Abstract
Heteroleptic coumarin-based silver(I) complexes with improved solubility profiles were synthesised using either triphenylphosphine or an N-heterocyclic carbene as adduct ligands, and were fully characterised using IR and NMR spectroscopy, elemental analysis, and, where possible, X-ray crystallography. The triphenylphosphine adducts formed well-resolved structures, where the oxyacetate ligands asymmetrically chelated the silver(I) ion in a bidentate chelating mode, and the silver(I) ion was also bound to two triphenylphosphine ligands. The solubility profile and photostability of the adducts were considerably improved compared to those of previously isolated simple coumarin silver(I) complexes. Analysis of the coumarin N-heterocyclic carbene(NHC) silver(I) adduct indicated that it likely formed as a complex aggregate species with an overall stoichiometry of 1:1:1 coumarin:Ag(I):NHC. The Kirby Bauer assay and broth microdilution assays were used to assess the silver(I) complexes’ and adducts’ antimicrobial activity against pathogenic strains of Pseudomonas aeruginosa, Escherichia coli, and MRSA. Interestingly, the formation of more soluble complexes did not increase the activity of the silver(I) complexes and, in effect, made them less effective antimicrobial agents, particularly against Escherichia coli and Pseudomonas aeruginosa, although they retained their activity against MRSA.
1. Introduction
The alarming speed at which bacteria evolve, acquire, and develop novel mechanisms of resistance to evade the drug-mediated killing associated with currently used antibiotic drug therapies is leading to a global health threat, with the potential to become the next pandemic [1]. Today, the problem posed by Antimicrobial Resistance (AMR) is considered one of the top ten global health threats, with approximately 13·7 million infection-related deaths reported in 2019 [2]. Moreover, by 2050, this problem is projected to cause more than 10 million global deaths annually [3]. The recent COVID-19 pandemic has exacerbated this worldwide health problem, as critical antibiotics have been overused in the treatment of secondary infections in patients associated with severe COVID-19 illness [4].
In 2022, a Global Burden of Disease study identified Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa as the six leading causes (73%) of AMR-associated deaths in 2019 [3]. Previous studies from our group, which investigated the antimicrobial activity of metal-based coumarin complexes against Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus), showed that silver(I) complexes of coumarin-derived ligands were particularly active against these pathogens [5,6,7,8]. The effectiveness of any silver drug as an antibacterial agent is linked to its bioavailability, and while silver(I) compounds have shown excellent antibiotic activity, their therapeutic use has been limited to date, primarily as a topical agent. Silver nitrate and silver sulfadiazine, commonly used topical antibacterials, have a very rapid bactericidal action, but they lose their efficacy as the complexes are ionic in nature, leading to a labile silver(I) ion being formed [9]. Quick release is optimal to kill surrounding bacteria, but the wound site remains exposed to possible re-infection once the initial burst of silver(I) ions dissipates.
The previously isolated series of coumarin-based silver(I) complexes, while effective as antibacterial agents, are largely polymeric in nature, and their solubility in aqueous solutions and photostability are limited. The choice of using coumarin-derived ligands is because coumarin is a privileged scaffold, and its many derivatives occur widely in nature. Many coumarin derivatives, both plant-derived and synthetic, have been found to have a range of therapeutic properties [10]; however, metal complexes of coumarin derivatives are often polymeric in nature and have poor solubility [11]. A 2023 review of recent advances in the use of coumarin-hybrid complexes has identified a number of such compounds which are currently in clinical trials for a range of diseases and medical conditions [12]. The approach of using complexes with multiple pharmacophores to address the challenges posed by our previous studies, namely their lack of solubility in aqueous media, led to our most recent work, where we reported the synthesis and characterisation of N-heterocyclic carbene (NHC) adducts of silver(I) coumarin carboxylate complexes [13]. N-heterocyclic carbenes (NHC), due to their excellent σ-donating properties and π-back bonding ability, are widely used as ancillary ligands to stabilise both the main group and transition metals [14,15], while the addition of triphenylphosphine (TPP) ligands has been shown to improve the photostability of silver(I) carboxylate complexes [16]. The introduction of a second ligand to the coumarin carboxylate complexes improved the solubility and photostability of the resulting adducts, and the formation of heteroleptic triphenylphosphino and NHC adducts improved their antimicrobial activity against some strains [13]. The synthesis of bis-triphenylphosphino-derived adducts proved to be straightforward. However, the synthesis and isolation of 1:1:1 coumarin:silver:NHC adducts proved difficult with very low yields, and the product of the reaction between the NHC and the silver coumarin carboxylates under Schlenk conditions resulted in a heteroleptic complex with two silver ions forming a strong argentophilic Ag(I)−Ag(I) interaction, with one silver(I) ion bound to two coumarin carboxylate ligands and the second ion bound to two carbene ligands in a Schlenk-like equilibrium complex. Isolation via an ionic carbene route led to higher yields of adducts, but more complex aggregates were formed. MS studies indicated that the complexes dissociated in polar solvents into ion-pair aggregates; therefore, an alternative strategy was proposed in this work to allow for the successful isolation of 1:1:1 coumarin: silver:NHC adducts which would have improved bioavailability, solubility, and photostability profiles.
The approach used in this study was to use the complex, 2-(8-acetyl-2-oxo-2H-chromen-7-yl)oxyacetato silver(I), as shown in Figure 1(ii), which has been previously synthesised and reported by our group to have credible antimicrobial activity against the clinically important MRSA and P. aeruginosa with MIC50 values of 33.0 and 22.7 µM respectively [7]. The acetate group at position C7 of this complex is comparable to that of the acetate function present in the antimicrobial compound known as SBC3, shown in Figure 1(i), synthesised using the ionic route by O’Beirne et al. [17]. SBC3 has excellent antimicrobial activity against bacterial strains of P. aeruginosa and E. coli, with MIC values of 3.13 and 6.25 μg/mL, respectively, while its activity against MRSA showed an MIC value of 12.5 μg/mL [17]. This compound also has high solubility in aqueous media and good stability in light. Thus, using 2-(8-acetyl-2-oxo-2H-chromen-7-yl)oxyacetatosilver(I) instead of the silver acetate moiety in SBC3 was proposed as a strategy to increase antimicrobial activity, with the possibility of dual-mode activity arising from the presence of both carbene and coumarin moieties, in addition to improving the physicochemical and bioavailability characteristics of the resulting adducts. The pKa values of the carboxylate group will not be greatly different for the oxyacetate moiety relative to the coumarin-3-carboxylate moiety used in previous studies, but there would be less steric hindrance around the silver(I) ion from the oxyacetate binding group.
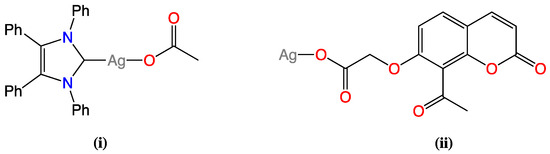
Figure 1.
Chemical structures of silver(I) complexes (i) SBC3 and (ii) 2-(8-acetyl-2-oxo-2H-chromen-7-yl)oxyacetatesilver(I).
To address the challenges associated with the previously poor solubility of promising lead compounds, we synthesised bis-TPP adducts for a previous series of coumarin silver(I) complexes [13], and their resulting improved physicochemical properties also prompted the synthesis of bis-TPP adducts in this study. We have also extended the biological studies to look at the activity of coumarin-derived silver(I) complexes against reference laboratory strains of E. coli, P. aeruginosa and MRSA.
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterisation of 2-(2-Oxo-2H-chromen-7-yl)-oxyacetatobistriphenylphosphinosilver(I) Complexes
The silver(I) complexes of coumarin-7-oxyacetate ligands 3 and 4, shown in Scheme 1, were prepared by previously published methods from ligands 1 and 2, with details given in the Supplementary Information. Adducts 5 and 6 were obtained by reacting complexes 3 and 4 in a 1:2 molar ratio with triphenylphosphine, using either ethanol or dichloromethane (DCM) as the reaction solvent. Both adducts were isolated as white-coloured powders in yields of 71 and 78%, respectively. Recrystallisation of 5 from DCM/ethanol afforded colourless crystals suitable for X-ray crystallographic analysis, while crystals of 6 were obtained through slow diffusion of pentane into a saturated solution of 6 in DCM.
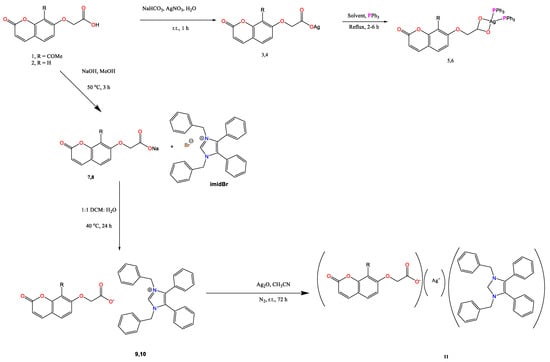
Scheme 1.
Synthetic protocols used toward the synthesis of bis-triphenylphosphino adducts (5 and 6) and substituted coumarin-7-oxyaceto-silver-NHC* complexes (11) R = -COCH3 for 5, 7, 9, and 11 and R = H for 6, 8, and 10.
The bis-triphenylphosphino adducts 5 and 6 were analysed by IR, 1H and 13C NMR spectroscopy, and elemental analysis. The main IR bands of the TPP adducts are presented in Table 1, showing a shift in the position of the IR bands for the carbonyl stretch of the lactone functionality, as well as the asymmetric and symmetric stretches of the carboxylate group relative to the silver(I) complexes 3 and 4 (Table S4.2—Supplementary Data). The Δν(oco) values for the silver(I) TPP adducts were in the range of ca. 125–150 cm−1 and are typical for bidentate chelating bonding [13,17]. In addition, bands typical of those found in TPP (P-C band: 795–650 cm−1 and P-Ph: 1130–1090 cm−1) [17] were present in the IR spectra of each of the TPP adducts, further confirming their formation. Microanalytical data confirmed the inclusion of a molecule of DCM for complex 6.

