Therapy of Tumors. Front. Bioeng. Biotechnol. 2022, 10, 846446. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Ma, G.; Karatasos, K.; Seitsonen, J.; Ruokolainen, J.; Koffi, C.R.; Hassan, H.A.F.M.; Al-Jamal, W.T. Liposome-Templated Indocyanine Green J-Aggregates for in Vivo near-Infrared Imaging and Stable Photothermal Heating. Nanotheranostics 2020, 4, 91–106. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Guo, Z.; Fu, M.; Tang, H.; Zhu, H.; Shen, G.; He, Y.; Lei, P. Establishment of a HTfr MAb-Functionalized HPPS Theranostic Nanoplatform. Nanotheranostics 2020, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Wang, X.; Liu, X.; Cheng, H.; Wang, P.; Shen, Y.; Xie, A.; Zhu, M. Self-Assembled Au4Cu4/Au25NCs@liposome Tumor Nanotheranostics with PT/Fluorescence Imaging-Guided Synergetic PTT/PDT. J. Mater. Chem. B 2021, 9, 6396–6405. [Google Scholar] [CrossRef]
- Amrahli, M.; Centelles, M.; Cressey, P.; Prusevicius, M.; Gedroyc, W.; Xu, X.Y.; So, P.W.; Wright, M.; Thanou, M. Mr-Labelled Liposomes and Focused Ultrasound for Spatiotemporally Controlled Drug Release in Triple Negative Breast Cancers in Mice. Nanotheranostics 2021, 5, 125–142. [Google Scholar] [CrossRef]
- Fan, Y.; Tu, W.; Shen, M.; Chen, X.; Ning, Y.; Li, J.; Chen, T.; Wang, H.; Yin, F.; Liu, Y.; et al. Targeted Tumor Hypoxia Dual-Mode CT/MR Imaging and Enhanced Radiation Therapy Using Dendrimer-Based Nanosensitizers. Adv. Funct. Mater. 2020, 30, 1909285. [Google Scholar] [CrossRef]
- Wang, K.; Tu, Y.; Yao, W.; Zong, Q.; Xiao, X.; Yang, R.M.; Jiang, X.Q.; Yuan, Y. Size-Switchable Nanoparticles with Self-Destructive and Tumor Penetration Characteristics for Site-Specific Phototherapy of Cancer. ACS Appl. Mater. Interfaces 2020, 12, 6933–6943. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, P.; Bai, L.; Ma, J.; Li, S.; Zhang, F.; Liu, S.; Wu, Q.; Shen, H.; Liu, H. Biodegradable Nanocomposite with Dual Cell-Tissue Penetration for Deep Tumor Chemo-Phototherapy. Small 2020, 16, e2000809. [Google Scholar] [CrossRef]
- Ouyang, Z.; Li, D.; Xiong, Z.; Song, C.; Gao, Y.; Liu, R.; Shen, M.; Shi, X. Antifouling Dendrimer-Entrapped Copper Sulfide Nanoparticles Enable Photoacoustic Imaging-Guided Targeted Combination Therapy of Tumors and Tumor Metastasis. ACS Appl. Mater. Interfaces 2021, 13, 6069–6080. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tian, Y.; Zhang, Y.; Xu, J.; Wang, Y.; Guan, X.; Li, T.; Yang, H.; Li, S.; Qin, X.; et al. Acid-Triggered Charge-Convertible Graphene-Based All-in-One Nanocomplex for Enhanced Genetic Phototherapy of Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2020, 9, e1901187. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, M.Z.; Aung, Y.Y.; Ahmad, M.A.; Kristanti, A.N.; Sakti, S.C.W.; Arjasa, O.P.; Lee, H.V. In Vivo Study of Chalcone Loaded Carbon Dots for Enhancement of Anticancer and Bioimaging Potencies. Nanotheranostics 2023, 7, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Meher, M.K.; Unnikrishnan, B.S.; Tripathi, D.K.; Packirisamy, G.; Poluri, K.M. Baicalin Functionalized PEI-Heparin Carbon Dots as Cancer Theranostic Agent. Int. J. Biol. Macromol. 2023, 253, 126846. [Google Scholar] [CrossRef]