Table 1.
Main IR bands (cm−1), melting points (°C) and microanalytical data of adducts 5 and 6.
The NMR spectral data for adducts 5 and 6 are presented in Supplementary Information together with the data for silver(I) complexes 3 and 4 and ligands (Figure S3.1a,b and Table S4.3). A summary of the data is given in Table 2. An upfield movement of the signals for protons H5 and H11 (Figure 2) of adducts 5 and 6 was observed compared to the corresponding signals for protons of the silver(I) complexes 3 and 4. In particular, the signal for H11, which is closest to the site of adduct formation, experienced the greatest upfield chemical movement for both adducts (Table 2). The increased electron density around the central silver(I) ion provided by the two TPP ligands is likely responsible for this chemical shift movement in the signals for the coumarin ligand. For both adducts, new multiple proton signals were observed in the region 7.53–7.30 ppm, with relative integration of 30 protons, confirming the presence of two TPP ligands. The coumarin 13C NMR signals were less affected by adduct formation, with the formation of 5 and 6 confirmed mainly by the addition of four new carbon signals to the 13C NMR spectra, corresponding to the aromatic carbons of the triphenylphosphine ligand (Table S4.4 in supplemental data).

Table 2.
Selected 1H and 13C NMR spectral data (ppm) for compounds 3, 4 (R = Ag(I)), and 5, 6 (R = Ag-TPP2) recorded in DMSO-d6.
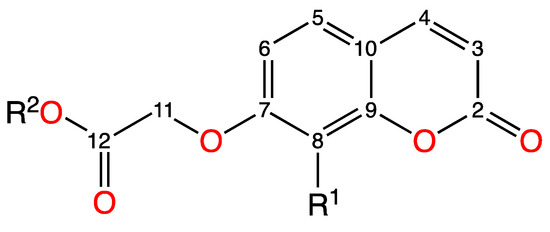
Figure 2.
General numbering system for the assignment of NMR spectra of 3–6.
The poor solubility of the parent silver(I) complexes 3 and 4 in common laboratory solvents other than dimethyl sulfoxide (DMSO) has been a limiting factor toward the potential development of these silver(I) complexes as therapeutic agents. The bis-TPP adducts 5 and 6 were readily soluble in DMSO at room temperature, and the solubility of the complexes also extended to toluene, chloroform, DCM, tetrahydrofuran and acetonitrile at room temperature. While heating to ~50 °C extends their solubility further to more polar solvents such as ethyl acetate, methanol, and ethanol. Both adducts remained stable in solution over 96 h in the absence of UV-Vis light (Figure S3.2a–d). However, upon exposure to ambient light conditions, degradation of five was observed to begin after 24 h, while complex 6 remained stable.
2.2. X-Ray Crystallography of Adducts 5 and 6
For each of the bis-triphenylphosphino adducts synthesised, crystals suitable for X-ray analysis were obtained. In the case of 5, clear colourless block-like crystals were obtained through recrystallisation from DCM/ethanol, while slow diffusion of pentane into a saturated solution of 6 in DCM at 4 °C afforded colourless rod-shaped crystals. Presented in Table 3 are the selected bond lengths and angles of each adduct, while the crystal data and structure refinement details of the two TPP adducts 5 and 6 are provided in Table S4.5 in the Supplementary Information. The X-ray crystal structures depicted in Figure 3 and Figure 4 show that the oxyacetate ligands asymmetrically chelate the silver(I) ion in a bidentate chelating mode in both complexes. Similar to the [bis-(triphenylphosphino)-(2-oxo-2H-chromen-3-carboxylato)]silver(I) complexes isolated previously [13], this binding mode is also consistent with the IR spectral data as both complexes produced a Δν(oco) of <150 cm−1. Both complexes grew as solvate forms with a disordered mixed solvent of DCM/ethanol with an occupancy of 25% per molecule of 5, while 6 had a single DCM molecule in the structure (Figures S3.3a and S3.4a)

Table 3.
Selected bond lengths (Å) and angles of [bis-triphenylphosphino)-(substituted-2-oxo-2H-chromen-7-oxyaceto]silver(I) adducts 5 and 6.
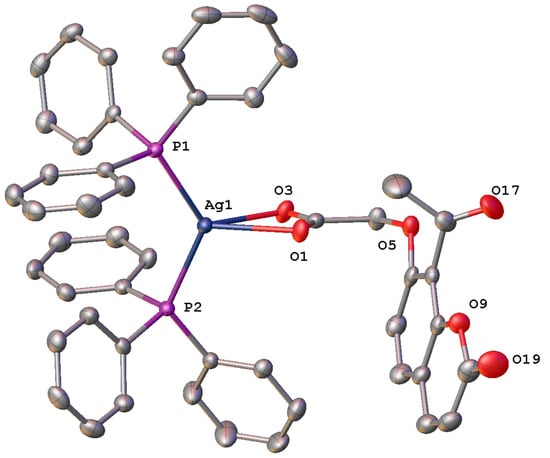
Figure 3.
Molecular structure of [bis-triphenylphosphino)-(8-acetyl-2-oxo-2H-chromen-7-oxyacetosilver(I)] (5) with displacement at 50% probability, hydrogen atoms and disordered solvent omitted for clarity. Heteroatoms labelled only. See Figure S3.3a.
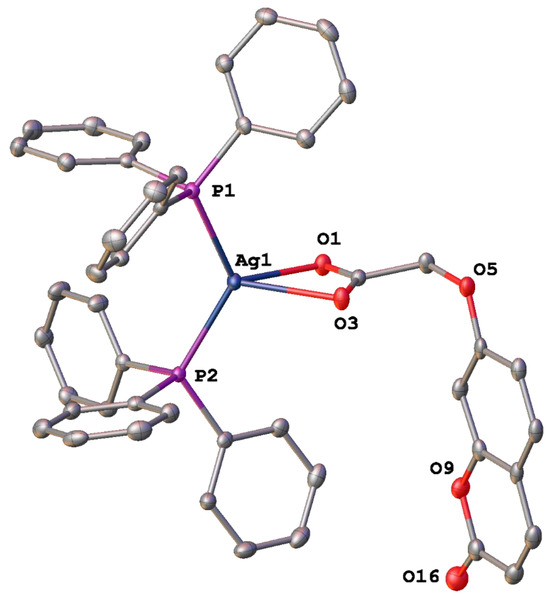
Figure 4.
Molecular structure of [bis-triphenylphosphino)-(2-oxo-2H-chromen-7-oxyacetosilver(I)] (6) with displacement at 50% probability, hydrogen atoms and solvent omitted for clarity. Heteroatoms labelled only. See Figure S3.4a.
The Ag-O bond lengths in these adducts, 5 (2.383 (2) and 2.538 (2) Å), 6 (2.4548 (16) and 2.4061 (16) Å) are noticeably longer than those expected for bond lengths such as these (2.2–2.4 Å) [18] but are comparable to other observed by other adducts of this type such as for the [bis-(triphenylphosphino)-(2-oxo-2H-chromen-3-carboxylato)]silver(I) complexes [13]. They are also comparable to the bis-triphenylphosphino silver(I) adduct reported by Edwards et al. [18], where the Ag-O bond lengths were reported as 2.495 (5) and 2.532 (5) Å.
In contrast, the Ag-P bond lengths of each of the adducts formed, 5 (2.4060 (8) and 2.4317 (8) Å) and 6 (2.4104 (6) and 2.4040 (6) Å), like the [bis-(triphenylphosphino)-(2-oxo-2H-chromen-3-carboxylato)]silver(I) complexes, are within the known range for triphenylphosphine bound silver, which is 2.352–2.521 Å [13,18,19].
Both adducts possess a similar P(2)-Ag-P(1) bond angle of 127.53(3)° and 126.62(2)° respectively, much larger than that typical bond angle of 109.5° displayed by a complex with a tetrahedral geometry [20]. They have adopted a distorted tetrahedral geometry likely caused by the crowded environment created by the six phenyl rings around the silver atom. The hydrogen bond geometries are presented in Table 4, and hydrogen bonding is shown in Figures S3.3b and S3.4b.

Table 4.
Hydrogen bond geometries (Å and °) observed in bis-triphenylphosphino)-(substituted-2-oxo-2H-chromen-7-oxyaceto]silver(I) adducts 5 and 6.
2.3. Synthesis of [(1,3-Dibenzyl-4,5-diphenyl imidazole-2-ylidene)–(8-acetyl-2-oxo-2H-chromene-7-yl)oxyaceto)(silver(I))] (11) via an Ion Exchange Route
Synthesis of the N-heterocyclic carbene (NHC), as shown in Scheme 1, was via the ionic route previously used by our group, as yields from these reactions are typically high [13]. To synthesise intermediate compounds 7 and 8, the sodium salts of the carboxylate ligands were first obtained by stirring the respective coumarin acetoxy acid with sodium hydroxide in a 1:1 molar ratio at 50 °C in methanol, forming 7 and 8 in yields of 83% and 91% respectively. Formation of the sodium salts was confirmed by IR spectroscopy and microanalysis as poor solubility did not allow for measurement of 1H and 13C NMR spectra. A summary of the main IR bands and microanalytical data is given in Table 5. A shift in the position of the carbonyl stretch of the lactone functionality compared to the corresponding acid, coupled with the loss of the broadband corresponding to the -OH stretch of the carboxylic acid in the region 3466–3046 cm−1, indicates the successful isolation of the sodium salts. The difference between the asymmetric and symmetric stretches of the carboxylate group of each of the compounds is greater than 200 cm−1, which indicates that the binding mode of these compounds is likely to be unidentate or ionic. The microanalytical data indicated that both contained one molecule of water.

Table 5.
Yield (%), main IR bands and microanalytical data of compounds 7–10.
The sodium salts 7 and 8 were reacted with imidBr, using a 1.5:1 mmol ratio to form ionic intermediates 9 and 10 in yields of 90 and 96%, respectively, with formation confirmed by 1H and 13C NMR spectroscopy and IR spectroscopy, while microanalytical data was obtained only for compound 9 as compound 10 was isolated repeatedly as an oil. Table 5 details the main IR bands observed for 9 and 10, including the bands corresponding to the C-N and C-C stretches of the imidazole ring of the NHC ligand at ~1555 and 1455 cm−1, respectively. Coupled with a slight shift in the IR bands corresponding to the νCO of the lactone function and the νasym and νsym stretches of the carboxylate group of the oxyacetate function, these observations indicated the formation of compounds 9 and 10.
The main details of the 1H and 13C NMR spectra of complexes 9 and 10 are given in Table 6, with the numbering system used for the assignment of the 1H and 13C NMR signals of these NHC+ intermediates shown in Figure 5 and the full data provided in Table S4.6 in the Supplementary Information. An example of a typical 1H NMR spectrum observed for these intermediate compounds is shown in Figure S3.5.

Table 6.
Comparison of 1H and 13C NMR spectral data for compounds 9 and 10 and precursors 1 and 2 and imidBr, showing the chemical shifts (ppm) of H5, H11, C11 and C12 recorded in DMSO-d6.
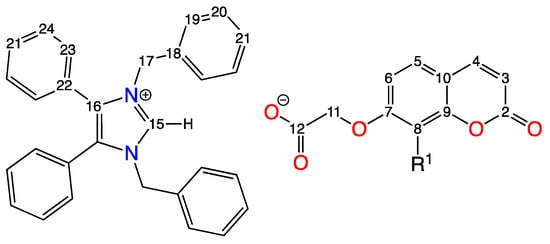
Figure 5.
Numbering system used for the assignment of the 1H and 13C NMR spectra of compounds 9 and 10, where R1 = -COCH3 for 9 (labelled C13 and C14) and = H for 10.
1H NMR signals corresponding to the coumarin moieties experienced an upfield movement, while those signals associated with the NHC+ were moved downfield compared to the respective precursors (Supplementary Information Table S4.6). In addition to this, the singlet at ~9.72 ppm, corresponding to the proton attached to the carbenic carbon of the NHC+ ligand (H15), for both compounds is present. Evidence that compounds 9 and 10 exist as ion pairs is provided by the lack of a signal in both 1H NMR spectra, corresponding to the proton of the coumarin carboxylic acid. When the 1H NMR spectra of 9 and 10 were compared to those of the corresponding acids 1 and 2, the signals for the H11 protons of both compounds, situated closest to the site of the ionic interaction between the coumarin oxyacetate and NHC+ moieties, moved upfield from 4.91 to 4.37 ppm and 4.83 to 4.38 ppm, respectively.
Signals corresponding to both coumarin and NHC+ moieties were present in both 13C NMR spectra, with full assignments provided in Supplementary Data, Table S4.6. Evidence of the ionic interaction between the ligands of compounds 9 and 10 is also provided by the presence of a C-H signal at 136.7 ppm and 137.3 ppm associated with the carbenic carbon (C15) of the NHC+ moiety for 9 and 10, respectively. Similarly, a downfield movement in the signal for the quaternary carbon associated with the carbonyl carbon of the carboxylate group of the coumarin (C11) close to the site of the ligand interaction, from 65.1 to 68.3 ppm and 64.8 to 68.6 ppm for 9 and 10, respectively, was observed, while a slight upfield movement in the C12 signal was also observed.
To synthesise the silver(I) complexes of 9 and 10, the ionic intermediate compounds were stirred in anhydrous acetonitrile containing a 0.2 mmol excess of silver oxide for 72 h at room temperature in a nitrogen-charged flask with the exclusion of light to isolate complex 11 in 82% yield. Despite repeated attempts, the silver(I) complex of 10 could not be isolated in its pure form. Adduct 11 was isolated as an amorphous solid; therefore, its formation was first confirmed by NMR spectroscopy. The 1H NMR spectrum of 11 is provided in Figure 6 with the same numbering system (Figure 5) used for the assignment of the 1H and 13C NMR spectra of 9. The H11 NMR signal of 11 moved downfield from 4.37 to 4.54 ppm, while all other chemical shifts of the protons associated with the coumarin ligand remained largely the same. In contrast, the NMR signals for the protons associated with the NHC* ligand of 11 moved upfield when compared to those of the ionic intermediate 9. In particular, the NMR signals for the protons of the two -CH2 groups (H17), closest to the silver ion, experienced the greatest shielding effect moving upfield from 5.42 to 5.33 ppm. The 13C NMR signals of the coumarin ligand experienced a slight upfield shift upon the formation of the carbene adduct complex, similar to the carbon signals of the NHC* ligand.
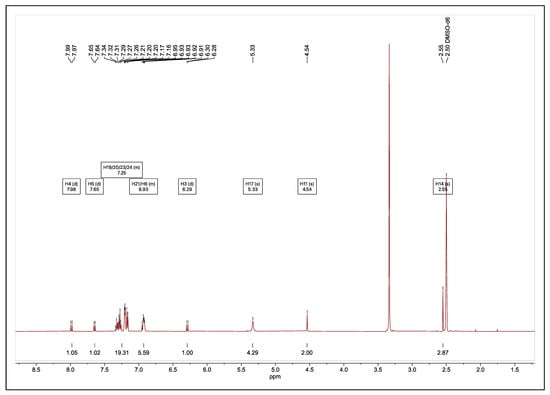
Figure 6.
1H NMR spectrum of [(8-acetyl-C-7-oxyaceto)(Ag)(NHC)] (11) recorded in DMSO-d6.
When the main IR bands of 11 (Table 5) were compared to those of the corresponding ionic intermediate, the appearance of a new band at 1398 cm−1, corresponding to an asymmetric stretch of this carboxylate group, indicated the formation of the complex. The difference in the symmetric and asymmetric stretches of the carboxylate function is slightly >200 cm−1, which would typically indicate an unidentate coordination mode of the coumarin ligand. Previous coumarin−carbene heteroleptic complexes isolated via the in situ generation of a free carbene under Schlenk conditions, followed by reaction with a coumarin silver(I) complex, resulted in the formation of a complex with two silver ions, forming a strong argentophilic Ag(I)−Ag(I) interaction with one silver(I) ion bound via unidentate coordination to two coumarin carboxylate ligands, and the other silver ion bound to two carbene ligands. Isolation via the ionic carbene route used here led to more complex aggregates, and the IR spectra of the amorphous solids indicated that monodentate or ionic bonding of the carboxylate to the silver ions is likely in all cases, given the values of Δν(oco) of >200 cm−1 recorded. However, the broad IR bands observed for the asymmetric and symmetric carboxylate bands do not preclude different coordination modes for adduct complexes in the solid state. Microanalytical data indicate that the complex formed is consistent with an empirical formulation of a 1:1:1 ratio of coumarin:Ag:NHC. Extensive efforts were made to form crystals of the complex, but these were not successful; therefore, MS analysis was performed to determine the exact structure of the complex. The MS data for 11 show the presence of a [NHC-Ag-NHC]+ moiety (Table S4.7); thus, as in the case of the previous coumarin−carbene silver(I) adducts isolated by ion exchange, the complexes may exist essentially as ion pairs held together by strong argentophilic interactions. However, such species may well form in the gas phase; therefore, the actual structure of the adduct complex remains unresolved. There was no evidence for the [coumarin-Ag-coumarin]− ion, as it appeared to be unstable in the MS experiments. However, in each case, the parent coumarin ligand was observed. The observed isotopic pattern for the [NHC-Ag-NHC]+ species of 11 was identical to that reported by Khe et al. for dinuclear silver(I)-NHC complexes [21]. In addition, it has been suggested by other workers that polar solvents such as methanol have been shown to stabilise the ionic [NHC-Ag-NHC]+ species and may possibly shift the equilibrium towards bis NHC-Ag complexes [22,23]. The photostability of 11 was examined by 1H NMR spectroscopy in deuterated DMSO and CDCl3 over a 96 h time period in the absence and presence of light and displayed excellent light stability in DMSO under both conditions (Figure S3.6a–d). Compared to the parent silver(I) complex 3, which was only soluble in hot DMSO, the solubility of 11 extended to multiple different solvents such as toluene, ethyl acetate, ethanol, methanol, dichloromethane and acetonitrile.
Isolation of the full suite of 7-acetoxy coumarin adduct complexes was not successful; thus, the Kirby Bauer method was used to assess the antibacterial activity of the 8-acetoxy-derived silver(I) complex 3 and its corresponding triphenylphosphino-derived adduct 5 and NHC carbene-derived adduct (11), and the results are given below. For comparison, we included coumarin-3-carboxylatosilver(I) [Coumarin-3-COOAg(I)] (12), a compound previously isolated by us which had shown excellent antimicrobial activity against MRSA and E. coli [24]. In addition, commonly prescribed antibiotics were included in these studies for reference.
2.3.1. Determination of Growth Inhibitory Activity of 8-Acetyl-C-7-oxyacetoAg(I)] and Its Triphenylphosphine and NHC Adducts Against MRSA and E. coli
The antimicrobial susceptibility of E. coli (ATCC-25922) and MRSA (ATCC-4330) to ligand 1, silver(I) complex 3, coumarin-3-COOAg(I) (12), and the bis-triphenylphosphino and NHC adducts 5 and 11, respectively, were determined via the Kirby Bauer disk diffusion assay [25]. Ligand 1 was found to be inactive against both E. coli and MRSA, with the diameters of the zones of inhibition measuring 0 mm. In addition, commercially available coumarin-3-carboxylic acid and triphenylphosphine were also found to have no inhibitory activity on the growth of the bacterial strains tested.