- Ricciardi, L.; Chatterjee, S.; Palermo, G.; Szerb, E.I.; Sanna, A.; Palermo, F.; Pieroni, N.; Fratini, M.; Bartolino, R.; Cedola, A.; et al. Hybrid Nanoparticles as Theranostics Platforms for Glioblastoma Treatment: Phototherapeutic and X-Ray Phase Contrast Tomography Investigations. J. Nanotheranostics 2022, 3, 1–17. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, M.; Fu, G.; Zhang, L.; Yu, H.; Yan, X.; Liu, F.; Sun, P.; Jia, X.; Liu, X.; et al. Ti3C2MXene Nanosheets Functionalized with NaErF4:0.5%Tm@NaLuF4Nanoparticles for Dual-Modal Near-Infrared IIb/Magnetic Resonance Imaging-Guided Tumor Hyperthermia. ACS Appl. Nano Mater. 2022, 5, 8142–8153. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Zhao, R.; Zhou, Y.; Feng, L.; Gai, S.; Yang, P. Pt Decorated Ti3C2Tx MXene with NIR-II Light Amplified Nanozyme Catalytic Activity for Efficient Phototheranostics. ACS Nano 2022, 16, 3105–3118. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Alamzadeh, Z.; Beik, J.; Sarikhani, A.; Mousavi, M.; Irajirad, R.; Khani, T.; Davani, E.S.; Farashahi, A.; Ardakani, T.S.; et al. A 2D Nanotheranostic Platform Based on Graphene Oxide and Phase-Change Materials for Bimodal CT/MR Imaging, NIR-Activated Drug Release, and Synergistic Thermo-Chemotherapy. Nanotheranostics 2022, 6, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Fu, G.; Li, Z.; Liu, Y.; Qi, L.; Liu, K.; Zhao, Z.; Xue, M. Au-Fe3O4 Janus Nanoparticles for Imaging-Guided near Infrared-Enhanced Ferroptosis Therapy in Triple Negative Breast Cancer. J. Colloid. Interface Sci. 2024, 663, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, I.; Singh, H.; Khan, A.; Ahmed Mir, M.; Farooq, B.; Shawl, A.I.; Hassan, S.; Ashraf, S.S.; Samad, Y.A.; Muzamil, S. Metallic Nanoparticles for Imaging and Therapy. In Functional Smart Nanomaterials and Their Theranostics Approaches; Springer Nature: Singapore, 2024; pp. 65–86. [Google Scholar]
- Fernandes, D.A. Review on Metal-Based Theranostic Nanoparticles for Cancer Therapy and Imaging. Technol. Cancer Res. Treat. 2023, 22, 15330338231191493. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.; Shahbazi-Gahrouei, D.; Safari, A. Recent Metal Nanotheranostics for Cancer Diagnosis and Therapy: A Review. Diagnostics 2023, 13, 833. [Google Scholar] [CrossRef]
- Iqbal, A.; Iqbal, K.; Li, B.; Gong, D.; Qin, W. Recent Advances in Iron Nanoparticles: Preparation, Properties, Biological and Environmental Application. J. Nanosci. Nanotechnol. 2017, 17, 4386–4409. [Google Scholar] [CrossRef]