The data presented in Table 7 show the growth inhibitory effects (mm) of coumarin ligands, silver(I) complexes, and adducts synthesised at contents of 5 and 10 µg against E. coli and MRSA. For the complexes and adduct 11, a greater inhibitory effect was observed as the amount of compound to which both bacteria were exposed doubled. Silver(I) complexes 3 and 12 and the corresponding NHC adduct of 3, compound 11, were both shown to inhibit the growth of E. coli at both 5 and 10 µg. When compared to the reference compound SBC3, which possesses the same NHC ligand, they both inhibited the growth of E. coli more effectively at both concentrations with zones of inhibition > 6.9 mm. Despite this, the inhibitory effect of the silver(I) complexes and the NHC adduct tested did not compare to the activity of the control antibiotic, tetracycline, at either concentration.

Table 7.
Average diameter (mm) of the zone of inhibition of test compounds at 5 µg and 10 µg in DMSO (100%) against E. coli and MRSA.
The bis-TPP adduct 5 showed no inhibitory effect against the E. coli strain tested, though it showed similar activity against MRSA to the commercially available silver(I) nitrate and silver(II) oxide with zones of inhibition of 7.3 mm and 8.8 mm at concentrations of 5 and 10 µg, respectively, but was less active than the reference compound, SBC3, where the zone of inhibition at 5 µg and 10 µg disk content against MRSA were determined to be 10.2 and 12.3 mm respectively. Only silver(I) complex 12 matched the activity of SBC3, with zones of inhibition of 13.4 mm when tested against MRSA. Nevertheless, none of the complexes or adducts tested showed better activity against MRSA than the antibiotic control tetracycline.
In fact, the unsubstituted coumarin silver(I) complex 12 exhibited the best activity against MRSA, while it exhibited similar activity against E. coli as complex 3 and its corresponding NHC adduct 11, further indicating that the substituents do not greatly influence the activity of the coumarin scaffold in this case. In contrast, although bis-TPP complex 5 was inactive against E. coli, it displayed similar activity against MRSA as its original silver(I) complexes 3. Similar trends in activity were observed in a study carried out by Tacke et al. [26], where a bis-TPP adduct of a silver(I) benzoate complex was reported to be inactive against an E. coli strain, but showed good activity against MRSA.
To obtain a more accurate reflection of the antimicrobial activity of the complexes, the complexes synthesised were assessed by a further series of assays discussed below.
2.3.2. Determination of Antibacterial Activity of Synthesised Coumarin Silver(I) Complexes Using the Broth Microdilution Assay
Using the broth microdilution assay, the antibacterial activity of complexes 2-(2-oxo-2H-chromen-7-yl)- silver(I) complexes 3 and 4, ligand 2 and TPP adducts 5 and 6, and control complex 12 were determined against Escherichia coli (ATCC-25922), Pseudomonas aeruginosa (PA01 ATCCBAA-47), and methicillin-resistant Staphylococcus aureus (ATCC-43300) using the EUCAST guidelines [27]. Ligand 1 and coumarin-3-carboxylic acid have been extensively tested by our group for antimicrobial activity, and no activity has been noted [6,7,24]. The lack of definitive structural information regarding the exact mass of the NHC adduct 11 excluded that complex from this study. The relatively poor solubility of the silver(I) complexes in water meant that they were first dissolved in 100% DMSO before being diluted with MHB (Mueller Hinton Broth) to a stock concentration which contained 5% v/v DMSO. The highest concentration of DMSO present in the test solutions once prepared was 2.05% v/v; therefore, controls of DMSO from 0–2.05% v/v prepared in MHB were also tested. The positive and negative antibiotic controls used in this assay were amikacin and erythromycin. The test solutions of erythromycin were prepared using ethanol to aid the solubilisation of the antibiotic. The final concentration of ethanol to which the bacterial strains were exposed was 1.05%, and as with the DMSO control, it was found to have no inhibitory activity on the bacteria. The antibacterial activity of the complexes was initially screened using a single-broth microdilution experiment across a concentration range of 0–1024 µg/mL in triplicate. As precipitation of some of the complexes at a concentration of 256 µg/mL occurred, a final test concentration range of 0–128 µg/mL was determined, and then all silver(I) complexes, their TPP adducts, and controls were tested using the 0–128 µg/mL concentration range. Ligand 2 (Table S4.8) and triphenylphosphine showed no activity against the three strains of bacteria tested, which is common with the previously tested ligand 1 and coumarin-3-carbocylix acid.
The reference antibiotics used in this series of studies were amikacin and erythromycin. First used in the late 1970s, amikacin, a semi-synthetic aminoglycoside antibiotic, is primarily used to treat broad-spectrum bacterial infections [28]. Since its introduction in the clinic, this antibiotic has been listed as one of the most active aminoglycosides against antibiotic-resistant bacteria and remains one of the essential antibiotics used to treat infections caused by multidrug-resistant (MDR) organisms [29,30,31]. The second reference antibiotic used in this study was erythromycin. The broad-spectrum antimicrobial activity of this antibiotic, especially in the treatment of infections caused by gram-positive bacteria resistant to other drugs, was responsible for its popular use in the clinic after its discovery in 1952 [32]. However, due to its low solubility in water and instability in acidic conditions, the widespread use of this antibiotic quickly diminished. Another drawback of its use is the requirement of relatively large doses to elicit a bactericidal effect [33]. Since higher doses of antibiotics have been shown to promote drug resistance, it is no surprise that bacteria have developed multiple resistance mechanisms against the mode of action of this antibiotic [34]. Despite this, erythromycin is still widely used as a bacteriostatic antibiotic drug to treat bacterial infections, which often leads to the need for secondary antibiotic therapies to treat persistent infections [34]. As such, erythromycin was included in this study as a less effective and older antibiotic against the three bacterial strains, E. coli, P. aeruginosa and MRSA, while amikacin was included as the strong positive antibiotic control.
Figure 7, Figure 8 and Figure 9 show the results for the antimicrobial screening activity of the complexes. The coumarin-based ligands, ethanol, triphenylphosphine, and DMSO controls showed no activity against any of the bacterial strains tested. In addition, the bis-triphenylphosphino adducts, 5 and 6 tested, were found to have poor activity against the bacterial strains MRSA and E. coli, with MIC50 values > 88 µg/mL. However, Coumarin-3-COOAg and Coumarin-7-oxyacetoAg compounds had considerable activity at 16 µg/mL and 32 µg/mL, respectively, against E. coli and P. aeruginosa, as evidenced in Figure 7 and Figure 8 above.
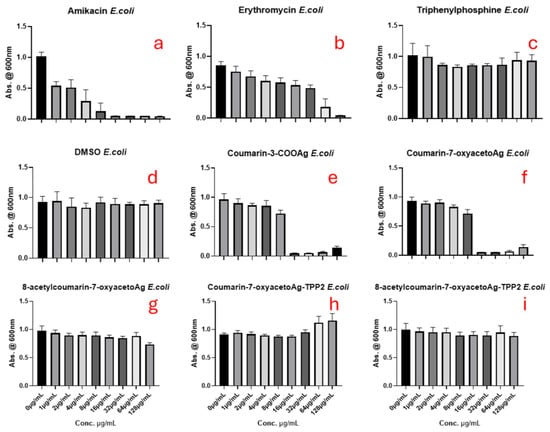
Figure 7.
The antimicrobial effects of (a) amikacin, (b) erythromycin, (c) triphenylphosphine (d) DMSO, (e) coumarin-3-COOAg (12), (f) coumarin-7-oxyacetoAg (4), (g) 8-acetylcoumarin-7-oxyacetoAg (3), (h) coumarin-7-oxyacetoAgTPP2 (6), and (i) 8-acetylcoumarin-7-oxyacetoAgTPP2 (5) on E. coli assessed by the micro broth dilution assay.
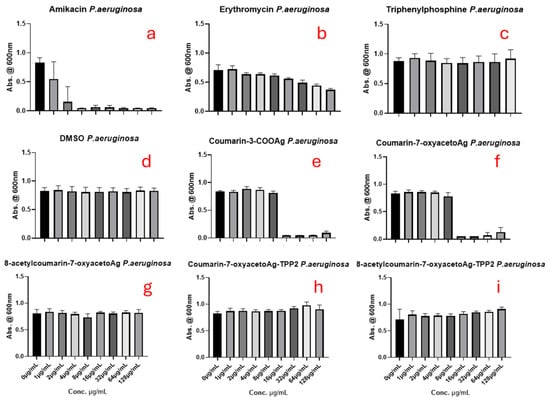
Figure 8.
The antimicrobial effects of (a) amikacin, (b) erythromycin, (c) triphenylphosphine (d) DMSO, (e) coumarin-3-COOAg (12), (f) coumarin-7-oxyacetoAg (4), (g) 8-acetylcoumarin-7-oxyacetoAg (3), (h) coumarin-7-oxyacetoAgTPP2 (6) and (i) 8-acetylcoumarin-7-oxyacetoAgTPP2 (5) on P. aeruginosa assessed by the micro broth dilution assay.
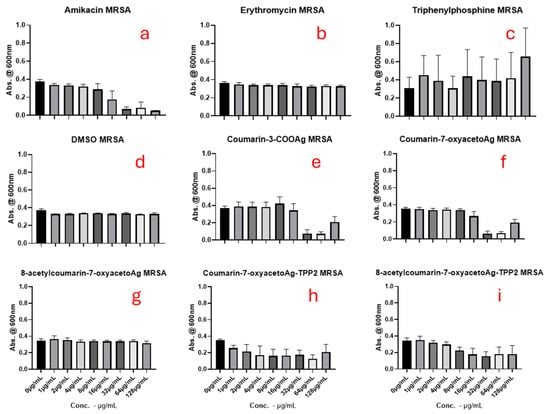
Figure 9.
The antimicrobial effects of (a) amikacin, (b) erythromycin, (c) triphenylphosphine (d) DMSO, (e) coumarin-3-COOAg (12), (f) coumarin-7-oxyacetoAg (4), (g) 8-acetylcoumarin-7-oxyacetoAg (3), (h) coumarin-7-oxyacetoAgTPP2 (6) and (i) 8-acetylcoumarin-7-oxyacetoAgTPP2 (5) on MRSA assessed by the micro broth dilution assay.
It was noted that complex 3 was inactive against all bacterial strains, which contradicts the findings of a previous study, where 3 had excellent antimicrobial activity against strains of MRSA and P. aeruginosa [7]. However, as the strains of bacteria used in this study differed from those used previously to screen 3, it would appear as if the complex is strain-specific, which is concerning. An additional concern is that this result contradicts the findings of the Kirby Bauer disk diffusion assay, where 3 was found to be the most effective in inhibiting the growth of E. coli, indicating that the bacterial growth media may also play a role.
Of all the complexes tested, complex 4 and the reference complex 12 were found to inhibit the growth of the three strains tested most effectively when compared to the untreated control, with activities at higher concentrations comparable to the control amikacin in the case of E. coli. The activity of complexes 4 and 12 against MRSA was better than that of the antibiotic control amikacin and, except against E. coli, performed better than erythromycin. Overall, these two silver(I) complexes offer excellent therapeutic potential against E. coli, P. aeruginosa, and MRSA, since their antibacterial activities are better than those of erythromycin and similar to those of amikacin. However, adduct formation, either through the formation of TPP adducts or the NHC carbene adduct, did not significantly improve their antimicrobial activity.
3. Materials and Methods
3.1. General Experimental Procedures
Melting point Analysis: All melting points were recorded on a Stuart Scientific SMP1 melting point apparatus. All values were taken up to 300 °C with a temperature ramp of 0.5 °C per minute and are uncorrected.
Infrared Spectroscopy: All samples were prepared as KBr discs, and infrared spectra were recorded in the region of 4000–400 cm−1 using an IR Prestige-21 Schimadzu infrared spectrometer (Supplied by Masons Technology Ltd., Dublin, Ireland).
Nuclear Magnetic Resonance Spectroscopy: NMR spectra were obtained using a Bruker Advance III 500 MHz spectrometer (Bruker Corporation, Billerica, MA, USA), with all chemical shifts reported as parts per million (ppm, δ). This instrument operated at 500 MHz with a typical resolution of 0.28 Hz for 1H NMR and 125 MHz with a typical resolution of 0.45 Hz for 13C NMR. All spectra were recorded in either DMSO-d6 or CDCl3. Signal assignments were aided using standard spectral experiments such as DEPT 90, DEPT 135 and HSQC.
Elemental Analysis: The percentage compositions (%C, %H, %N) of some compounds were determined at the Microanalytical laboratory by Ronan Crowley at University College Dublin using an Exeter CE440 elemental analyser (Exeter Analytical, Warwick, UK). Other compounds were analysed by Carmel O’Flaherty and Maryanne Ryan at the Microanalytical laboratory in NUI Maynooth using a Flash EA1112 series NC analyser (ThermoFisher Scientific, Dublin, Ireland), with Eager 300 software.
X-ray Crystallography: Crystals 5 and 6 were mounted on a MiTeGen micromount with NVH immersion oil. Data were collected from a shock-cooled single crystal at 150(2) K for 5 and 100(2) K for 6 on a Bruker D8 Quest ECO three-circle diffractometer ((Bruker AXS, Karlsruhe, Germany) with a sealed X-ray tube using graphite as the monochromator and a Bruker PHOTON 50 detector. The diffractometer was equipped with an Oxford Cryostream 800 low-temperature device (Oxford Cryosystems Ltd., Oxford, UK) and used Mo Kα radiation (λ = 0.71073 Å). All data were integrated with SAINT, and multi-scan absorption correction using SADABS was applied [35,36]. The structure was solved by dual methods using SHELXT v2018/2 and refined by full-matrix least-squares methods against F2 by SHELXL using Olex2 [37,38,39]. All non-hydrogen atoms were refined using anisotropic displacement parameters. All hydrogen atoms were refined isotropic on calculated positions using a riding model with their Uiso values constrained to 1.5 times the Ueq of their pivot atoms for terminal sp3 carbon atoms and 1.2 times for all other carbon atoms. The disordered moieties were refined using bond length restraints and displacement parameter restraints.
In 5, the solvent site is occupied with two different solvents, CH2Cl2 (13% occupied) and EtOH (12%), with a combined total of 25% occupancy, modelled with geometric (DFIX) and displacement restraints (RIGU, SIMU, ISOR). In 6, the solvent site CH2Cl2 is fully occupied.
See Table S4.5 for crystal data and structural details. Crystallographic data for the structures reported here have been deposited at the Cambridge Crystallographic Data Centre [40]. CDC 2396768-2396769 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
Atomic Absorption Spectroscopy: The silver content of the synthesised complexes was obtained using an iCE 3000 AA02184306 atomic absorption spectrometer (Agilent Technologies Ireland Ltd., Cork, Ireland) at a wavelength of 328.1 nm with a bandpass width of 0.5 nm. The samples for analysis were digested by boiling in concentrated nitric acid (10 mL) for 1 h. The samples were then diluted with water (50 mL). This stock solution was further diluted to 1/10 and filtered prior to aspiration. Five working standards (2, 3, 5, 7, and 10 ppm) were prepared from a silver standard from Sigma Aldrich (1000 ppm) to generate a calibration curve. All standards and samples were analysed in triplicate.
Liquid Chromatography/Mass Spectroscopy (LC/MS): Analysis of compounds was performed using an Agilent Technologies 6530 Accurate Mass Q-TOF LC/MS system (Agilent Technologies Ireland Ltd., Cork, Ireland) tuned in positive mode at 100–2500 M/Z, and data were interpreted using Mass Hunter Software packages-version 2.8. Samples for analysis were dissolved in mobile phase using HPLC-grade solvent and analysed on a C18 Phenomenex column using either acetonitrile: water: 0.1% acetic acid or methanol: water: 0.1% formic acid mobile phases with a flow rate of 0.4 mL/min. The injection volume was set to 1.0 µL. Silver(I) complexes were analysed by continuous infusion using a 50:50 methanol: water mobile phase.
Reagents and Solvents: All reagents were purchased from Sigma (Scientific laboratory Supplies, Dublin, Ireland), TCI chemicals (TCI Europe, Zwijndrecht, Belgium), Fisher Scientific (ThermoFisher Scientific, Dublin, Ireland)) or Fluorochem (Fluorochem Eu Ltd., Dublin, Ireland) and used without further purification. All solvents were purchased from Lennox and used again without further purification. LC-MS- and HPLC-grade solvents were purchased from Merck (Scientific laboratory Supplies, Dublin, Ireland). Stocks of anhydrous solvents were prepared by drying over appropriately sized molecular sieves, which were then purged with nitrogen prior to use in the reaction.
3.2. Synthesis of [(Bis-triphenylphosphino)-((8-acetyl-2-oxo-2H-chromene-7-yl)oxy]aceto Silver(I) (5) [8-AcetylC-oxyaceto-Ag-(TPP)2]
To a solution of triphenylphosphine (0.5245 g, 2.00 mmol) in dichloromethane, 8-acetylcoumarin-7-oxyaceto silver(I) (0.369 g, 1.00 mmol) complex was added. The reaction suspension was stirred for 6 h under reflux. Upon cooling, the precipitated solid was filtered under vacuum, washed with cold solvent, and a white-coloured solid was isolated and dried in a vacuum oven at 50 °C for several days. Recrystallisation from DCM/ethanol produced crystals suitable for X-ray crystallographic analysis. Molecular formula: C49H39O6AgP2; Yield: 0.63 g (71%); M.P. (°C):141.2–144.5; 1H NMR (DMSO-d6) (ppm): 7.93 (d, 1H, J = 9.6 Hz, H4), 7.50–7.43 (m, 6H, HTPP), 7.42 (d, 1H, J = 8.8 Hz, H5), 7.38–7.27 (m, 25H, HTPP), 6.80 (d, 1H, J = 8.8 Hz, H6), 6.27 (d, 1H J = 9.6 Hz, H3), 4.52 (s, 2H, H11), 2.53 (s, 3H, H16); 13C NMR (DMSO-d6) (ppm): 198.8 (C15), 170.5 (C12), 159.6 (C2), 158.3 (C7), 150.2 (C9), 144.4 (C4), 132.9–132.8 (d, -CHortho, TPP), 131.6–131.4 (QC, TPP), 129.8 (C5), 129.1 (s, -CHpara, TPP), 128.4–128.3 (d, -CHmeta, TPP), 117.8 (C8), 111.9 (C3), 111.4 (C10), 108.9 (C6), 67.5 (C11), 31.4 (C16); IR (KBr, cm−1): 342, 3054, 1731, 1601, 1479, 1435, 1402, 1353, 1291, 1263, 1229, 1138, 1093, 1027, 997, 833, 744, 694, 515, 501; CHN (%): theory: C = 65.86, H = 4.40, Ag = 11.97; found: C = 66.00, H = 4.31, Ag = 12.07.
3.3. Synthesis of [Bis-triphenylphosphino)-((2-oxo-2H-chromene-7-yl)oxy]aceto Silver(I) (6) [C-7-oxyaceto-Ag-(TPP)2]
Using the general synthesis described above, this compound was synthesised using the following reagents: coumarin-7-oxyaceto silver(I) (0.327 g, 1.00 mmol) and triphenylphosphine (0.524 g, 2.00 mmol) with dichloromethane as the reaction solvent. A white-coloured solid was isolated. Crystals suitable for X-ray crystallographic analysis were obtained by the slow diffusion of pentane into dichloromethane. Molecular formula: C47H37O5P2Ag; Yield: 0.66 g (78%); M.P. (°C): 139.2–140.1; 1H NMR (DMSO-d6) (ppm): 7.96 (d, 1H, J = 9.5 Hz, H4), 7.53–7.35 (m, 37H, H5, HTPP), 6.82 (dd, 1H, J = 8.7, 2.5 Hz, H6), 6.74 (d, 1H, J = 2.4 Hz, H8), 6.24 (d, 1H, J = 9.5 Hz, H3), 4.42 (s, 2H, H11); 13C NMR (DMSO-d6) (ppm): 170.7 (C12), 162.3 (C2), 160.4 (C7), 155.3 (C9), 144.4 (C4), 133.6–133.5 (d, -CHortho, TPP), 131.9–131.7 (QC, TPP), 130.6 (C5), 129.1–129.0 (d, -CHmeta, TPP), 112.9 (C6), 111.9 (C3), 111.8 (C10), 101.2 (C8), 67.9 (C11); IR (KBr, cm−1): 3051, 1722, 1608, 1581, 1505, 1479, 1435, 1404, 1329, 1278, 1227, 1194, 1121, 1095, 1041, 843, 749, 693, 515, 503; CHN (%): theory: based on C47H37O5P2Ag.CH2Cl2: C = 61.56, H = 4.20, Ag = 11.52; found: C = 62.59, H = 4.14, Ag = 11.71.