- Chala, G.H. Review on Green Synthesis of Iron-Based Nanoparticles for Environmental Applications. J. Chem. Rev. 2023, 5, 1–14. [Google Scholar]
- Luo, S.; Qin, S.; Oudeng, G.; Zhang, L. Iron-Based Hollow Nanoplatforms for Cancer Imaging and Theranostics. Nanomaterials 2022, 12, 3023. [Google Scholar] [CrossRef]
- Shang, L.; Zhou, X.; Zhang, J.; Shi, Y.; Zhong, L. Metal Nanoparticles for Photodynamic Therapy: A Potential Treatment for Breast Cancer. Molecules 2021, 26, 6532. [Google Scholar] [CrossRef] [PubMed]
- Navyatha, B.; Nara, S. Gold Nanotheranostics: Future Emblem of Cancer Nanomedicine. Nanobiomedicine 2021, 8, 18495435211053945. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud. Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Gupta, D.; Roy, P.; Sharma, R.; Kasana, R.; Rathore, P.; Gupta, T.K. Recent Nanotheranostic Approaches in Cancer Research. Clin. Exp. Med. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Gawel, A.M.; Betkowska, A.; Gajda, E.; Godlewska, M.; Gawel, D. Current Non-Metal Nanoparticle-Based Therapeutic Approaches for Glioblastoma Treatment. Biomedicines 2024, 12, 1822. [Google Scholar] [CrossRef] [PubMed]
- Łopuszyńska, N.; Węglarz, W.P. Contrasting Properties of Polymeric Nanocarriers for MRI-Guided Drug Delivery. Nanomaterials 2023, 13, 2163. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Zhang, L. Current Advances in Polymer-Based Nanotheranostics for Cancer Treatment and Diagnosis. ACS Appl. Mater. Interfaces 2014, 6, 21859–21873. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional Nanomedicine with Silica: Role of Silica in Nanoparticles for Theranostic, Imaging, and Drug Monitoring. J. Colloid. Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Redox-Responsive Mesoporous Silica Nanoparticles for Cancer Treatment: Recent Updates. Nanomaterials 2021, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Muthu, M.S.; Feng, S.S. Theranostic Liposomes for Cancer Diagnosis and Treatment: Current Development and Pre-Clinical Success. Expert. Opin. Drug Deliv. 2013, 10, 151–155. [Google Scholar] [CrossRef]
- Lee, W.; Im, H.J. Theranostics Based on Liposome: Looking Back and Forward. Nucl. Med. Mol. Imaging 2019, 53, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, L. Different Nano-Delivery Systems for Delivery of Nutraceuticals. Food Biosci. 2021, 43, 101258. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in Drug Delivery and Targeting: Drug-Dendrimer Interactions and Toxicity Issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Pérez-Ferreiro, M.; Abelairas, A.M.; Criado, A.; Gómez, I.J.; Mosquera, J. Dendrimers: Exploring Their Wide Structural Variety and Applications. Polymers 2023, 15, 4369. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Singh, R.K.; Kim, H.W. Carbon-Based Nanomaterials as an Emerging Platform for Theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of Graphene and Its Disorders: A Review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef]
- Shi, S.; Chen, F.; Ehlerding, E.B.; Cai, W. Surface Engineering of Graphene-Based Nanomaterials for Biomedical Applications. Bioconjug Chem. 2014, 25, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano. 2020, 14, 7760–7782. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef]
- Wulf, V.; Bisker, G. Integrating Single-Walled Carbon Nanotubes into Supramolecular Assemblies: From Basic Interactions to Emerging Applications. ACS Nano 2024, 18, 29380–29393. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. MXenes in Cancer Nanotheranostics. Nanomaterials 2022, 12, 3360. [Google Scholar] [CrossRef]
- Dutta, G.; Manickam, S.; Sugumaran, A. Stimuli-responsive hybrid metal nanocomposite—A promising technology for effective anticancer therapy. Int. J. Pharm. 2022, 624, 121966. [Google Scholar] [CrossRef] [PubMed]
- Shabatina, T.; Vernaya, O.; Shumilkin, A.; Semenov, A.; Melnikov, M. Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications. Materials 2022, 15, 3602. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]

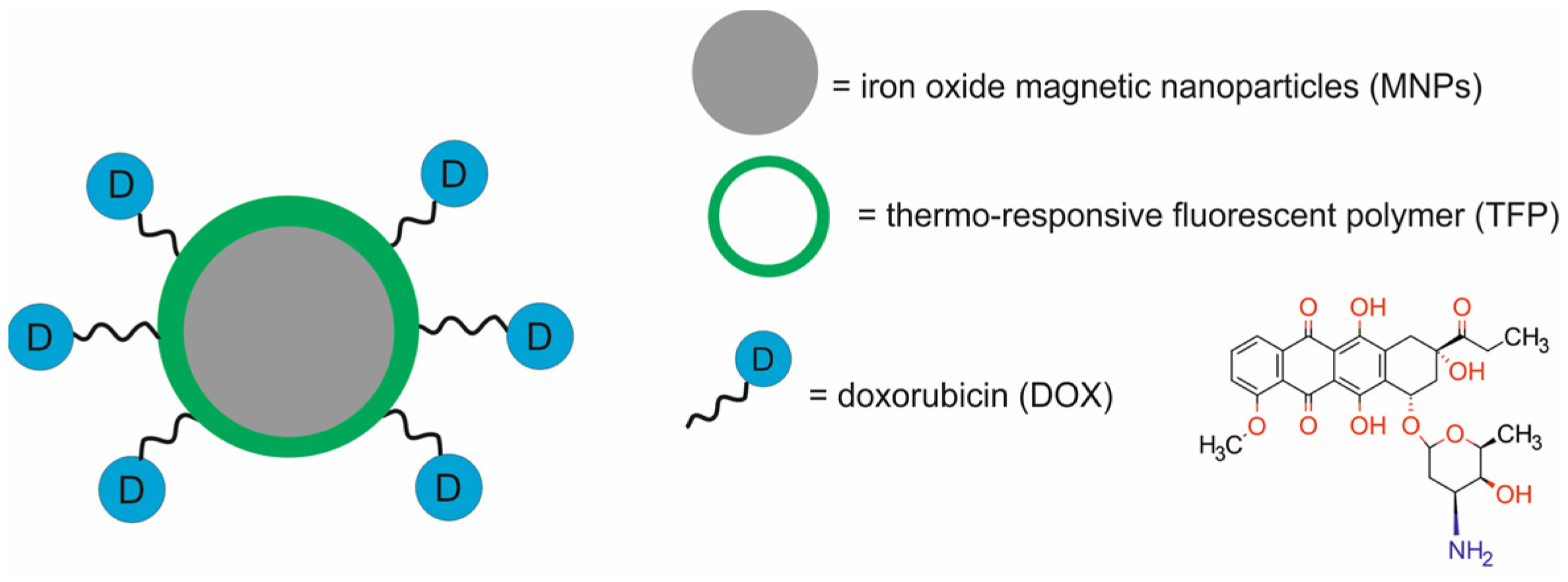

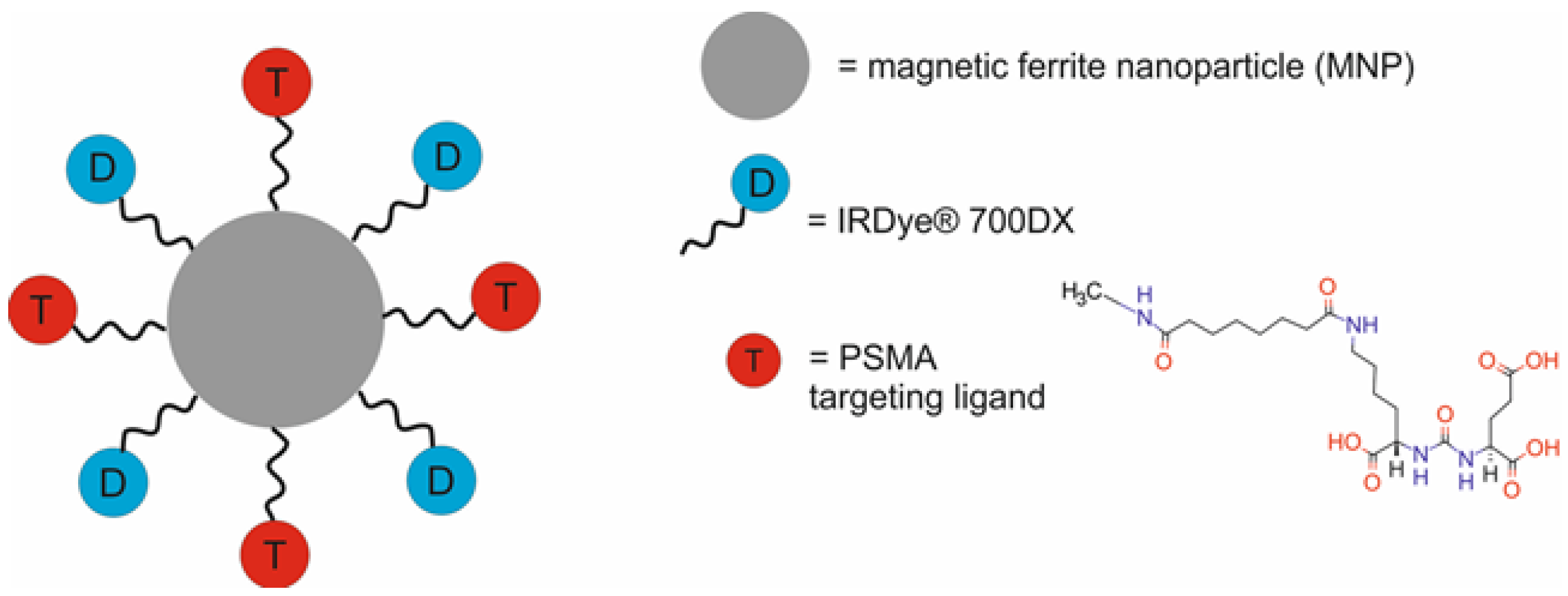
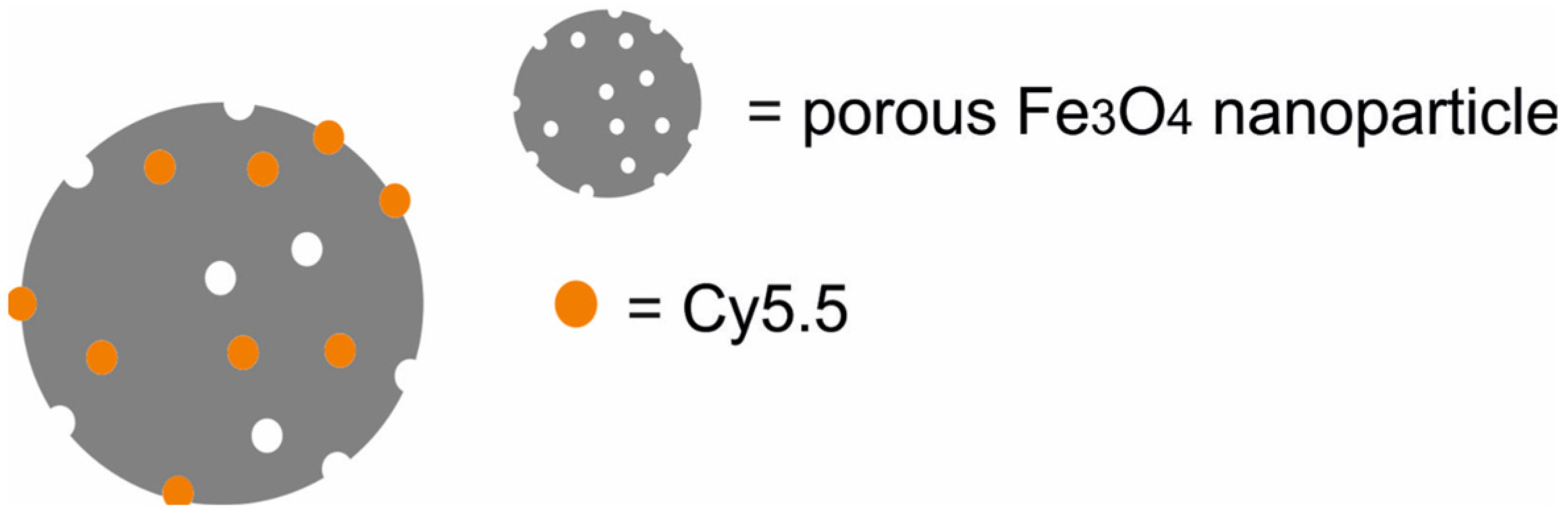
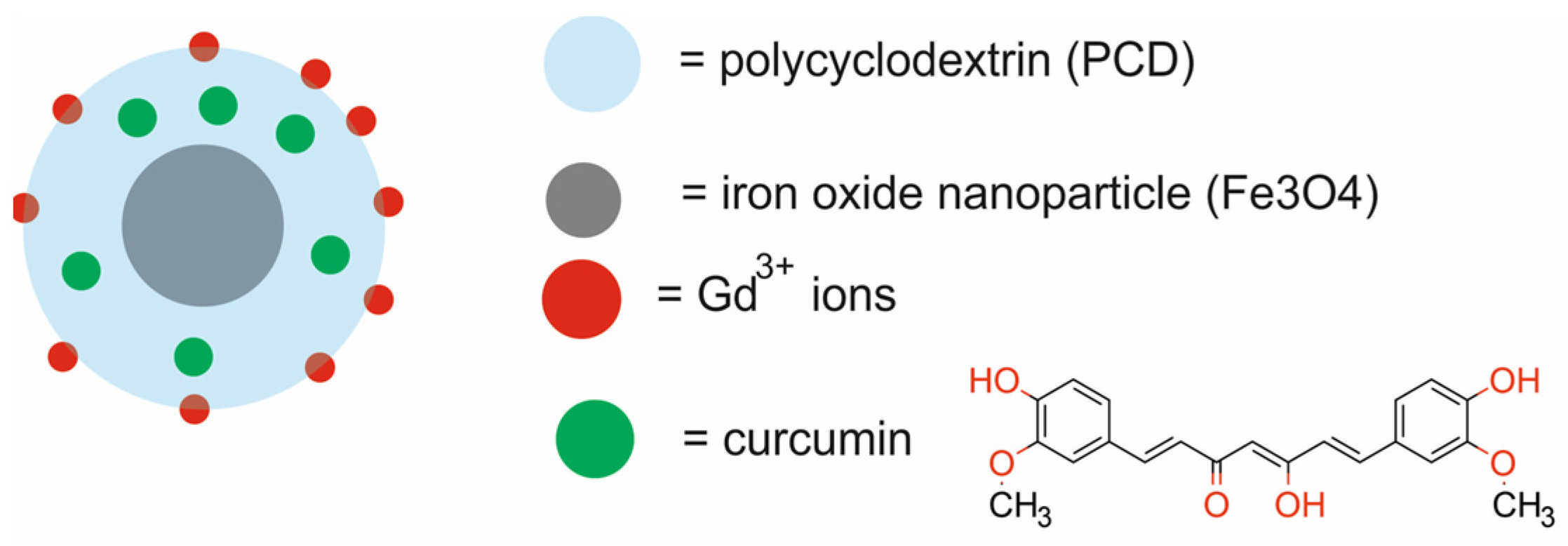




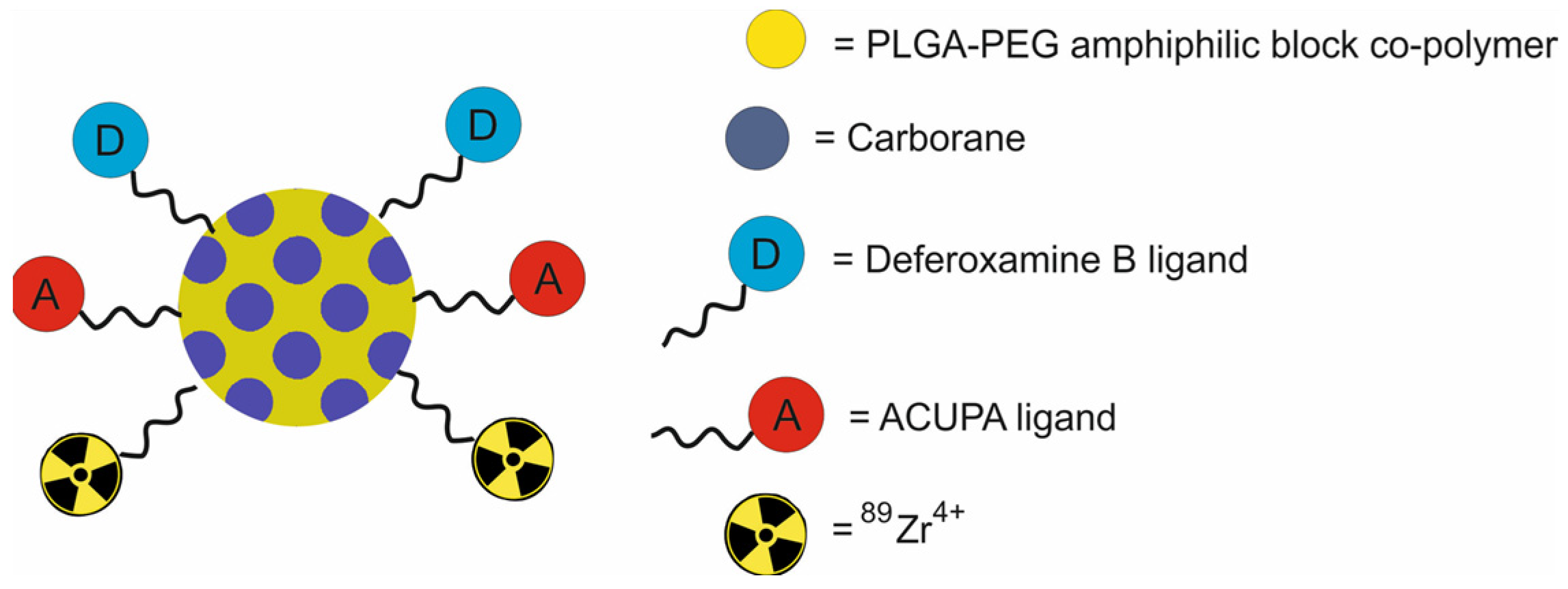





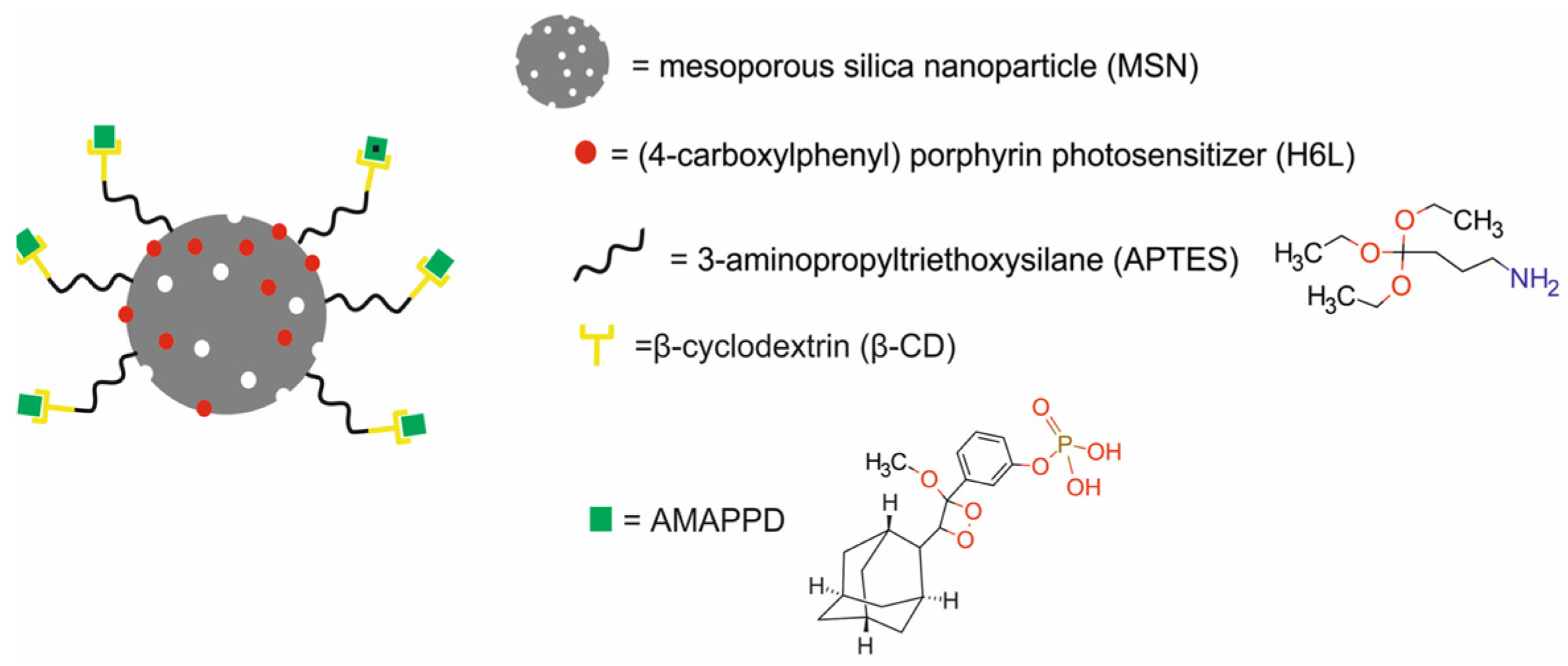
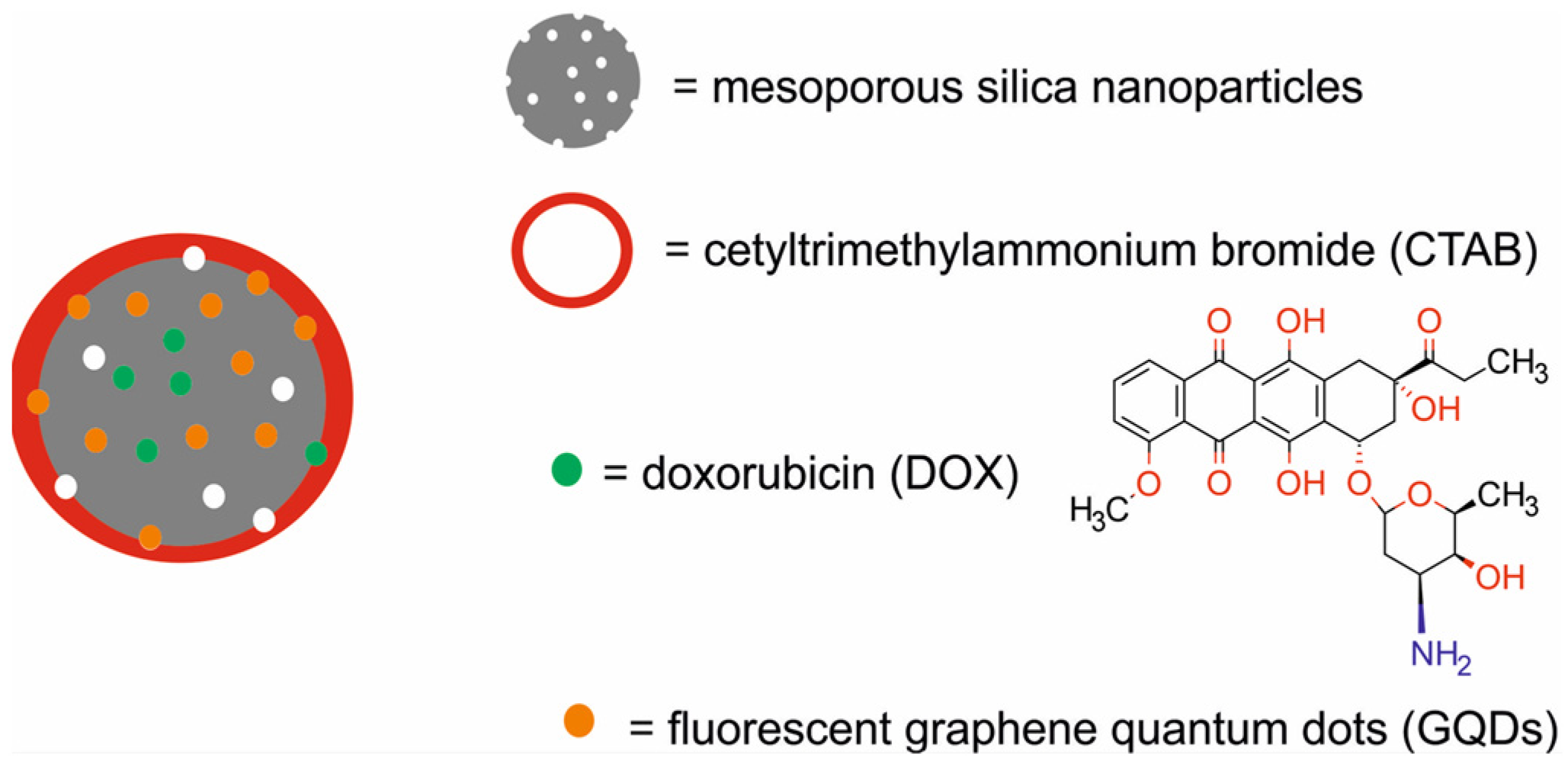

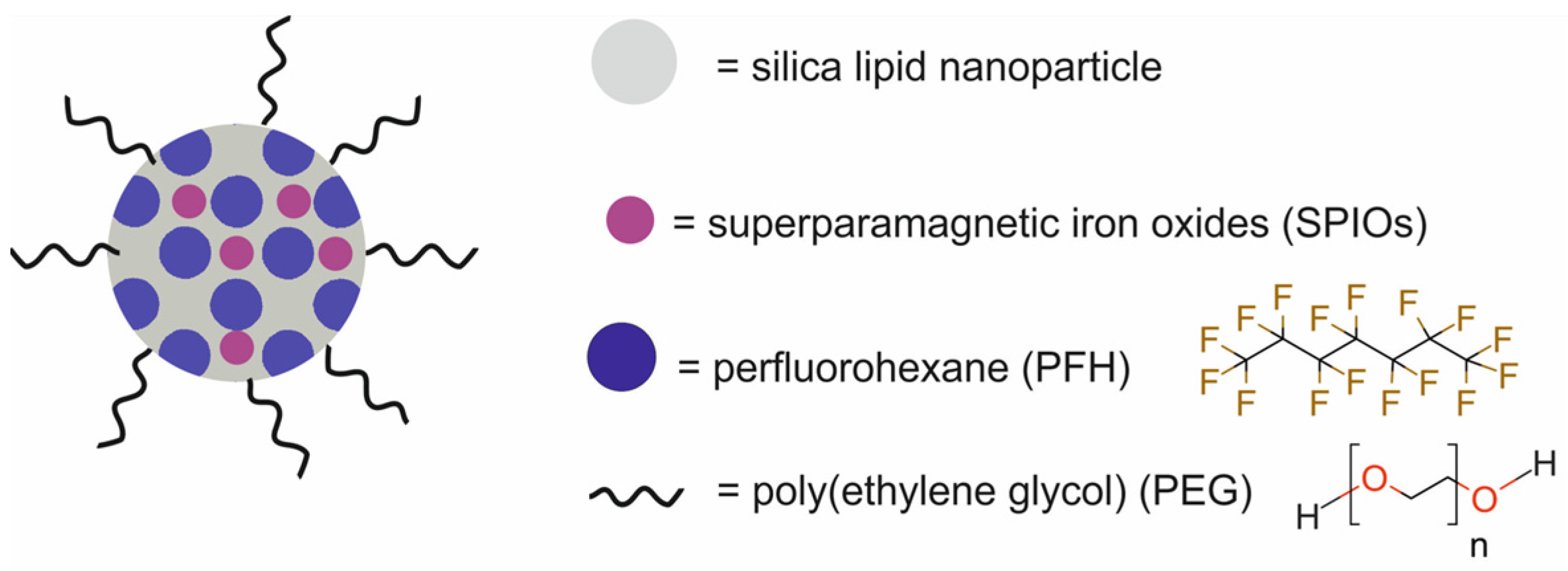





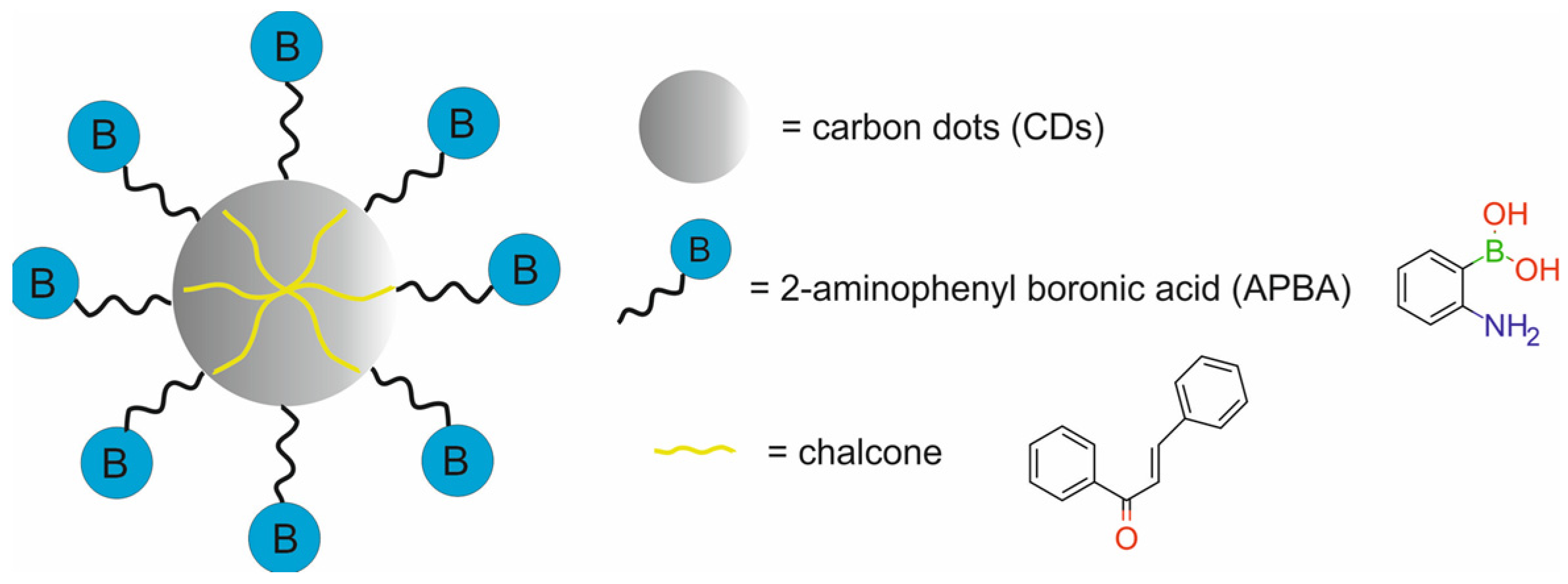
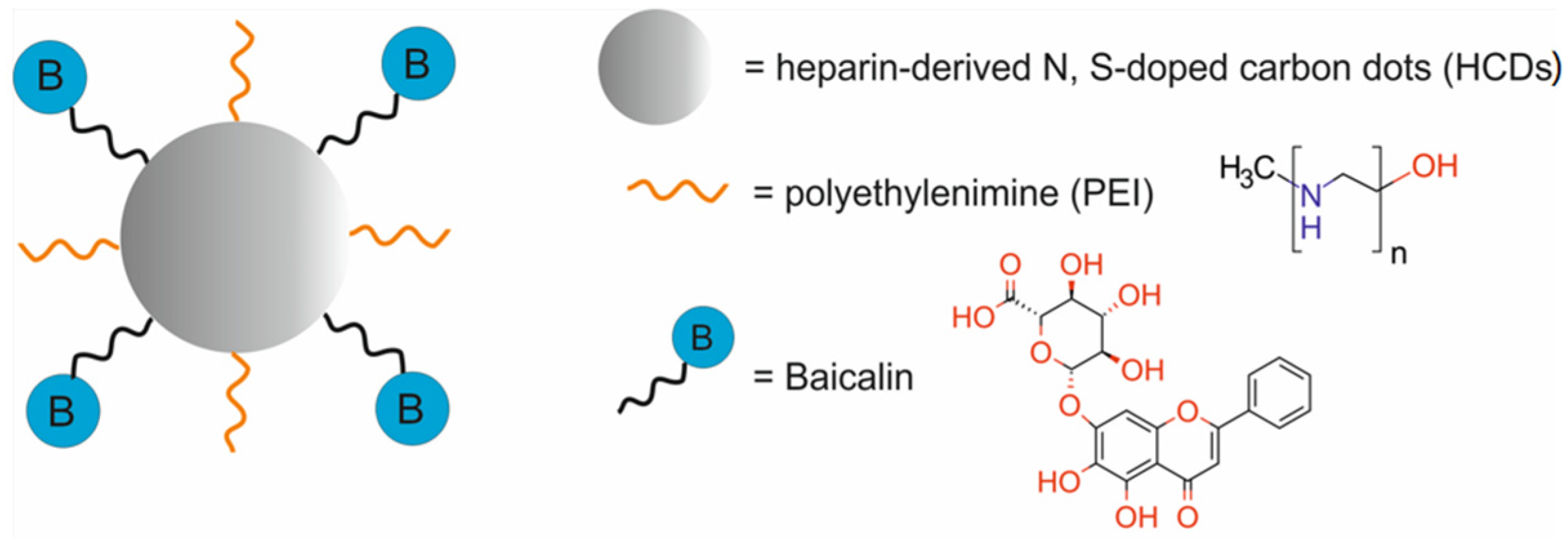
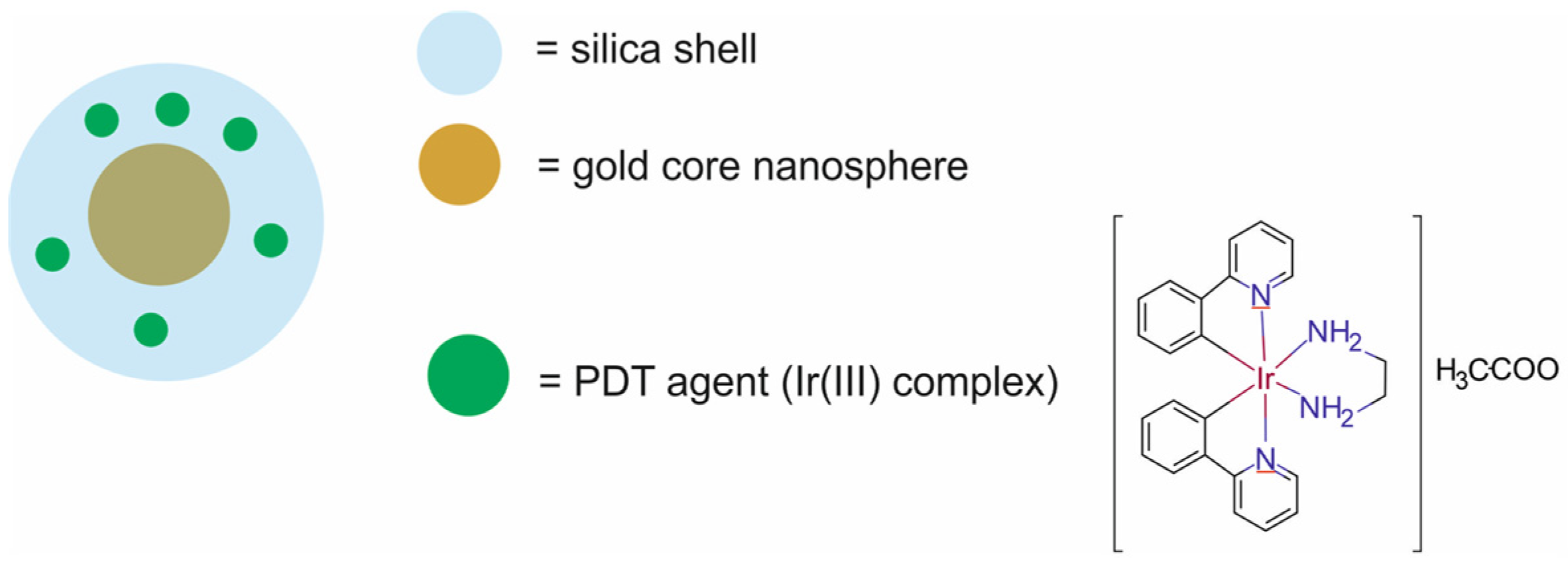
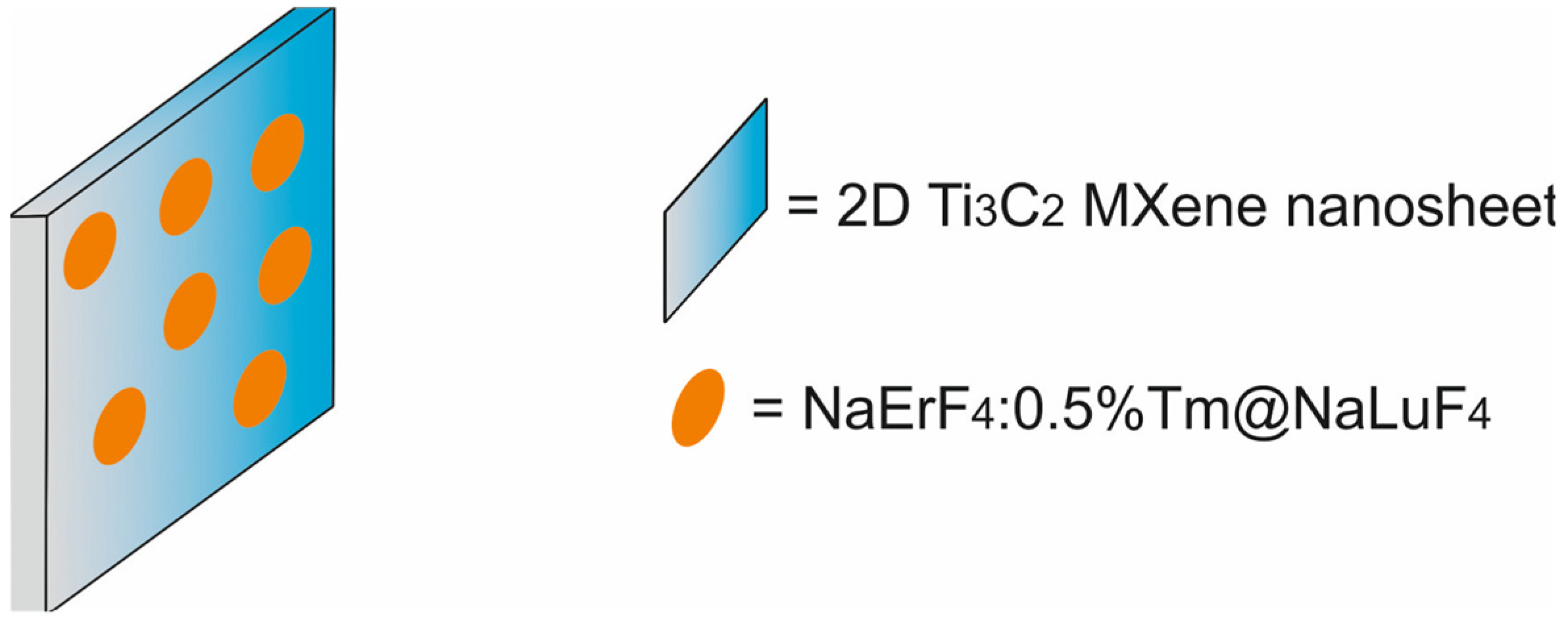



| Type of NPs | Composition of Nanotheranostic System and Final Compound | NP Size (nm) | Therapeutic Modality | Imaging Modality | Cell Type/Animal Models | Ref. |
|---|---|---|---|---|---|---|
| iron-based NPs | Fe3O4 NPs coated with thermo-responsive fluorescent polymer and loaded with doxorubicin [Dox-TFP-MNPs] | ~135 | CTX | FI/MRI | human dermal fibroblasts (HDFs), normal prostate epithelial cells (PZ-HPV-7), human skin cancer A431 and G361 cells, human prostate cancer PC3 and LNCaP cells, NOD SCID mice bearing PC3-KD xenografts | [45] |
| Fe3O4 NPs modified with alendronate-β-cyclodextrin conjugate and cross-linked by curcumin [CUR/ALN-β-CD-SPIONs] | 200 | CTX | MRI | Balb/c mice bearing murine 4T1 xenografts | [46] | |
| ferrite NPs modified with PEG and functionalized with YC-9 [YC-9-MNPs] | 147 ± 8 | PDT | FI/MRI | human prostate cancer PSMA(+) PC3 PIP and PSMA(−) PC3 flu cells, non-obese diabetic severe-combined immune deficient gamma (NSG) mice bearing PSMA(+) PC3 PIP or PSMA(−) PC3 flu xenografts | [47] | |
| Fe3O4 NPs loaded with Cy5.5 [Fe3O4-Cy5.5] | 190 | PTT | MRI/NIRF/PAI | rat glioma C6 cells, bone marrow macrophages, rats bearing C6 glioma xenografts | [48] | |