3.4. Synthesis of Intermediate Compounds Required for the Isolation of [(1,3-Dibenzyl-4,5-diphenyl imidazole-2-ylidene)–(substituted-2-oxo-2H-chromene-7-yl)]oxyaceto Silver(I) Complexes
3.4.1. General Synthesis of Sodium Salts of 2H-Chromene-2-One Derived Ligands
The appropriately substituted 2H-chromene-2-one-derived ligand was stirred in methanol at 50 °C until it was fully dissolved. To this, a methanolic solution of sodium hydroxide was added, with a suspension forming upon addition. The resulting suspension was stirred at 50 °C for 6 h. Upon cooling, the product was isolated via vacuum filtration, washed with cold methanol (2 × 10 mL) followed by cold ethyl acetate (2 × 10 mL) and dried in a vacuum oven at 50 °C for 24 h.
8-Acetylcoumarin-7-Oxyaceto Sodium Salt (7) [8-acetyl-C-7-oxyacetoNa]
Using the general synthesis described above, this compound was synthesised using the following reagents: 7-methoxycoumarin-3-carboxylic acid (1.10 g, 5.00 mmol) and sodium hydroxide (0.20 g, 5.00 mmol). An off-white coloured powder was obtained. Molecular Formula: C13H9O6Na; Yield: 1.06 g, 83%; M.P. (°C): >300; IR (KBr, cm−1): 3482, 3431, 3397, 1728, 1715, 1609, 1557, 1489, 1420, 1402, 1294, 1260, 1225, 1140, 1908, 729, 773, 514; C13H9O6Na.H2O: CHN (%): theory: C = 51.67, H = 3.19; found: C = 51.63, H = 3.48.
Coumarin-7-Oxyaceto Sodium Salt (8) [C-7-oxyacetoNa]
Using the general synthesis described above, this compound was synthesised using the following reagents: coumarin-7-oxyacetic acid (1.50 g, 6.82 mmol) and sodium hydroxide (0.27 g, 6.82 mmol). A white-coloured powder was obtained. Molecular Formula: C11H7O5Na; Yield: 1.49 g (91%); M.P. (°C): >300; IR (KBr, cm−1): 3438, 3092, 3056, 2949, 2923, 1741, 1617, 1508, 1444, 1429, 1402, 1347, 1283, 1274, 1236, 1201, 1164, 1127, 1055, 995, 838, 821, 688, 619; C11H7O5Na.H2O CHN (%): theory: C = 50.78, H = 3.49 found: C = 49.69, H = 3.71.
3.5. General Synthesis of [(1,3-Dibenzyl-4,5-diphenyl-imidazole-2-ylidene)+-(substituted)-(2-oxo-2H-chromene-7-yl)oxy]acetate]− Ionic Intermediates
In a biphasic solution of dichloromethane and deionised water (1:1, 40 mL), the appropriate substituted coumarin sodium salt (1.50 mmol) and [imid Br] were stirred vigorously at 40 °C for 24 h. Upon cooling, the reaction mixture was transferred to a separation funnel, and the dichloromethane layer containing the product was collected. The remaining water layer was extracted with fresh dichloromethane (2 × 10 mL). The dichloromethane layers were collected and pooled together and washed gently with deionised water (2 × 10 mL). The dichloromethane layer was collected, and the solvent was removed under reduced pressure in a water bath heated to 30 °C to obtain a hygroscopic amorphous fluffy solid or oil.
3.5.1. [1,3-Dibenzyl-4,5-diphenylimidazole-2-ylidene)]+-[(8-acetylcoumarin-7-oxyacetate)− (9) [8-acetylcou-7-oxyacetNHC]
This ionic intermediate was synthesised using the method described above, using the following reagents: [imid Br] (0.4814 g, 1.00 mmol) and 8-acetyl-C-7-oxyacetoNa (0.426 g, 1.50 mmol) A white coloured amorphous solid was obtained. Molecular Formula: C42H34O6N2; Yield: 0.596 g (90%); 1H NMR (DMSO-d6) (ppm): 9.72 (s, 1H, H15), 7.99 (d, 1H, J = 9.5 Hz, H4), 7.64 (d, 1H, J = 8.7 Hz, H5), 7.46–7.26 (m, 18H, H19/20/23/24), 7.08 (m, 4H, H21/25), 6.90 (d, 1H, J = 8.7 Hz, H6), 6.28 (d, 1H, J = 9.5 Hz, H3), 5.42 (s, 4H, H17), 4.37 (s, 2H, H11), 2.57 (s, 3H, H14); 13C NMR (DMSO-d6) (ppm): 199.6 (C13), 168.3 (C12), 159.7 (C2), 158.8 (C7), 150.2 (C9), 144.5 (C4), 136.7 (C15), 134.1 (C16), 131.8 (C22), 130.8 (C19), 130.2 (C5), 129.8 (C20), 128.8 (C24), 128.5 (C23), 127.8 (C21), 124.9 (C18), 118.3 (C8), 112.3 (C3), 111.8 (C10), 109.8 (C6), 68.3 (C11), 50.5 (C17), 32.1 (C14); IR (KBr, cm−1): 3401, 3059, 3032, 1724, 1601, 1553, 1489, 1456, 1445, 1402, 1534, 1288, 1263, 1226, 1140, 1096, 1020, 930, 837, 764, 7--, 635, 598; CHN (%): theory: C = 72.19, H = 5.48, N = 4.01; found: C = 71.85, H = 5.21, N = 4.15.
3.5.2. [1,3-Dibenzyl-4,5-diphenylimidazole-2-ylidene)]+-[Coumarin-7-oxyacetate]− (10) [cou-7-oxyacetNHC]
This ionic intermediate was synthesised using the method described above using the following reagents: [imid Br] (0.4814 g, 1.00 mmol) and cou-7-oxyacetoNa (0.363 g, 1.5 mmol). A colourless oil was obtained. Molecular Formula: C40H32O5N2; Yield: 0.594 g (96%); 1H NMR (DMSO-d6) (ppm): 9.73 (s, 1H, H15), 7.96 (d, 1H, J = 9.9 Hz, H4), 7.57 (d, 1H, J = 8.7 Hz, H5), 7.45–7.26 (m, 17H, H19/20/23/24), 7.12–7.04 (m, 4H, H21/25), 6.85 (dd, 1H, J = 8.6, 2.4 Hz, H6), 6.76 (d, 1H, J = 2.5 Hz, H8), 6.24 (d, 1H, J = 9.5 Hz, H3), 5.42 (s, 4H, H17), 4.38 (s, 2H, H11); 13C NMR (DMSO-d6) (ppm): 169.1 (C12), 163.1 (C7), 160.9 (C2), 155.7 (C9), 144.9 (C4), 137.3 (C15) 134.5 (C16), 132.3 (C22), 131.3 (C19), 130.6 (C20), 129.5 (C5), 129.3 (C21), 129.3 (C25), 128.9 (C24), 128.3 (C23), 125.4 (C18), 113.3 (C6), 112.2 (C3), 112.1 (C10), 101.7 (C8), 68.6 (C11), 50.9 (C17); IR (NaCl plate, cm−1): 3049, 1728, 1613, 1554, 1498, 1455, 1446, 1401, 1354, 1335, 1277, 1232, 1192, 1157, 1133, 1123, 1076, 1045, 1021, 979, 928, 892, 839.
3.6. Synthesis of [(1,3-Dibenzyl-4,5-diphenyl imidazole-2-ylidene)–(8-acetoxy-2-oxo-2H-chromene-7-oxyaceto)] Silver(I) (11) via an Ion Exchange Route
8-acetyl-C-7-oxyacetoNHC intermediate (0.596 g, 0.90 mmol) was dissolved in anhydrous acetonitrile (5 mL), and the solution was degassed with nitrogen. This solution was injected into a flask containing a suspension of silver oxide (0.127 g, 0.55 mmol) in anhydrous acetonitrile, with the resulting suspension stirred under nitrogen in the absence of light at room temperature for 72 h. Following this time, the reaction suspension was filtered by gravity to remove the excess silver oxide. The filtrate was collected and filtered by gravity, followed by filtration through a grade 4 sinter funnel. The final filtrate was collected, and the solvent was removed under reduced pressure in a water bath heated to 35 °C to obtain a light brown-coloured amorphous fluffy solid. Molecular formula: C42H33O6N2Ag, Yield: 0.56 g, 82%; 1H NMR: (500 MHz) (DMSO-d6) (ppm): 7.98 (d, 1H, J = 9.5 Hz, H4), 7.65 (d, 1H, J = 8.7 Hz, H5), 7.35–7.15 (m, 19H, H19/21/23/24), 6.96–6.90 (m, 6H, H16/21), 6.29 (d, 1H, J = 9.5 Hz, H3), 5.33 (s, 4H, H17), 4.54 (s, 2H, H11), 2.55 (s, 3H, H14); 13C NMR (DMSO-d6) (ppm): 199.4 (C13), 170.6 (C12), 159.6 (C2), 158.3 (C7), 150.2 (C9), 144.3 (C4), 136.7 (C15), 132.2 (C16), 130.6 (C5), 129.9 (C23), 129.2 (C24), 128.5 (C22), 127.7 (C19), 127.5 (C18), 126.8 (C21), 118.4 (C8), 112.6 (C3), 112.1 (C10), 109.7 (C6), 67.9 (C11), 52.6 (C17), 32.1 (C14); IR (KBr, cm−1): 3061, 3030, 1728, 1601, 1558, 1489, 1447, 1398, 1352, 1287, 1263, 1227, 1140, 1060, 1022, 926, 835, 768, 735, 700; CHN (%): theory: C = 65.55 H = 4.32, N = 3.64, Ag = 14.02; found: C = 66.62, H = 4.35, N = 3.87, Ag = 14.42.
3.7. Details of Bacterial Strains Used for Biological Studies
The Escherichia coli (ATCC-25922) and methicillin-resistant Staphylococcus aureus (ATCC-43300) test strains used were obtained from frozen stocks of the collaborative group in UCD, while the Pseudomonas aeruginosa (PA01 ATCCBAA-47) test strain was obtained from frozen stocks from the microbiological lab in TU Dublin Tallaght. Fresh frozen stocks of each of the test strains were made by culturing each strain individually in Tryptone Soy Broth (TSB) and storing them with 10% glycerol at −80 °C. Prior to their use in the experiment, they were passaged twice on a Tryptone Soy Agar plate (TSA).
The general method for Kirby Bauer disk diffusion as per the EUCAST guidelines was previously published [24].
Preparation of Microtitre Test Plates for the Broth Microdilution Assay
The 96-well microtitre plates were prepared five days prior to the experiment being carried out. Once the plates were loaded with the silver(I) complexes and controls, they were stored in a freezer at −20 °C for a maximum of five days before being used. Prior to being used in the experiment, the plates were thawed in an incubator at 37 °C. The concentration (0–256 µg/mL) of the suspensions of each silver(I) complex was prepared individually and loaded (50 µL) onto a 96-well microtitre plate. Separately, the antibiotic, DMSO and ethanol controls were serially diluted across the microtitre plate with MHB (50 µL) so that concentrations of 0–256 µg/mL were obtained. MHB was also included in each microtitre plate as a negative control in triplicate.
The bacteria test strains were cultured overnight in MHB (10 mL) for 18 h. Fresh MHB (100 mL) was inoculated with the overnight culture, and the bacteria grew to the mid-log phase (OD between 0.6 and 0.8). Once the mid-log phase was reached, the cultures were diluted with MHB to a concentration of 1 × 106 CFU/mL and loaded onto the microtitre plates already containing the complexes. The final volume in each well was 100 µL, the final concentration of the inoculum was 5 × 105 CFU/mL, and the final test concentration range obtained was 0–128 µg/mL. The plates were then incubated in a stack of three plates in an aerobic environment at 37 °C with agitation (~65 rpm) for 24 h. After this time, the microtitre plates were analysed on a multi-plate reader at an absorbance of 600 nm. The average OD at 600 nm for each complex tested was obtained, and the results are presented in the following section. Replicate experiments (n = 3) were carried out on independent days with independent overnight cultures of the test strains of bacteria. Six replicates of each complex were tested, giving a total of eighteen readings per complex, while the antibiotics and controls were tested in triplicate, giving a total of nine readings each.
4. Conclusions
This work detailed the synthesis and characterisation of heteroleptic complexes of coumarin, firstly the bis-triphenylphosphino adducts of acetoxy substituted and unsubstituted coumarin-7-oxyaceto silver(I) complexes 5 and 6. As the bis-TPP methoxy substituted coumarin-3-carboxylato silver(I) adducts were isolated previously, the bis-TPP coumarin-7-oxyaceto silver (I) adducts displayed an overall increased solubility profile when compared to the parent silver(I) complexes and relatively good photostability in DMSO-d6. The antimicrobial activity of the complexes was assessed via two different techniques, the Kirby Bauer disc diffusion assay and the broth microdilution assay, with the introduction of the TPP ligands reducing the activity of the complexes against E. Coli and P. aeruginosa in the case of the coumarin-7-oxyaceto derivative in both cases. All of these studies were actually prompted by the previously reported antimicrobial activity of the 8-acetylcoumarin-7-oxyacetosilver(I) complex [7], but the activity of that complex was very limited in these studies. Our previous work was carried out using different strains of these pathogenic bacteria; nevertheless, the results were surprising.
Analysis of 11 by 1H and 13C NMR spectroscopy, IR spectroscopy, microanalysis, and AAS indicated that this complex was likely formed as a complex aggregate species with an overall stoichiometry of 1:1:1 coumarin:Ag(I):NHC. However, despite forming these complex aggregates, the silver(I) adduct exhibited better photostability and increased solubility when compared to the parent silver(I) complex. Our ultimate goal of achieving water solubility was not achieved in this case. The Kirby Bauer method was used to determine the antimicrobial activity of the adduct complex, with the heteroleptic complex formation not adversely affecting its activity against E. coli and MRSA. Indeed, given its likely large molecular mass, the adduct complex has good antimicrobial activity at low concentrations, but our lack of a clear structural definition of the complex’s exact mass would likely hinder its development for further studies as a systemic therapeutic.
In future work, exploring the potential of combining these novel compounds with established antibiotics, such as erythromycin, could be a promising avenue to develop effective adjunct or combination therapies, enhancing their antimicrobial efficacy and addressing complex bacterial resistance mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29245917/s1. Scheme S1: Overall synthetic scheme for isolation of ligands 1 and 2 and complexes 3 and 4. Figure S1.1: Numbering system used for the assignment of 1H and 13C NMR spectra of 7-acetyl-2-(2-oxo- 2H-chromene-2-one). Figure S1.2: Numbering system used for the assignment of 1H and 13C NMR spectra of 8-acetyl-7-hydroxy- (2-oxo-2H-chromene-2-one). Figure S1.3: Numbering system used for the assignment of 1H and 13C NMR spectra of ethyl 2-(2-oxo-2H-chromene-substituted-yl)oxy acetates, where R = -COCH3 and -CO = C15 and -CH3 = C16. Figure S1.4: Numbering system used for the assignment of 1H and 13C NMR spectra of 2-(2-oxo-2H-chromen-substituted-yl)oxy acetic acid ligands and their corresponding silver(I) complexes, where R = -COCH3 and -CO = C15 and -CH3 = C16. Figure S1.5: Numbering system used for the assignment of 1H spectra of 1,3-dibenzyl-4,5-diphenyl-imidazolium bromide. Figure S3.1 (a): 1H NMR spectrum of [8acetyl-C-7-oxyaceto Ag(TPP2)] (5); recorded in DMSO-d6. Figure S3.1(b): 1H NMR spectrum of [C-7-oxyaceto Ag(TPP2)] (6); recorded in DMSO-d6. Figure S3.2a: 1H NMR solution photostability study of [8acetyl-C-7-oxyacetoAg(TPP2)] (5) in the presence of UV/Vis light over a 96 h period in DMSO-d6. Figure S3.2b: 1H NMR solution photostability study of [8acetyl-C-7-oxyacetoAg(TPP2)] (5) in the absence of UV/Vis light over a 96 h period in DMSO-d6. Figure S3.2c: 1H NMR solution photostability study of [C-7-oxyacetoAg(TPP2)] (6) in the absence of UV/Vis light over a 96 h period in DMSO-d6. Figure S3.2d: 1H NMR solution photostability study of [C-7-oxyacetoAg(TPP2)] (6) in the presence of UV/Vis light over a 96 h period in DMSO-d6. Figure S3.3a. Molecular structure of [bis-triphenylphosphino)-(8-acetyl-2-oxo-2H-chromen-7-oxyaceto]silver(I) (5) showing disordered the disordered solvent site with two different solvents, CH2Cl2 (13% occupied) and EtOH (12%), combined total 25% occupancy. Displacement shown at 50% probability. Heteroatoms labelled only. Figure S3.3b. Molecular structure of [bis-triphenylphosphino)-(8-acetyl-2-oxo-2H-chromen-7-oxyaceto]silver(I) (5) showing H-bonding interactions. Figure S3.4a Molecular structure of [bis-triphenylphosphino)-(2-oxo-2H-chromen-7-oxyaceto]silver(I) (6) showing the fully occupied CH2Cl2 solvent site. Displacement shown at 50% probability. Heteroatoms labelled only. Figure S3.4b Molecular structure of [bis-triphenylphosphino)-(2-oxo-2H-chromen-7-oxyaceto]silver(I) (6) showing H-bonding interactions. Figure S3.5: 1H NMR spectrum of [|(8-acetylcou-7-oxyacet)(NHC)] (10) recorded in DMSO-d6. Figure S3.6 a: Stability of 11 examined in DMSO-d6 (absence of light). Figure S3.6 b: Stability of 11 examined in DMSO-d6 (presence of UV/Vis light). Figure S3.6 c: Stability of 11 examined in CDCl3 (absence of light). Figure S3.6 d: Stability of 11 examined in CDCl3 (presence of UV/Vis light). Table S4.1: Compound names and abbreviations. Table S4.2: Experimental yields (%), melting points (°C ) and main IR bands of compounds 1–4. Table S4.3: Experimental and literature values of 1–6, showing chemical shifts (ppm), multiplicity patterns and J values (Hz); recorded in DMSO-d6; OacetH gp. (oxyacetic acid group) = -OCH2COOH; Acet. gp (acetyl group) = -COOCH3. Table S4.4: 13C NMR data of 1–6 showing the chemical shifts (ppm) recorded in DMSO-d6. Table S4.5: Crystallographic and structural refinement data for 5 and 6. Table S4.6: NMR spectral data for compounds 9–11 showing the chemical shifts (ppm), multiplicity patterns and J-values (Hz); recorded in DMSO-d6; *n/o = not observed. Table S4.7: Table of MS Data for Adduct 11. Table S4.8. Growth Curves for ligand 2 against E. coli, MRSA and P. aeruginosa, measured using the Micro Broth Dilution Assay.