| Fe3O4 NPs coated with polycyclodextrin, functionalized with gadolinium ions and loaded with curcumin [CUR-Fe3O4@PCD-Gd] | 57 | CTX | MRI | human mammary epithelial MCF 10A cells, murine breast cancer 4T1 cells, BABL/c mice, Balb/c mice bearing murine 4T1 xenografts | [49] | |
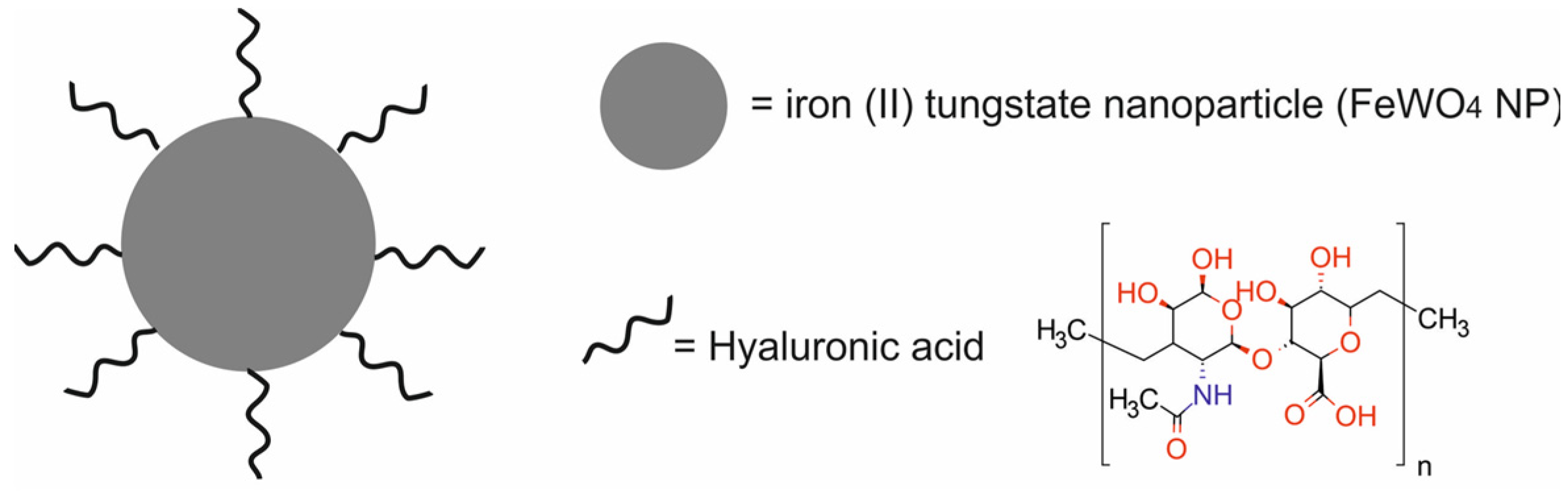
| iron (II) tungstate NPs functionalized with hyaluronic acid [HA-FeWO 4 NPs] | 91.4 | PTT | MRI/CT | murine breast cancer 4T1 cells, human normal breast MCF-10A cells, Balb/c mice, Balb/c mice bearing murine 4T1 xenografts | [50] | |
| gold NPs | Au nanorods coated with manganese dioxide nanosheet and cancer cell membrane and loaded with doxorubicin [CM-DOX-GMNPs] | 164.5 ± 0.7 | PTT/CTX | MRI/PTI | murine breast cancer 4T1 cells, BABL/c mice, Balb/c mice bearing murine 4T1 xenografts | [51] |
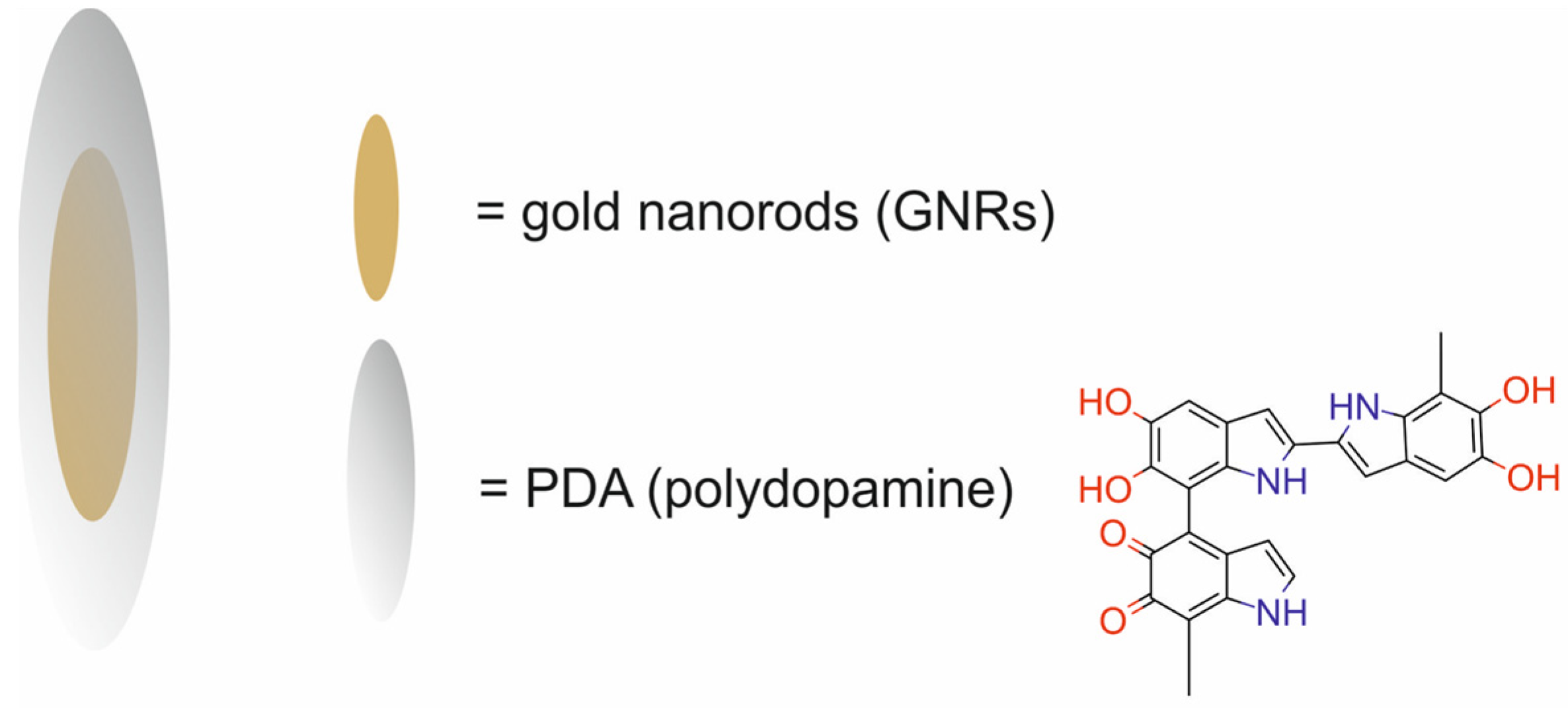
| Au nanorods modified with poly(ethylene glycol) and coated with polydopamine [GNR@PDAs] | 51.1 ± 4.2 × 7.2 ± 1.2 | PTT | PAI | human ovarian adenocarcinoma SKOV3 cells | [52] | |
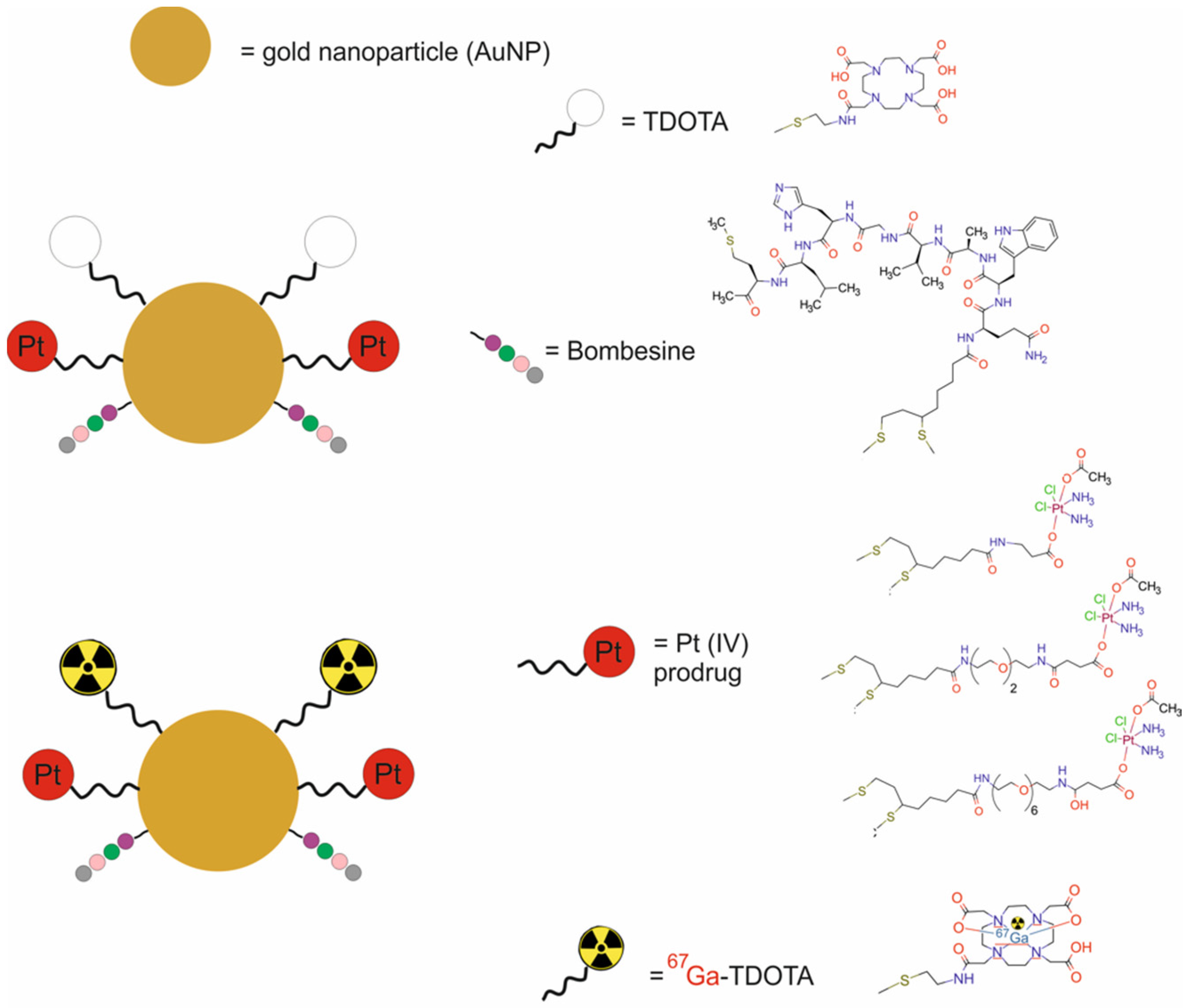
| Au NPs modified with poly(ethylene glycol) and DOTA and functionalized with bombesin analog (BBN [7,8,9,10,11,12,13,14]) and a Pt(IV) and labeled with 67Ga [67Ga-AuNP-BBN-Pt1] | 104.1–112.5 | CTX | SPECT | human prostate cancer PC3 cells, non-tumoral prostate RWPE-1 cells, Balb/c-Nude mice bearing PC3 xenografts | [53] | |
| Au NPs modified with L-Cys-DTPA ligand and propargylamine crosslinker and labeled with AlexaFluor 647 [Au(L-Cys DTPA) (Propargylamine)-AF647] | 1–2 | RT | FI | rat 9LGS gliosarcoma cells | [54] | |
| polymeric NPs | NIR absorbing semiconducting polymer NPs with iodine-grafted amphiphilic copolymer [SNP-1] | 20–30 | PDT | FI/CT | Lewis lung carcinoma cells, c57bl/6 mice bearing Lewis cell xenografts | [55] |
| polymer NPs composed of polysaccharide sodium alginate, modified with caspase 3 enzyme responsive activatable imaging probe and loaded with β- cyclodextrin-adamantane complex with kinase inhibitor [SPNs] | 200 | therapy by inhibition of PI3K/mTOR signaling pathway | FRET | murine BRAFV600E melanoma D4M cells, murine breast cancer 4T1 cells, C57B/L6 mice bearing D4M xenografts | [56] | |
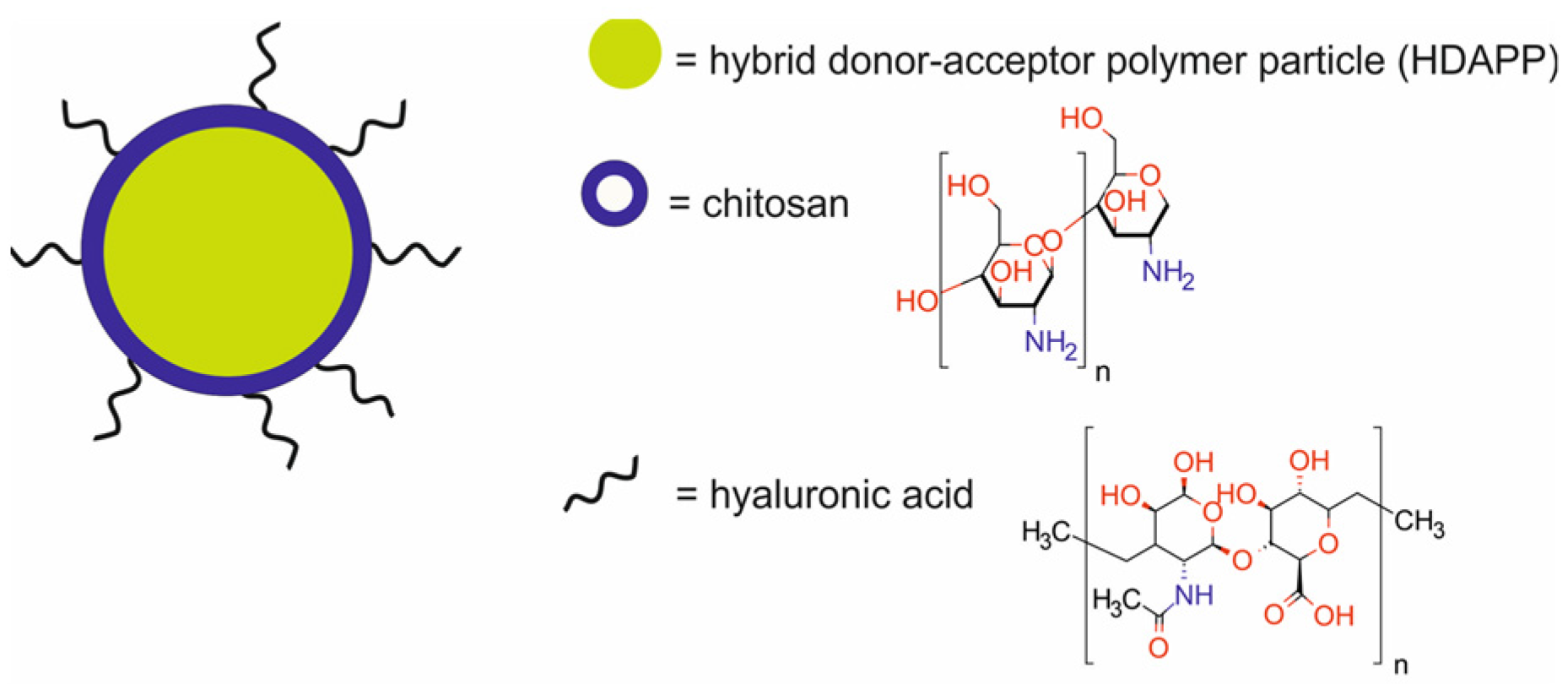
| photothermal hybrid donor-acceptor polymer NPs modified with chitosan and functionalized with hyaluronic acid [HA-HDAPPs] | 172–242 | PTT | FI | murine colorectal carcinoma CT26.WT-Fluc-Neo (CT26) cells transduced with lentivirus encoded with firefly luciferase and a neomycin resistance gene, BALB/c mice bearing CT26.WT-Fluc-Neo (CT26) xenografts | [57] | |
| PLGA-b-PEG NPs functionalized with deferoxamine B and ACUPA, loaded with carborane and labeled with 89Zr [[89Zr]DFB(25)ACUPA(75)] | ~160 | BNCT | PET | human prostate adenocarcinoma PC3-flu and PC3-pip cells transfected with PSMA, nu/nu athymic mice bearing PC3-flu and PC3-pip xenografts | [58] | |
| polymer NPs loaded with gadolinium-porphyrin embedded in polyethyleneimine [Gd–PNPs] | 200 nm | PDT | MRI/FI | human ovarian carcinoma Hela cells, murine colorectal carcinoma CT 26 cells, zebrafish animal model, BALB/c mice bearing murine CT26 xenografts | [59] | |
| polymeric NPs loaded with PTX-TIBA (2,3,5-Triiodobenzoic acid) prodrug [SSTPTX] | ~81–103 | CTX | CT | murine breast cancer 4T1 cells, Balb/c mice bearing murine 4T1 xenografts | [60] | |
| CuNC(Octa)-loaded PEG-PCL micelles [CuNC(Octa)-loaded micelles] | 35–110 | PTT | PAI | murine breast cancer 4T1 cells, Balb/c mice bearing 4T1 xenografts | [61] | |
| chitosan NPs loaded with palbociclib and ultra-small magnesium nanoclusters and functionalized with Folic acid and estrone [PB-UMN-CS-ES-FA-NPs] | 198.2 ± 1.43 | CTX | FI | human breast cancer MCF-7 cells, human breast cancer T-47D cells, SD rats with breast tumors induced by using 7,12 Dimethylbenzathracene (DMBA) | [62] | |
| silica NPs | mesoporous organosilica NPs, doped with iron, modified with silane–PEG, functionalized with transferrin and loaded with doxorubicin [DOX@Fe-HMON-Tf] | 71 | CDT/CTX | MRI | human hepatoma cancer HepG2 cells, human fetal hepatocyte L-02 cells, Balb/c nude mice, bearing HepG2 xenografts | [63] |
| mesoporous silica NPs modified with APTES, β-cyclodextrin and AMPPD and loaded with (4-carboxylphenyl) porphyrin (H6L) [MSN@H6L@β-CD@AMPPD NPs] | 70 | CL-PDT | NIRF | human normal liver HL-7702 cells, human hepatocarcinoma SMCC-7721 cells, human normal breast MCF-10A cells, murine breast cancer 4T1 cell line, BALB/c nude mice bearing SMCC-7721 xenografts | [64] | |
| mesoporous silica NPs decorated with graphene quantum dots and loaded with doxorubicin [DOX-carbanosilica] | 120−140 | PTA/CTX | NIFR | murine normal fibroblast L929 cells, murine breast cancer 4T1 cells, Balb/c mice bearing e 4T1 xenografts | [65] | |
| fibrous silica NPs modified with trimethoxysilyl-propyldiethylenetriamine and functionalized with chlorambucil, organotin metallodrug, folic acid and Alexa Fluor 647 [FS-DT-Chl-FA-Sn-AX] | 427.9 ± 20.9 | CTX | FI | human breast cancer MDA-MB-231 cells, NOD Scid IL2 receptor gamma chain KO mice bearing MDA-MB-231 xenografts | [66] | |
| silica-lipid NPs modified with poly(ethylene glycol) and loaded with iron oxides and perfluoropentane [SSPN] | 289 | HIFU | MRI/USI | human ovarian carcinoma Hela cells, BALB/c mice bearing CT26 xenografts | [67] | |
| liposomes | DSCP (1,2-distearoyl-sn-glycero-3-phosphati dylcholine-[methoxy(polyethylene glycol)-2000) liposomes with indocyanine green J-aggregate, incorporated in lipid bilayer and loaded with doxorubicin [DOX-DSPC-IJA-HBS] | 140–170 | PTA/CTX | NIFR | BALB/c mice bearing CT 26 xenografts, BALB/c mice bearing 4T1 xenografts, NSG mice bearing C4-2B xenografts | [68] |
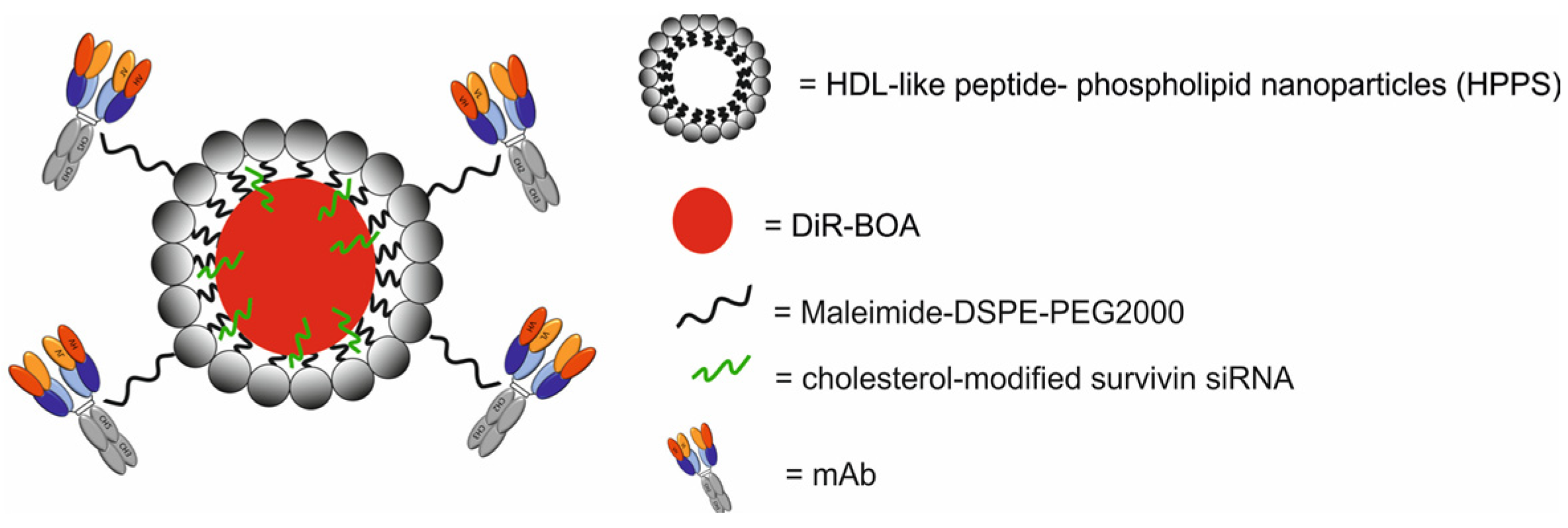