Author Contributions
Conceptualization, F.K. and B.S.C.; Methodology, E.M., B.T., G.C., E.C. and M.T.; Software, B.T.; Formal analysis, E.M., B.T., G.C., E.C., F.K. and B.S.C.; Investigation, E.M. and B.T.; Resources, B.S.C.; Data curation, E.M. and B.S.C.; Writing—original draft, E.M. and B.S.C.; Writing—review & editing, B.T., G.C., E.C., M.T. and F.K.; Supervision, G.C., E.C., M.T., F.K. and B.S.C.; Project administration, F.K. and B.S.C.; Funding acquisition, F.K. and B.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the HEA Funded TU4D INITIATIVE 2018 (PHEA-1705) and the Centre of Applied Science for Health, TU Dublin.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- UNEP. Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance; UNEP: Nairobi, Kenya, 2023. [Google Scholar]
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, P.; Kumar, M. Antimicrobial Resistance Ignited by COVID-19 Pandemic: SOS for Antimicrobial Stewardship. In Wastewater Surveillance for COVID-19 Management; Kumar, M., Kuroda, K., Mukherjee, S., Ngiehm, L.D., Vithanage, M., Tyagi, V.K., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 323–336. [Google Scholar]
- Sullivan, M.; Kia, A.F.-A.; Long, M.; Walsh, M.; Kavanagh, K.; McClean, S.; Creaven, B.S. Isolation and characterisation of silver(I) complexes of substituted coumarin-4-carboxylates which are effective against Pseudomonas aeruginosa biofilms. Polyhedron 2014, 67, 549–559. [Google Scholar] [CrossRef]
- Jaiswal, S.; Bhattacharya, K.; Sullivan, M.; Walsh, M.; Creaven, B.S.; Laffir, F.; Duffy, B.; McHale, P. Non-cytotoxic antibacterial silver–coumarin complex doped sol–gel coatings. Colloids Surf. B Biointerfaces 2013, 102, 412–419. [Google Scholar] [CrossRef]
- Mujahid, M.; Trendafilova, N.; Arfa-Kia, A.F.; Rosair, G.; Kavanagh, K.; Devereux, M.; Walsh, M.; McClean, S.; Creaven, B.S.; Georgieva, I. Novel silver(I) complexes of coumarin oxyacetate ligands and their phenanthroline adducts: Biological activity, structural and spectroscopic characterisation. J. Inorg. Biochem. 2016, 163, 53–67. [Google Scholar] [CrossRef]
- Mujahid, M.; Kia, A.F.-A.; Duff, B.; Egan, D.A.; Devereux, M.; McClean, S.; Walsh, M.; Trendafilova, N.; Georgieva, I.; Creaven, B.S. Spectroscopic studies, DFT calculations, and cytotoxic activity of novel silver(I) complexes of hydroxy ortho-substituted-nitro-2H-chromen-2-one ligands and a phenanthroline adduct. J. Inorg. Biochem. 2015, 153, 103–113. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, W.; Qiao, K.; Feng, J.; Zhu, L.; Zhu, X. Bioavailability and toxicity of silver nanoparticles: Determination based on toxicokinetic–toxicodynamic processes. Water Res. 2021, 204, 117603. [Google Scholar] [CrossRef]
- Gupta, D.; Guliani, E.; Bajaj, K. Coumarin—Synthetic Methodologies, Pharmacology, and Application as Natural Fluorophore. Top. Curr. Chem. 2024, 382, 16. [Google Scholar] [CrossRef]
- Martin, A.; De Menezes, I.R.A.; Sousa, A.K.; Farias, P.A.M.; Dos Santos, F.A.V.; Freitas, T.S.; Figueredo, F.G.; Ribeiro-Filho, J.; Carvalho, D.T.; Coutinho, H.D.M.; et al. In vitro and in silico antibacterial evaluation of coumarin derivatives against MDR strains of Staphylococcus aureus and Escherichia coli. Microb. Pathog. 2023, 177, 106058. [Google Scholar] [CrossRef]
- Yildirim, M.; Poyraz, S.; Ersatir, M. Recent advances on biologically active coumarin-based hybrid compounds. Med. Chem. Res. 2023, 32, 617–642. [Google Scholar] [CrossRef]
- Mooney, E.; Tacke, M.; Müller-Bunz, H.; Bruno-Colmenárez, J.; Cooke, G.; Caraher, E.; Kelleher, F.; Creaven, B.S. Hybrid silver(I) coumarin-carbene and coumarin-triphenylphosphine complexes: Towards more effective antimicrobial therapies. Inorganica Chim. Acta 2024, 572, 122222. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) N-Heterocyclic Carbene Complexes: A Winning and Broad Spectrum of Antimicrobial Properties. Int. J. Mol. Sci. 2021, 22, 2497. [Google Scholar] [CrossRef]
- Napoli, M.; Saturnino, C.; Cianciulli, E.I.; Varcamonti, M.; Zanfardino, A.; Tommonaro, G.; Longo, P. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013, 725, 46–53. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Sato, M.; Amano, A.; Hara, A.; Tsuruta, S.; Sugie, A.; Nomiya, K. Light-stable and antimicrobial active silver(I) complexes composed of triphenylphosphine and amino acid ligands: Synthesis, crystal structure, and antimicrobial activity of silver(I) complexes constructed with hard and soft donor atoms (n∞{[Ag(L)(PPh3)]2} with L = α-ala− or asn− and n = 1 or 2). Inorganica Chim. Acta 2008, 361, 1267–1273. [Google Scholar] [CrossRef]
- Sharkey, M.A.; O’Gara, J.P.; Gordon, S.V.; Hackenberg, F.; Healy, C.; Paradisi, F.; Patil, S.A.; Schaible, B.; Tacke, M. Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3. Antibiotics 2012, 1, 25–28. [Google Scholar] [CrossRef]
- Edwards, D.A.; Harker, R.M.; Mahon, M.F.; Molloy, K.C. Aerosol-assisted chemical vapour deposition (AACVD) of silver films from triorganophosphine adducts of silver carboxylates, including the structure of [Ag(O2CC3F7)(PPh3)2]. Inorganica Chim. Acta 2002, 328, 134–146. [Google Scholar] [CrossRef]
- Whitcomb, D.R.; Rogers, R.D. The molecular structure of [bis-triphenylphosphine-silver(I) stearate], [((C6H5)3P)2Ag(O2C(CH2)16CH3)], solubilization of long alkyl chain silver carboxylates. J. Chem. Crystallogr. 1996, 26, 99–105. [Google Scholar] [CrossRef]
- Han, J.; Shen, Y.; Li, C.; Li, Y.; Pan, Y. Synthesis and characterization of triphenylphosphine stabilized silver α,β-unsaturated carboxylate: Crystal structure of [Ag(O2CCHC(CH3)2)(PPh3)2]. Inorganica Chim. Acta 2005, 358, 4417–4422. [Google Scholar] [CrossRef]
- Khe, J.M.; Fong, Z.; Lee, W.L.; Tan, K.W.; Ting, A.S.Y.; Cheow, Y.L. Synthesis, characterisation and biological evaluation of novel bisimidazolium mononuclear and dinuclear silver(I)-N-heterocyclic carbene complexes with long N-alkyl chains. J. Organomet. Chem. 2024, 1009, 123076. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Bildstein, B.; Malaun, M.; Kopacka, H.; Wurst, K.; Mitterböck, M.; Ongania, K.-H.; Opromolla, G.; Zanello, P. N,N‘-Diferrocenyl-N-heterocyclic Carbenes and Their Derivatives. Organometallics 1999, 18, 4325–4336. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterization and antimicrobial activity of a series of substituted coumarin-3-carboxylatosilver(I) complexes. Inorganica Chim. Acta 2006, 359, 3976–3984. [Google Scholar] [CrossRef]
- EUCAST Disk Diffusion Test Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 6 March 2024).
- O’Beirne, C.; Alhamad, N.F.; Ma, Q.; Müller-Bunz, H.; Kavanagh, K.; Butler, G.; Zhu, X.; Tacke, M. Synthesis, structures and antimicrobial activity of novel NHC∗- and Ph3P-Ag(I)-Benzoate derivatives. Inorganica Chim. Acta 2019, 486, 294–303. [Google Scholar] [CrossRef]
- Testing, T.E.C.o.A.S. EUCAST Reading Guide for Broth Microdilution. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 11 October 2024).
- Mahfouz, A.A.; Said, H.S.; Elfeky, S.M.; Shaaban, M.I. Inhibition of Erythromycin and Erythromycin-Induced Resistance among Staphylococcus aureus Clinical Isolates. Antibiotics 2023, 12, 503. [Google Scholar] [CrossRef]
- Brittain, D.C. Erythromycin. Med. Clin. North Am. 1987, 71, 1147–1154. [Google Scholar] [CrossRef]
- Fyfe, C.; Grossman, T.H.; Kerstein, K.; Sutcliffe, J. Resistance to Macrolide Antibiotics in Public Health Pathogens. Cold Spring Harb. Perspect. Med. 2016, 6, a025395. [Google Scholar] [CrossRef]
- Platon, V.-M.; Dragoi, B.; Marin, L. Erythromycin Formulations—A Journey to Advanced Drug Delivery. Pharmaceutics 2022, 14, 2180. [Google Scholar] [CrossRef]
- Harika, K.; Shenoy, V.P.; Narasimhaswamy, N.; Chawla, K. Detection of Biofilm Production and Its Impact on Antibiotic Resistance Profile of Bacterial Isolates from Chronic Wound Infections. J. Glob. Infect. Dis. 2020, 12, 129–134. [Google Scholar]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.B.; Dyavaiah, M.; Lin, X. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Mohamad, F.; Alzahrani, R.R.; Alsaadi, A.; Alrfaei, B.M.; Yassin, A.E.B.; Alkhulaifi, M.M.; Halwani, M. An Explorative Review on Advanced Approaches to Overcome Bacterial Resistance by Curbing Bacterial Biofilm Formation. Infect. Drug Resist. 2023, 16, 19–49. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bruker. SAINT, v8.40B; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Struct. Chem. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).