| DiR-BOA-loaded HDL-like peptide- phospholipid NPs, functionalized with anti-TfR mAb, loaded with cholesterol-modified survivin siRNA [DiR-BOA-HPPS-mAb/siRNA] | 29.26 ± 1.47 | therapy by inhibition of the survivin expression at RNA level and induction of apoptosis | NIFR | human hepatic carcinoma HepG2 cells, human glioblastoma U87 cells, human cervical cancer HeLa cells, human breast cancer MDA-MB-231 cells, CHO-hTfR (human TfR) cells which stably express hTfR-GFP, control CHOvec cells, Balb/c nude mice bearing CHO-hTfR cells and CHOvec cells | [69] | |
| lecithin/cholesterol liposomes co-loaded with Au4Cu4 and Au25 nanoclusters [Au4Cu4 /Au25@Lip] | 50 | PTT/PDT | PTI/FI | human cervical cancer HeLa cells, Kunming mice bearing H22 xenografts | [70] | |
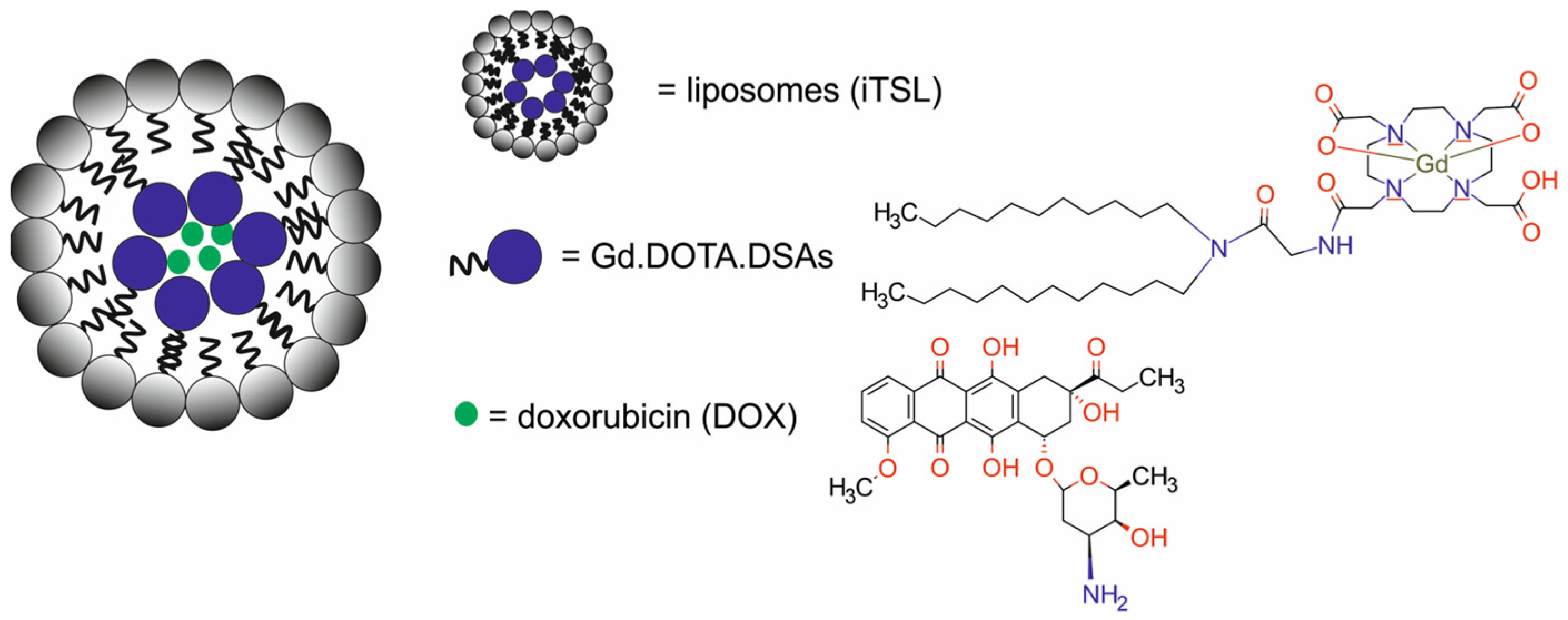
| imaging-thermosensitive liposomes conjugated with NIRF probe CF750.DSA, modified with DOTA and labeled with gadolinium-based contrast agents Gd.DOTA.DSA and loaded with doxorubicin [iTSL-DOX] | 179 ± 3 | MR-FUS/CTX | MRI/NIFR | human breast cancer MDA-MB-231 cells, CD-1 mice, athymic nude mice bearing MDA-MB-231 xenografts | [71] | |
| dendrimers | G5 PAMAM dendrimers entrapped with gold nanoparticles and conjugated with nitroimidazole via a PEG linker and with Gd(III) by DOTA [Gd-Au-DENPs-Nit] | 112.3 | RT | MRI/CT | human nasopharyngeal carcinoma CNE-1 cell and human nasopharyngeal carcinoma hypoxia-resistant CNE-1H cells, normal murine NIH3T3 cells, BALB/c nude mice bearing CNE-1H xenografts | [72] |
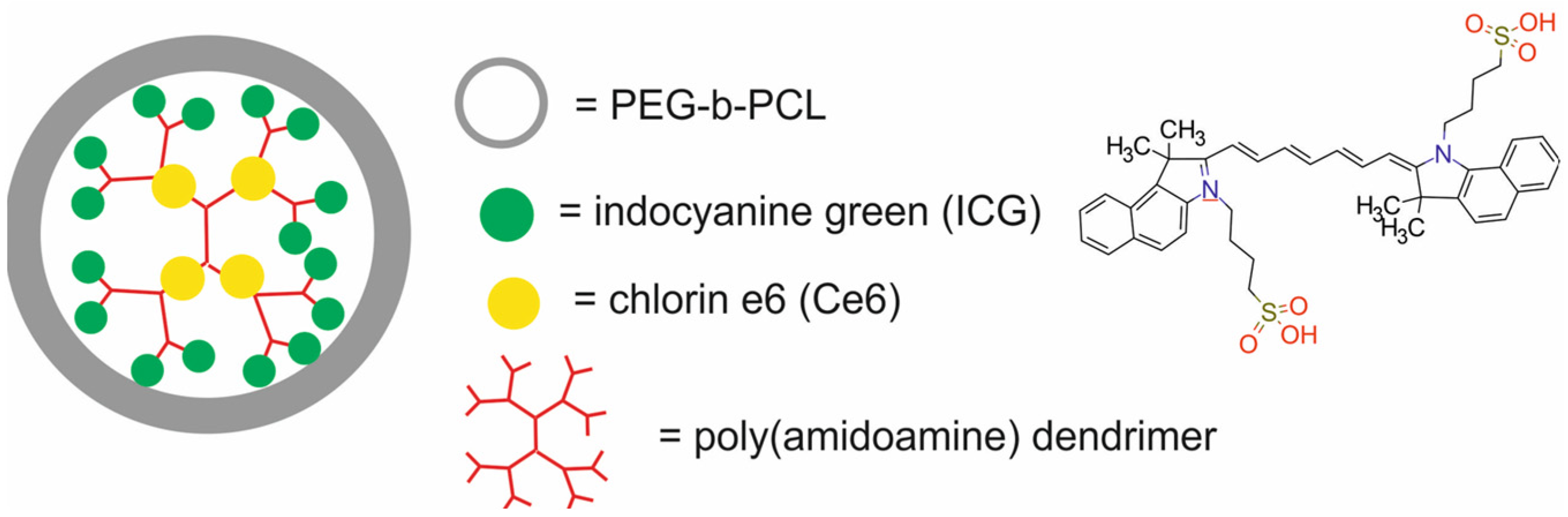
| PAMAM dendrimer-conjugated indocyanine green, bound to PEG-b-PCL polymer through a singlet oxygen-responsive thioketal bond and loaded with chlorin e6 (Ce6) [SNPICG/Ce6] | 118 → 10 | PTT/PDT | FI | murine breast cancer 4T1 cells, BALB/c nude mice bearing 4T1 xenografts | [73] | |
| G4 PAMAM dendrimers loaded with doxorubicin and copper sulfide and wrapped by amphiphilic gelatin [PRDCuS@AG] | 200−250 | PTT/CTX | PAI | murine fibroblast NIH-3T3 cells, murine sarcoma 4T1 cells, BALB/c nude mice bearing 4T1 xenografts | [74] | |
| G5 PAMAM dendrimers entrapped with CuS nanoparticles, modified with PEG, functionalized with RGD and 1,3-propane sultone and complexed with plasmid DNA-encoding pDNA-HIC1 [RGD-CuS DENPs/pDNA polyplexes] | 157.9 | PTT/gene therapy | PAI | human breast cancer MDA-MB-231 cells, BALB/c-nude mice bearing MDA-MB-231 xenografts | [75] | |
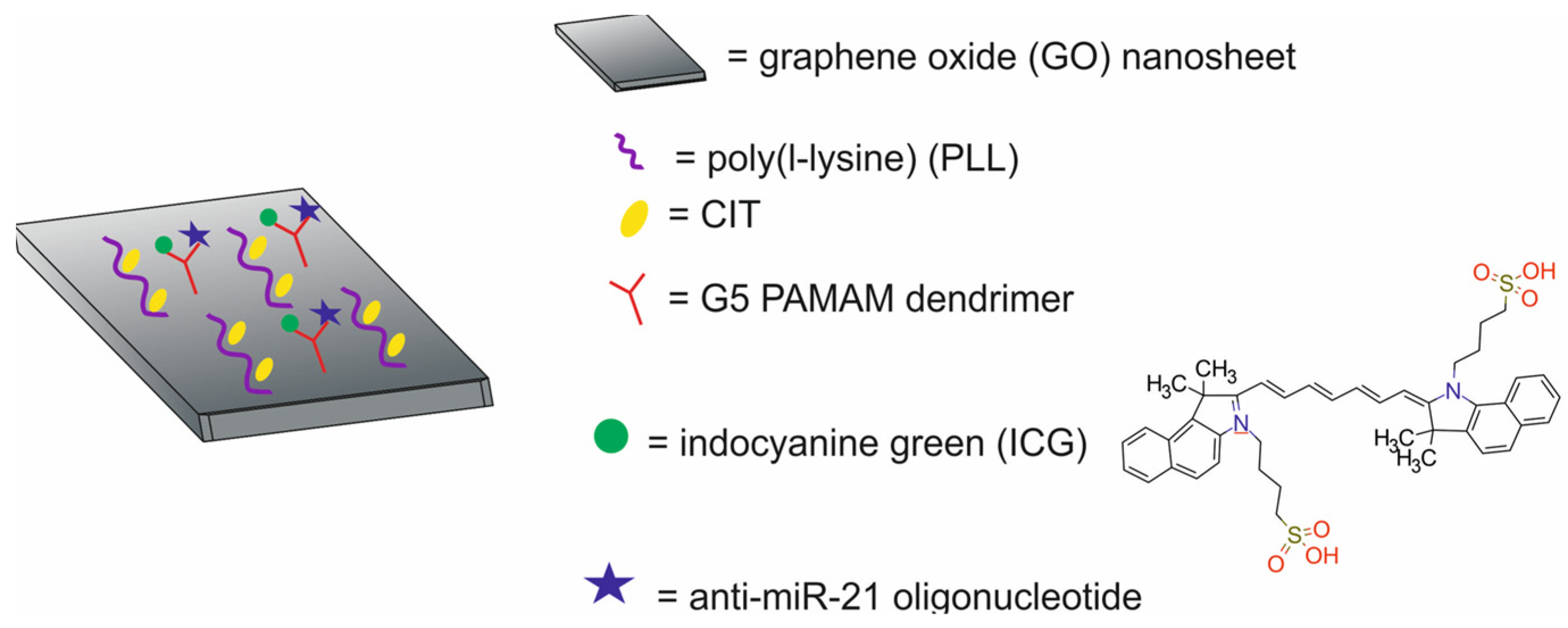
| carbon-based NPs | graphene oxide (GO) nanosheets modified with poly(l-lysine), conjugated with Cit and G4-PAMAM dendrimers loaded with anti-miR-21 oligonucleotide and indocyanine green [GPCP/miR-21i/ICG] | ~250 | PTT/gene therapy | PTI/FI | human breast cancer MDA-MB-231 cells, BALB/c-nude mice bearing MDA-MB-231 xenografts | [76] |
| carbon dots labeled with fluorescent 2-aminophenyl boronic acid and modified with chalcone [chalcone-APBA-CDs] | 8.721 | CTX | FI | human cervical cancer HeLa cells, fibrosarcoma cancer-bearing mice | [77] | |
| heparin-derived N, S-doped carbon dots, modified with polyethylenimine and loaded with baicalin [BA-PHCDs] | 13 ± 2 | CTX | FI | human lung cancer A549 cells, murine fibroblast NIH/3T3 cells | [78] | |
| core–shell and composite NPs | core–shell Si-Au NPs loaded with PDT agent Ir1 [Ir1- AuSiO2] | 40 ± 2 | PTT/PDT | LI | athymic mice bearing human glioblastoma Gli36D5 xenografts | [79] |
| 2D titanium carbide MXene nanosheets, functionalized with core–shell NaErF4:0.5%Tm@NaLuF4 (NaErF4) nanoparticles [NaErF4@ Ti3C2] | 200 × 4.5 | PTT | MRI/FI | human hepatoma HepG2 cells, BALB/c-nu mice bearing HepG2 xenografts | [80] | |
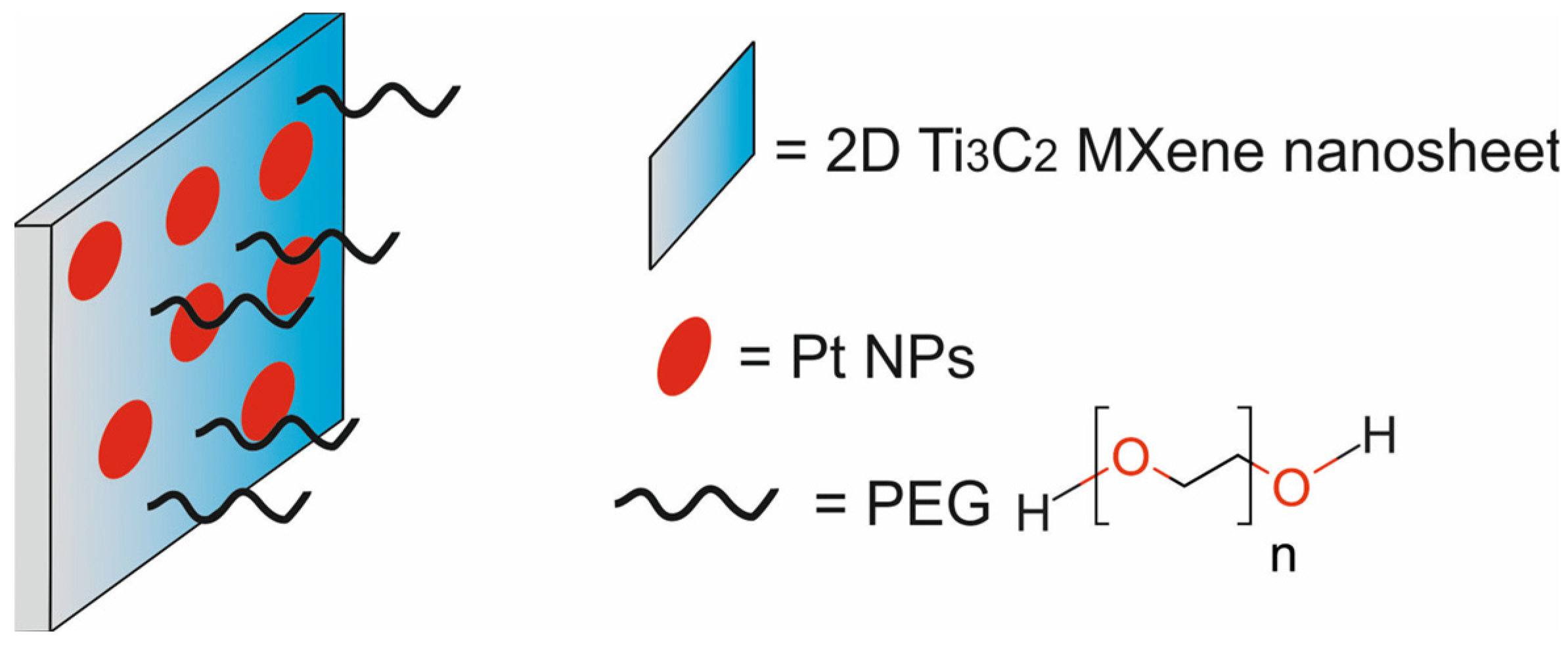
| 2D titanium carbide MXene nanosheets, decorated with platinum nanoparticles and modified with PEG [Ti3C2Tx-Pt-PEG] | 200 | PTT/enzyme therapy | PAI | murine breast cancer 4T1 cells, murine fibroblast L929 cells, BALB/c mice bearing 4T1 xenografts | [81] | |
| 2D stimuli-responsive nanocomposites comprising graphene oxide nanosheets decorated with Au NPs and Fe3O4 NPs, coated with doxorubicin-loaded 1-tetradecanol, and modified with alginate polymer [GO-SPIO-Au-DOX-TD-Alg NCs] | 40 | PTT/CXT | MRI/CT | murine colon adenocarcinoma CT26 cells, Balb/c mice bearing CT 26 xenografts | [82] | |
| copper sulfide NPs, coated with polyethyleneimine, loaded with indocyanine green and functionalized with folic acid [CuS-PEI-ICG-FA] | <10 | PTT | PAI | human cervical cancer HeLa cells, Balb/c mice bearing HeLa xenografts | [34] | |
| Au- Fe3O4 Janus NPs modified with DHCA and functionalized with RGD [GION@RGD] | 18.7 | PTT/therapy by NIFR-enhanced ferroptosis | MRI | murine breast cancer 4T1 cells, Balb/c nude mice bearing 4T1 xenografts | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lankoff, A.M.; Czerwińska, M.; Kruszewski, M. Advances in Nanotheranostic Systems for Concurrent Cancer Imaging and Therapy: An Overview of the Last 5 Years. Molecules 2024, 29, 5985. https://doi.org/10.3390/molecules29245985
Lankoff AM, Czerwińska M, Kruszewski M. Advances in Nanotheranostic Systems for Concurrent Cancer Imaging and Therapy: An Overview of the Last 5 Years. Molecules. 2024; 29(24):5985. https://doi.org/10.3390/molecules29245985
Chicago/Turabian StyleLankoff, Anna Małgorzata, Malwina Czerwińska, and Marcin Kruszewski. 2024. "Advances in Nanotheranostic Systems for Concurrent Cancer Imaging and Therapy: An Overview of the Last 5 Years" Molecules 29, no. 24: 5985. https://doi.org/10.3390/molecules29245985
APA StyleLankoff, A. M., Czerwińska, M., & Kruszewski, M. (2024). Advances in Nanotheranostic Systems for Concurrent Cancer Imaging and Therapy: An Overview of the Last 5 Years. Molecules, 29(24), 5985. https://doi.org/10.3390/molecules29245985







